The Golden Activity of Lysinibacillus sphaericus: New Insights on Gold Accumulation and Possible Nanoparticles Biosynthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Samples
2.2. L. sphaericus Strain Identification
2.3. Calibration Curve for Gold Determination (HAuCl4)
2.4. Metal Sorption in L. sphaericus Strains
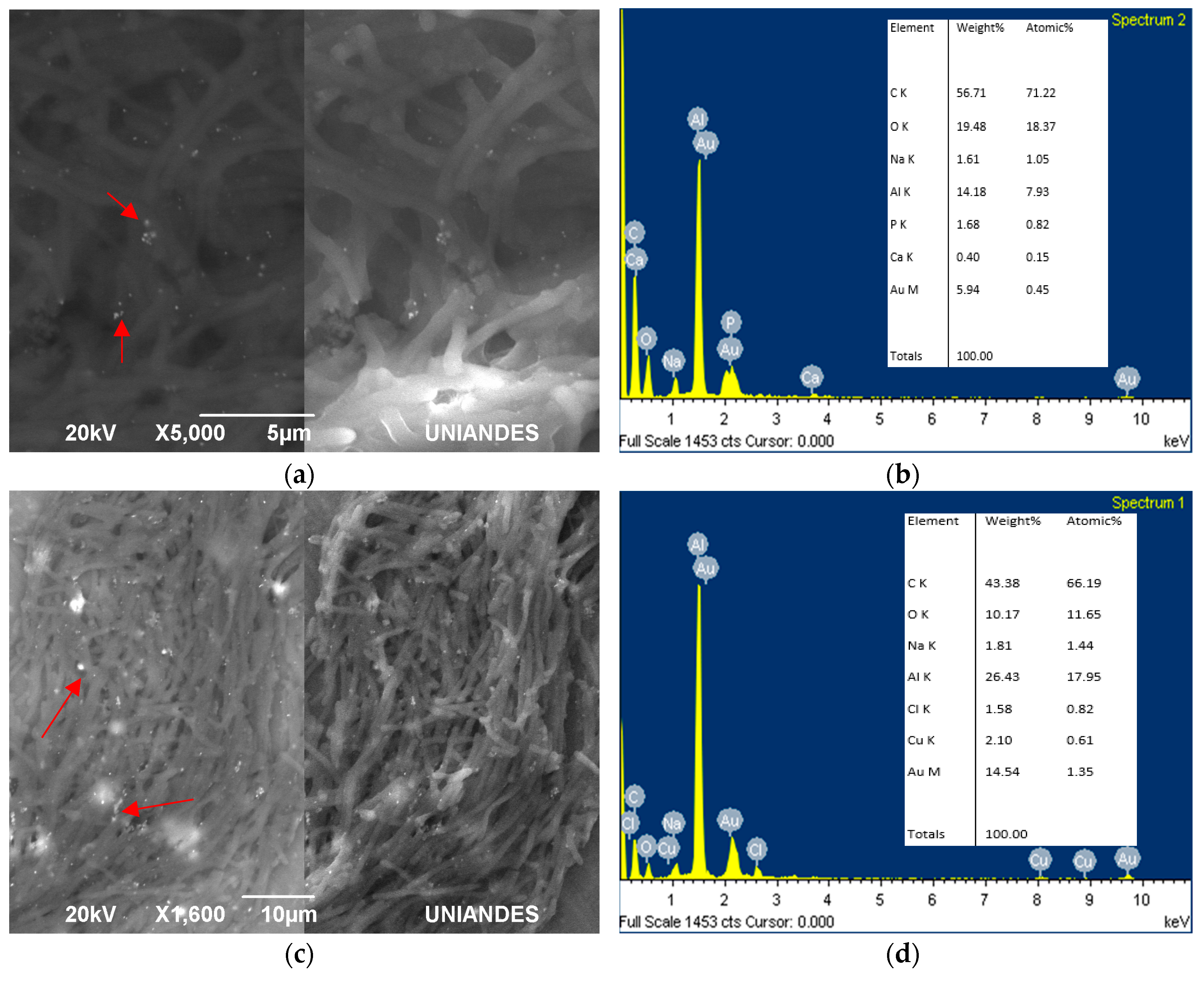
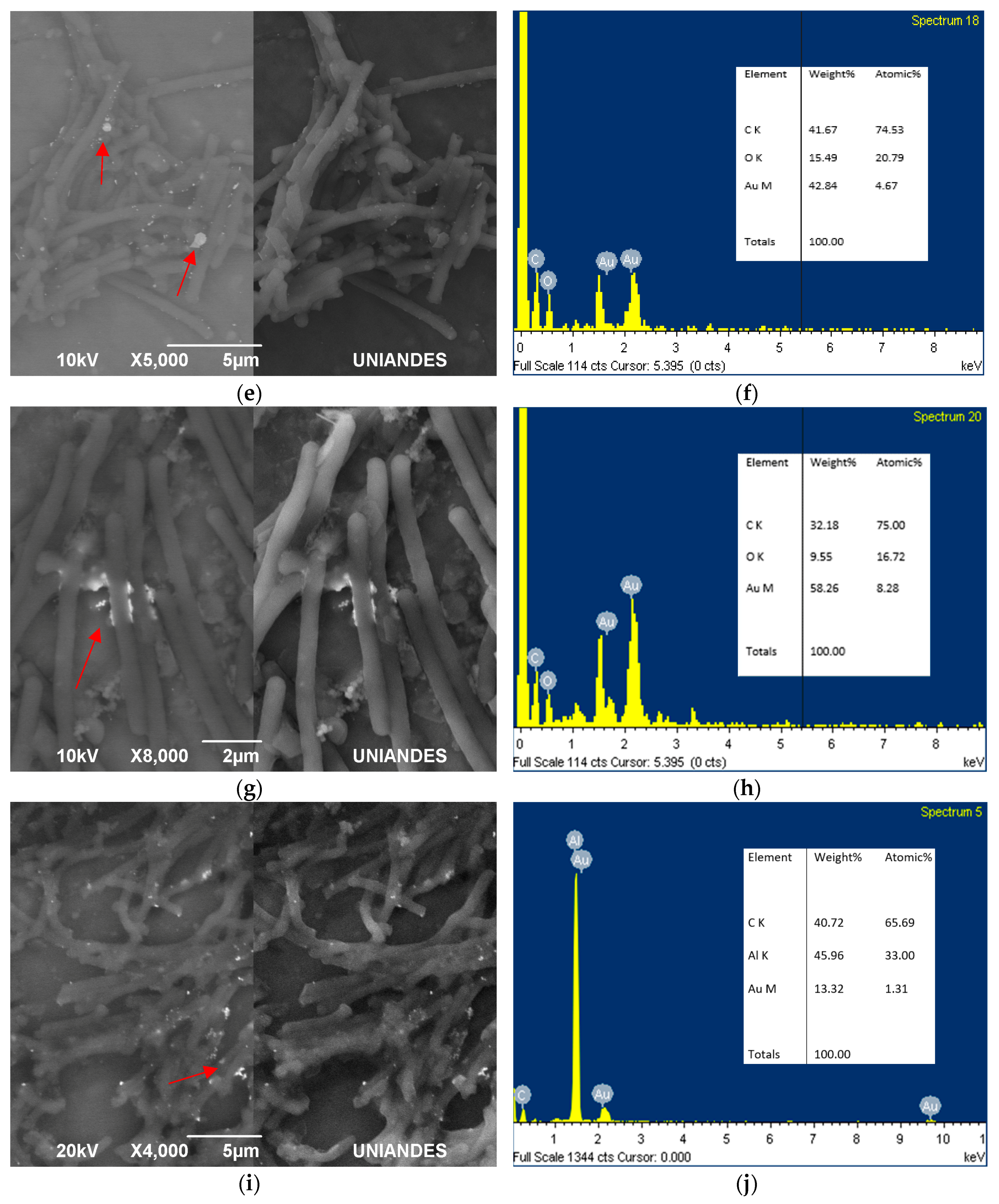
2.5. SEM and EDS Analysis
3. Results
3.1. Sporulated Microorganisms
3.2. Metal Sorption in Living Cells of L. sphaericus Strains
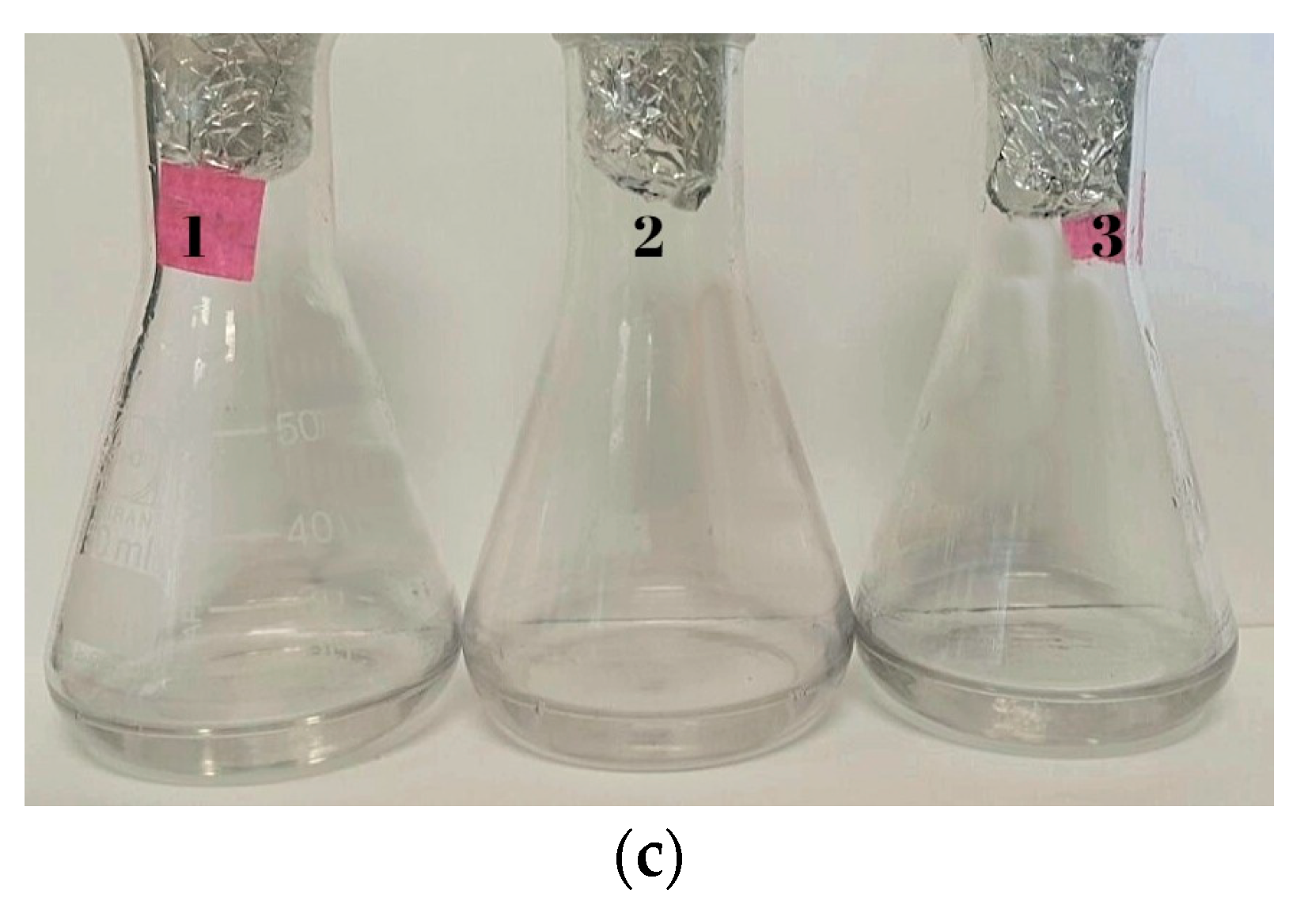
3.3. Biofabrication of Gold Nanoparticles (AuNPs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumah, A. Sustainability and gold mining in the developing world. J. Clean. Prod. 2006, 14, 315–323. [Google Scholar] [CrossRef]
- Giraldo, J.; Muñoz, J.C. La minería en Antioquia: Entre la informalidad y la criminalidad. In Informalidad e Ilegaidad en la Explotación del ORO y la MADERA en Antioquia; Universidad Eafit, Fundación Proantiquia, Centro de Análisis Político: Medellín, Colombia, 2012; pp. 25–100. ISBN 978-958-99013-2-8. [Google Scholar]
- Idrobo, N.; Mejía, D.; Tribin, A.M. Illegal gold mining and violence in Colombia. Peace Econ. Peace Sci. Publ. Policy 2014, 20, 83–111. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Carranza-Lopez, L.; Caballero-Gallardo, K.; Ripoll-Arboleda, A.; Muños-Sosa, D. Human exposure and risk assessment associated with mercury pollution in the Caqueta River, Colombian Amazon. Environ. Sci. Pollut. Res. Int. 2016, 23, 20761–20771. [Google Scholar] [CrossRef] [PubMed]
- Tarras-Wahlberg, N.H.; Flachier, A.; Lane, S.N.; Sangfors, O. Environmental impacts and metal exposure of aquatic ecosystems in rivers contaminated by small scale gold mining: The Puyango River basin, southern Ecuador. Sci. Total Environ. 2001, 278, 239–261. [Google Scholar] [CrossRef]
