SrCe0.9Sm0.1O3-α Compounded with NaCl-KCl as a Composite Electrolyte for Intermediate Temperature Fuel Cell
Abstract
:1. Introduction
2. Experimental Section
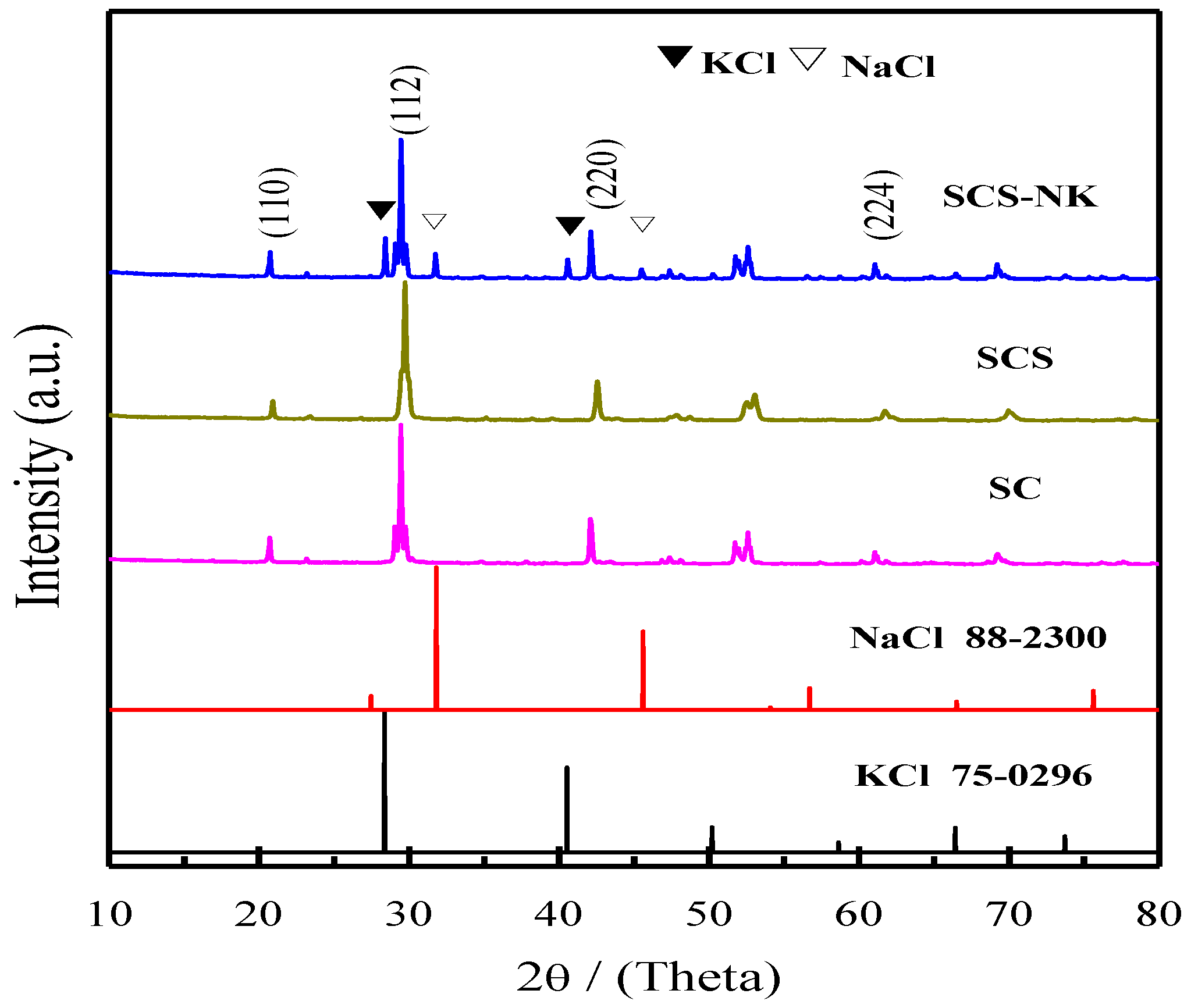
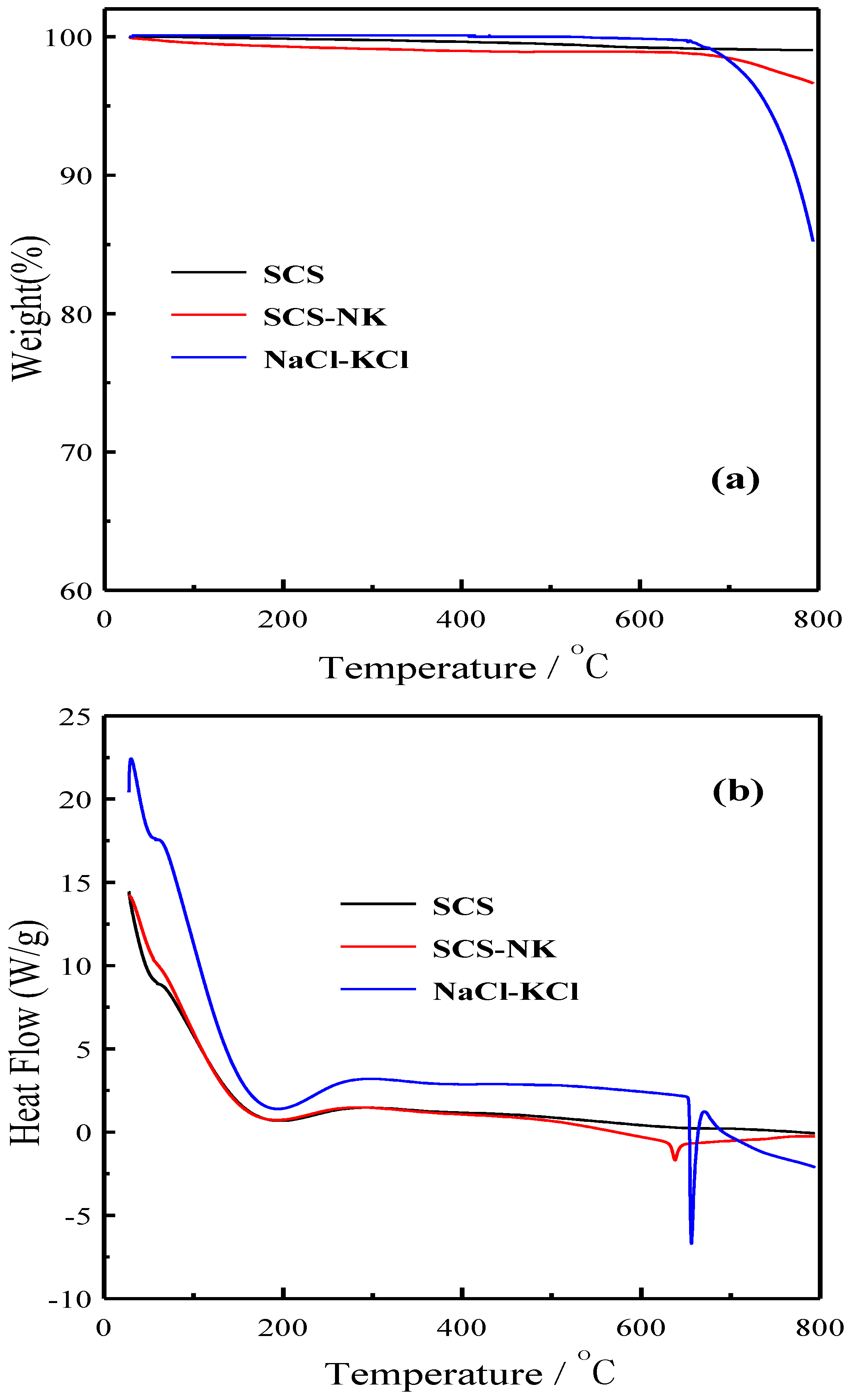
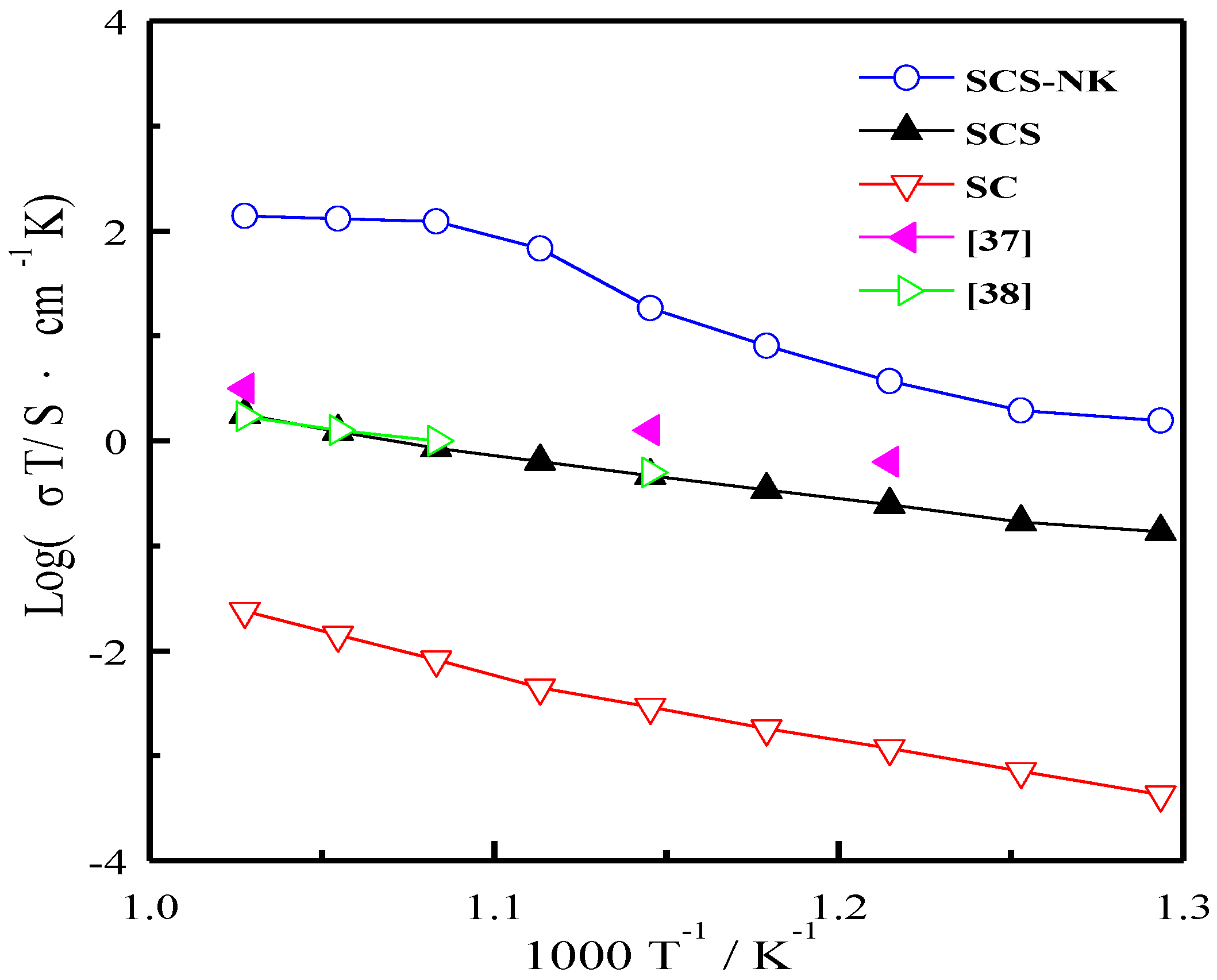
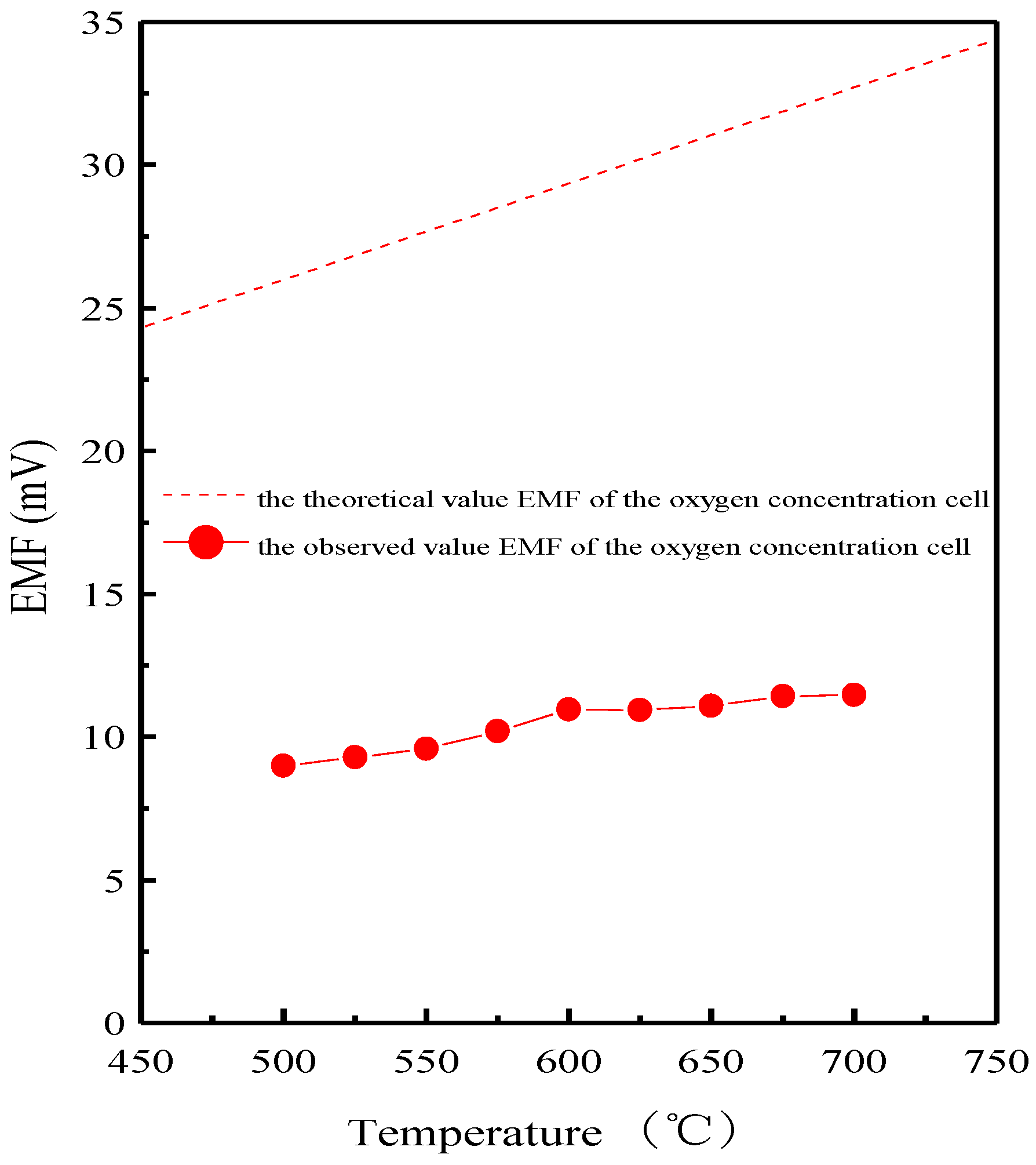
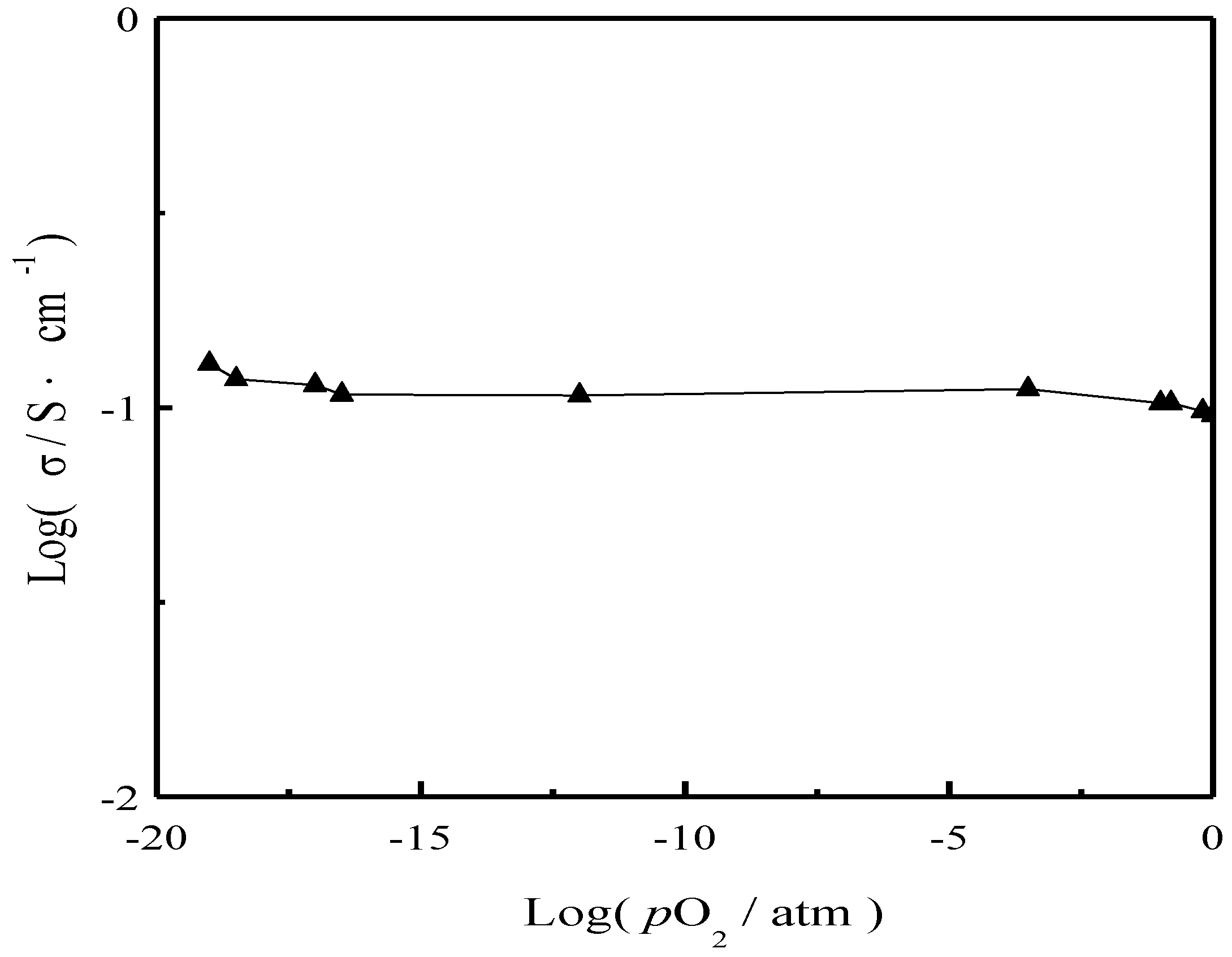
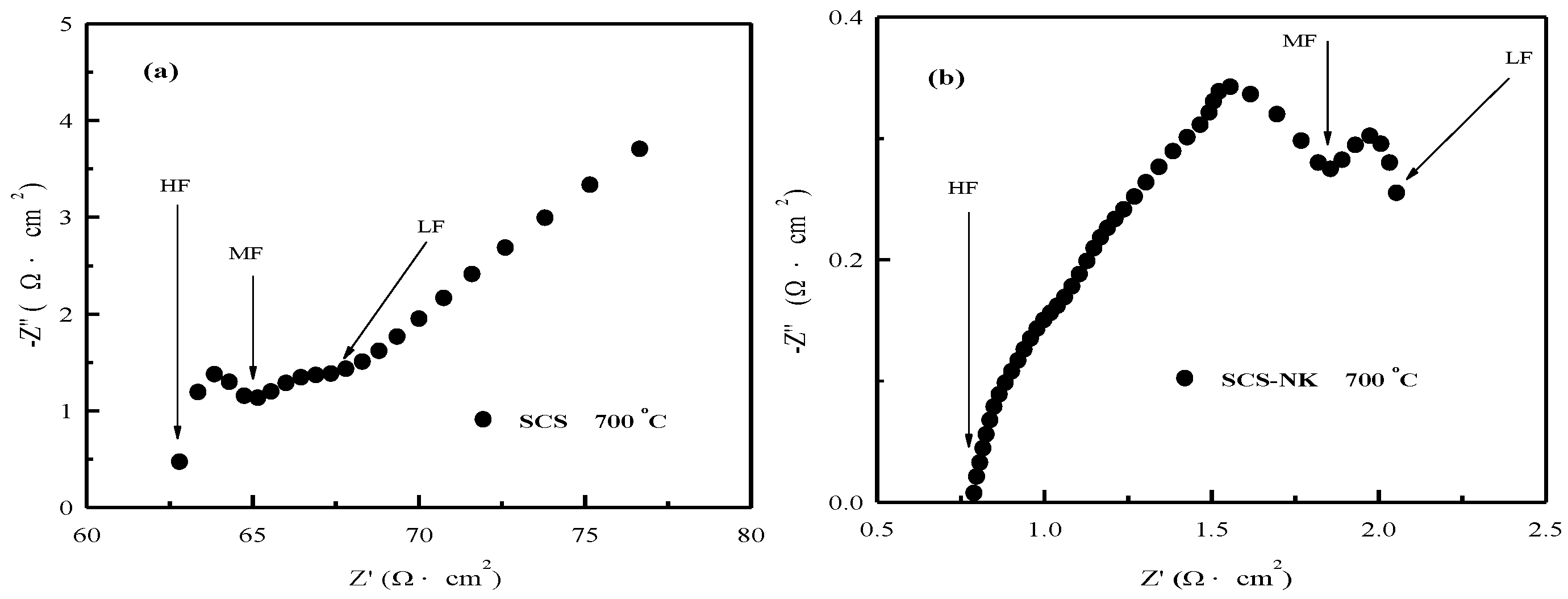
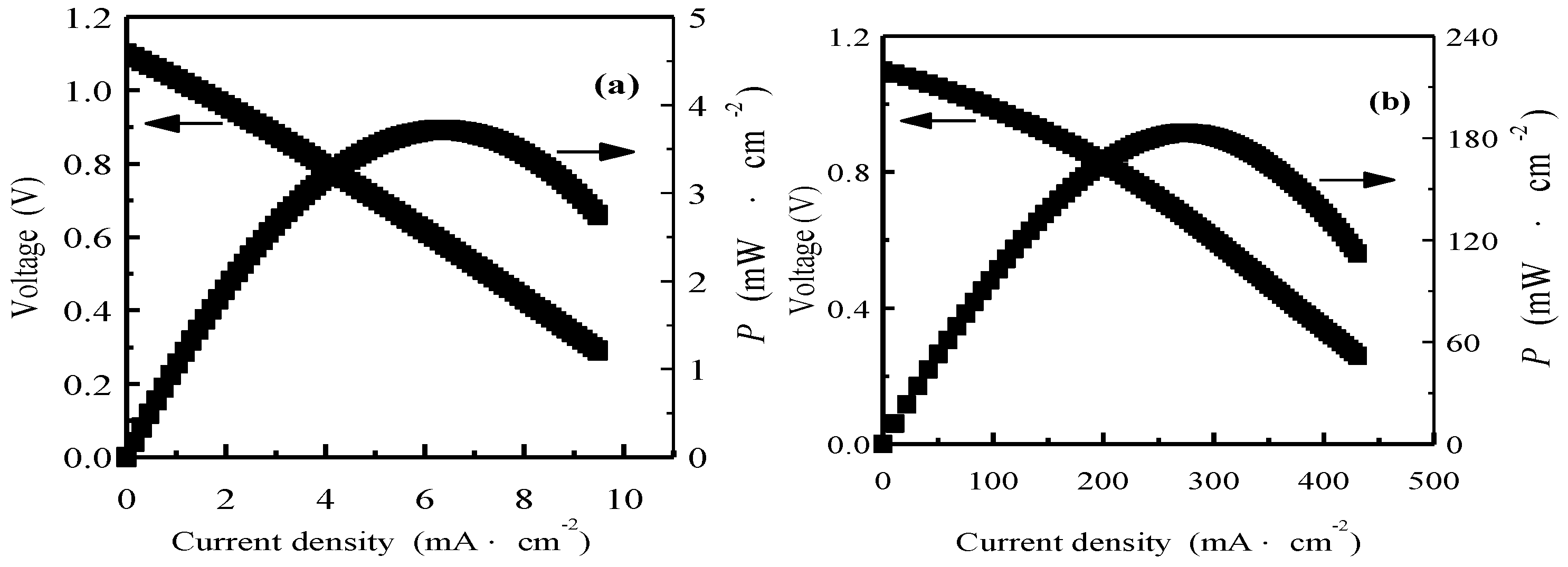
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Zhang, H.; Gao, M.; Li, Q.; Bian, W.; Tao, T.; Zhang, H. High-temperature wettability and interactions between Y-containing Ni-based alloys and various oxide ceramics. Materials 2018, 11, 749. [Google Scholar] [CrossRef] [PubMed]