- Takai, K.; Moser, D.P.; DeFlaun, M.; Onstott, T.C.; Fredrickson, J.K. Archaeal Diversity in Waters from Deep South African Gold Mines. Appl. Environ. Microbiol. 2001, 67, 5750–5760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, F.; Takai, K.; Hirayama, H.; Yamato, Y.; Nealson, K.H.; Horikoshi, K. Distribution and phylogenetic diversity of the subsurface microbial community in a Japanese epithermal gold mine. Extremophiles 2003, 7, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, G.; Osman, S.; Kukkadapu, R.; Engelhard, M.; Vaishampayan, P.A.; Andersen, G.L.; Sani, R.K. Microbial and Mineralogical Characterizations of Soils Collected from the Deep Biosphere of the Former Homestake Gold Mine, South Dakota. Microb. Ecol. 2010, 60, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, G.; Osman, S.; Vaishampayan, P.A.; Stetler, L.D.; Sani, R.K. Microbial Diversity in Uranium Mining-Impacted Soils as Revealed by High-Density 16S Microarray and Clone Library. Microb. Ecol. 2010, 59, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, H.; Wang, L. Bacterial Diversity in Linglong Gold Mine, China. Geomicrobiol. J. 2016, 34, 267–273. [Google Scholar] [CrossRef]
- Schippers, A.; Breuker, A.; Blazejak, A.; Bosecker, K.; Kock, D.; Wright, T.L. The biogeochemistry and microbiology of sulfidic mine waste and bioleaching dumps and heaps, and novel Fe (II)-oxidizing bacteria. Hydrometallurgy 2010, 104, 342–350. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.I.; Sun, Q. Archaeal and bacterial communities in acid mine drainage from metal-rich abandoned tailing ponds, Tongling, China. Trans. Nonferr. Met. Soc. China 2014, 24, 3332–3342. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Yin, H.Q.; Liu, Y.; Xie, M.; Qiu, G.Z.; Liu, X.D. Diversity of microbial community at acid mine drainages from Dachang metals-rich mine, China. Trans. Nonferr. Met. Soc. China 2010, 20, 1097–1103. [Google Scholar] [CrossRef]
- Baker, B.J.; Moser, D.P.; MacGregor, B.J.; Fishbain, S.; Wagner, M.; Fry, N.K.; Jackson, B.; Speolstra, N.; Loos, S.; Takai, K.; et al. Related assemblages of sulphate-reducing bacteria associated with ultradeep gold mines of South Africa and deep basalt aquifers of Washington State. Environ. Microbiol. 2003, 5, 267. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.M.; Johnson, D.B. Acidophilic sulphate-reducing bacteria: Candidates for bioremediation of acid mine drainage. Process Metall. 1999, 9, 709–718. [Google Scholar] [CrossRef]
- Hao, C.; Wang, L.; Gao, Y.; Zhang, L.; Dong, H. Microbial diversity in acid mine drainage of Xiang Mountain sulfide mine, Anhui Province, China. Extremophiles 2010, 14, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Korehi, H.; Blothe, M.; Sitnikova, M.A.; Dold, B.; Schippers, A. Metal mobilization by iron-and sulfur-oxidazing bacteria in multiple extreme mine tailings in the Atacama Desert, Chile. Environ. Sci. Technol. 2013, 47, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B. Acidophilic microbial communities: Candidates for bioremediation of acidic mine effluents. Int. Biodeterior. Biodegrad. 1995, 35, 41–58. [Google Scholar] [CrossRef]
- Hallberg, K.B. New perspectives in acid mine drainage microbiology. Hydrometallurgy 2010, 104, 448–453. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.R.; Dussan, J. Mathematical modelling of toxic metal uptake and efflux pump in metal-resistant bacterium Bacillus cereus isolated from heavy crude oil. Water Air Soil. Pollut. 2015, 226, 112–114. [Google Scholar] [CrossRef]
- Velasquez, L.; Dussan, J. Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J. Hazard. Mater. 2009, 167, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Das, A.P.; Ghosh, S. Bioleaching of Manganese from mining waste materials. Mater. Today Commun. 2018, 5, 2381–2390. [Google Scholar] [CrossRef]
- Tuzen, M.; Uluozlu, O.D.; Usta, C.; Soylak, M. Biosorption of copper(II), lead(II), iron(III) and cobalt(II) on Bacillus sphaericus-loaded Diaion SP-850 resin. Anal. Chim. Acta 2007, 581, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Reith, F.; Zammit, C.M.; Rogers, S.L.; McPhail, D.C.; Brugger, J. Potential utilisation of micro-organisms in gold processing: A review. Miner. Process. Extr. Metall. Rev. 2012, 121, 251–260. [Google Scholar] [CrossRef]
- Reith, F.; Etschmann, B.; Grosse, C.; Moors, H.; Benotmane, M.A.; Monsieurs, P.; Doonan, C.; Vogt, S.; Lai, B.; Martinez-Criado, G.; et al. Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans. Proc. Natl. Acad. Sci. USA 2009, 106, 17757–17762. [Google Scholar] [CrossRef] [PubMed]