- Sadeghifar, H. In-plane and through-plane electrical conductivities and contact resistances of a Mercedes-Benz catalyst-coated membrane, gas diffusion and micro-porous layers and a Ballard graphite bipolar plate: Impact of humidity, compressive load and polytetrafluoroethylene. Energy Convers. Manag. 2017, 154, 191–202. [Google Scholar]
- Kondoh, J. Origin of the hump on the left shoulder of the X-ray diffraction peaks observed in Y2O3-fully and partially stabilized ZrO2. J. Alloys Compd. 2004, 375, 270–282. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Bahrami, M.; Djilali, N. A statistically-based thermal conductivity model for fuel cell Gas Diffusion Layers. J. Power Sources 2013, 233, 369–379. [Google Scholar] [CrossRef]
- Xia, C.; Qiao, Z.; Feng, C.; Kim, J.; Wang, B.; Zhu, B. Study on zinc oxide-based electrolytes in low-temperature solid oxide fuel cells. Materials 2018, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Sadeghifar, H.; Djilali, N.; Bahrami, M. Effect of Polytetrafluoroethylene (PTFE) and micro porous layer (MPL) on thermal conductivity of fuel cell gas diffusion layers: Modeling and experiments. J. Power Sources 2014, 248, 632–641. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Djilali, N.; Bahrami, M. A new model for thermal contact resistance between fuel cell gas diffusion layers and bipolar plates. J. Power Sources 2014, 266, 51–59. [Google Scholar] [CrossRef]
- Ueno, T.; Hirata, Y.; Shimonosono, T. Analysis of compressive deformation behavior of wet powder compacts of nanometer-sized yttria-stabilized zirconia particles. Ceram. Int. 2016, 42, 1926–1932. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Rioja-Monllor, L.; Nakamura, T.; Ricote, S.; O’Hayre, R.; Amezawa, K.; Einarsrud, M.; Grande, T. Effect of cation ordering on the performance and chemical stability of layered double perovskite cathodes. Materials 2018, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Sadeghifar, H.; Djilali, N.; Bahrami, M. Thermal conductivity of a graphite bipolar plate (BPP) and its thermal contact resistance with fuel cell gas diffusion layers: Effect of compression, PTFE, micro porous layer (MPL), BPP out-of-flatness and cyclic load. J. Power Sources 2015, 273, 96–104. [Google Scholar] [CrossRef]
- Dankeaw, A.; Poungchan, G.; Panapoy, M.; Ksapabutr, B. In-situ one-step method for fabricating three-dimensional grass-like carbon-doped ZrO2 films for room temperature alcohol and acetone sensors. Sensor. Actuators B Chem. 2017, 242, 202–214. [Google Scholar] [CrossRef]
- Sammes, N.; Phillips, R.; Smirnova, A. Proton conductivity in stoichiometric and sub-stoichiometric yittrium doped SrCeO3 ceramic electrolytes. J. Power Sources 2004, 134, 153–159. [Google Scholar] [CrossRef]
- Okuyama, Y.; Isa, K.; Lee, Y.S.; Sakai, T.; Matsumoto, H. Incorporation and conduction of proton in SrCe0.9-xZrxY0.1O3-δ. Solid State Ion. 2015, 275, 35–38. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Liu, X.; Zhao, X.; He, D.; Qiu, H.; Qiu, Q.; Wang, S.; Jiang, L. Low temperature synthesis of Yb doped SrCeO3 powders by gel combustion process. Int. J. Hydrogen Energy 2013, 38, 12921–12926. [Google Scholar] [CrossRef]
- Xing, W.; Dahl, P.I.; Roaas, L.V.; Fontaine, M.-L.; Larring, Y.; Henriksen, P.P.; Bredesen, R. Hydrogen permeability of SrCe0.7Zr0.25Ln0.05O3-α membranes (Ln = Tm and Yb). J. Membr. Sci. 2015, 473, 327–332. [Google Scholar] [CrossRef]