- Southam, G.; Saunders, J.A. The geomicrobiology of ore deposits. Econ. Geol. 2005, 100, 1067–1084. [Google Scholar] [CrossRef]
- Reith, F.; Lengke, M.F.; Falconer, D.; Craw, D.; Southam, G. Winogradsky review—The geomicrobiology of gold. ISME 2007, 1, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Lengke, M.F.; Ravel, B.; Fleet, M.E.; Wanger, G.; Gordon, R.A.; Southam, G. Mechanisms of gold bioaccumulations by filamentous cyanobacteria from gold(III)-chloride complex. Environ. Sci. Technol. 2006, 40, 6304–6309. [Google Scholar] [CrossRef] [PubMed]
- Lozano, L.C.; Dussan, J. Metal tolerance and larvicidal activity of Lysinibacillus sphaericus. World J. Microbiol. Biotechnol. 2013, 29, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Manchola, L.; Dussan, J. Lysinibacillus sphaericus and Geobacillus sp. Biodegradation of Petroleum Hydrocarbons and Biosurfactant Production. Remediation 2014, 25, 85–100. [Google Scholar] [CrossRef]
- Pollmann, K.; Raff, J.; Merroun, M.; Fahmy, K.; Selenska-Pobell, S. Metal binding by bacteria from uranium mining waste piles and its technological applications. Biotechnol Adv. 2006, 24, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, A.C.A.; Pereira, F. Bioaccumulation of copper, zinc, cadmium and lead by Bacillus sp., Bacillus cereus, Bacillus sphaericus and Bacillus subtilis. Braz. J. Microbiol. 2001, 32, 1–5. [Google Scholar] [CrossRef]
- Lapage, S.P.; Shelton, J.E.; Mitchell, T.G. Methods in Microbiology; Academic Press: Cambridge, MA, USA, 1970; p. 116. [Google Scholar]
- Fox, J.G.; Yan, L.L.; Dewhirst, F.E.; Paster, B.J.; Shames, B.; Murphy, J.C.; Hayward, A.; Belcher, J.C.; Mendes, E.N. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J. Clin. Microbiol. 1995, 33, 445–454. [Google Scholar] [PubMed]
- Santana-Martinez, J.C.; Silva, J.J.; Dussan, J. Efficacy of Lysinibacillus sphaericus against mixed-cultures of field-collected and laboratory larvae of Aedes aegypti and Culex quinquefasciatus. Bull. Entomol. Res. 2018, 53, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Moawed, E.A.; El-Shahat, M.F. Synthesis, characterization of low density polyhydroxy polyurethane foam and its application for separation and determination of gold in water and ores samples. Anal. Chim. Acta 2013, 788, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Castillo González, C.M. Caracterización Molecular De La Resistencia A Mercurio Mercúrico De Bacterias Nativas Colombianas; Undergraduated, Universidad de los Andes: Bogotá, Colombia, 2007. [Google Scholar]
- Shaw, D.R.; Dussan, J. Transcriptional analysis and molecular dynamics simulations reveal the mechanism of toxic metals removal and efflux pumps in Lysinibacillus sphaericus OT4b.31. Int. Biodeterior. Biodegrad. 2017, 127, 46–61. [Google Scholar] [CrossRef]
- Romero de Pérez, G. Método de procesamiento de los tejidos para microscopía electrónica de transmisión. In Microscopía Electrónica de Transmisión (MET) Área Biomédica: Teoría y Práctica; Academia de Ciencias Exactas, Físicas y Naturales: Madrid, Spain, 2003; pp. 264–265. [Google Scholar]
- Cifuentes, D.P.; Ocampo, M.; Curtidor, H.; Vanegas, M.; Forero, M.; Patarroyo, M.E.; Patarroyo, M.A. Mycobacterium tuberculosis Rv0679c protein sequences involved in host-cell infection: Potential TB vaccine candidate antigen. BMC Microbiol. 2010, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Pum, D.; Sleytr, U.B. The application of bacterial S-layers in molecular nanotechnology. Trends Biotechnol. 1999, 17, 8–12. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Györvary, E.; Pum, D. Crystallization of S-layer protein lattices on surfaces and interfaces. Prog. Org. Coat. 2003, 47, 279–287. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Deepak, V.; Kumar, S.; Pandian, S.R.K; Gurunathan, S. Biological synthesis of gold nanocubes from Bacillus licheniformis. Bioresour. Technol. 2009, 100, 5356–5358. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Ng, I.S. Biofabrication of gold nanoparticles by Shewanella species. Bioresour. Bioprocess. 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Sá-Pereira, P.; Rodrigues, M.; Simões, F.; Domingues, L.; Videira e Castro, I. Bacterial activity in heavy metals polluted soils: Metal efflux systems in native rhizobial strains. Geomicrobiol. J. 2009, 26, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Nies, D.H. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 2003, 27, 313–339. [Google Scholar] [CrossRef] [Green Version]
- Gerbino, E.; Carasi, P.; Mobili, P.; Serradell, M.A.; Gómez-Zavaglia, A. Role of S-layer proteins in bacteria. World J. Microbiol. Biotechnol. 2015, 31, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, K.; Merroun, M.; Pollmann, K.; Raff, J.; Savchuk, O.; Hennig, C.; Selenska-Pobell, S. Secondary structure and Pd (II) coordination in S-layer proteins from Bacillus sphaericus studied by infrared and X-ray absorption spectroscopy. Biophys. J. 2009, 91, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of nanoparticles using microbes-A review. Colloids Surf. B 2014, 121, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Shedbalkar, U.; Singh, R.; Wadhwani, S.; Gaidhani, S.; Chopade, B.A. Microbial synthesis of gold nanoparticles: Current status and future prospects. Adv. Colloid Interface Sci. 2014, 209, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Khlebtsov, N.G. Gold Nanoparticles in Biology and Medicine: Recent Advances and Prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustos, M.C.; Ibarra, H.; Dussán, J. The Golden Activity of Lysinibacillus sphaericus: New Insights on Gold Accumulation and Possible Nanoparticles Biosynthesis. Materials 2018, 11, 1587. https://doi.org/10.3390/ma11091587
Bustos MC, Ibarra H, Dussán J. The Golden Activity of Lysinibacillus sphaericus: New Insights on Gold Accumulation and Possible Nanoparticles Biosynthesis. Materials. 2018; 11(9):1587. https://doi.org/10.3390/ma11091587
Chicago/Turabian StyleBustos, María Camila, Humberto Ibarra, and Jenny Dussán. 2018. "The Golden Activity of Lysinibacillus sphaericus: New Insights on Gold Accumulation and Possible Nanoparticles Biosynthesis" Materials 11, no. 9: 1587. https://doi.org/10.3390/ma11091587