- Matskevich, N.I.; Wolf, T.; Vyazovkin, I.V.; Adelmann, P. Preparation and stability of a new compound SrCe0.9Lu0.1O2.95. J. Alloys Compd. 2015, 628, 126–129. [Google Scholar] [CrossRef]
- Uchida, H.; Maeda, N.; Iwahara, H. Relation between proton and hole conduction in SrCeO3-based solid electrolytes under water-containing atmospheres at high temperatures. Solid State Ion. 1983, 11, 117–124. [Google Scholar] [CrossRef]
- Yuan, W.; Xiao, C.; Li, L. Hydrogen permeation and chemical stability of In-doped SrCe0.95Tm0.05O3-α membranes. J. Alloys Compd. 2014, 616, 142–147. [Google Scholar] [CrossRef]
- Shrivastava, U.N.; Duncan, K.L.; Chung, J.N. ExperimentallyExperimentaly validated numerical modeling of Eu doped SrCeO3 membrane for hydrogen separation. Int. J. Hydrogen Energy 2012, 37, 15350–15358. [Google Scholar] [CrossRef]
- Shawuti, S.; Gulgun, M.A. Solid oxide-molten carbonate nano-composite fuel cells: Particle size effect. J. Power Sources 2014, 267, 128–135. [Google Scholar] [CrossRef]
- Zhu, B.; Li, S.; Mellander, B.E. Theoretical approach on ceria-based two-phase electrolytes for low temperature (300–600 °C) solid oxide fuel cells. Electrochem. Commun. 2008, 10, 302–305. [Google Scholar] [CrossRef]
- Rajesh, S.; Macedo, D.A.; Nascimento, R.M.; Souza, G.L.; Figueiredo, F.M.L.; Marques, F.M.B. One-step synthesis of composite electrolytes of Eu-doped ceria and alkali metal carbonates. Int. J. Hydrogen Energy 2013, 38, 16539–16545. [Google Scholar] [CrossRef]
- Kim, J.-T.; Lee, T.-H.; Park, K.-Y.; Seo, Y.; Kim, K.B.; Song, S.-J.; Park, B.; Park, J.-Y. Electrochemical properties of dual phase neodymium-doped ceria alkali carbonate composite electrolytes in intermediate temperature. J. Power Sources 2015, 275, 563–572. [Google Scholar] [CrossRef]
- Fu, Q.X.; Zhang, W.; Peng, R.R.; Peng, D.K.; Meng, G.Y.; Zhu, B. Doped ceria–chloride composite electrolyte for intermediate temperature ceramic membrane fuel cells. Mater. Lett. 2002, 53, 186–192. [Google Scholar] [CrossRef]
- Fu, Q.X.; Zha, S.W.; Zhang, W.; Peng, D.K.; Meng, G.Y.; Zhu, B. Intermediate temperature fuel cells based on doped Ceria-LiCl-SrCl2 composite electrolyte. J. Power Sources 2002, 104, 73–78. [Google Scholar] [CrossRef]
- Shi, R.; Liu, J.; Wang, H.; Wu, F.; Miao, H. Intermediate temperature fuel cell durability of Eu-doped SrCeO3-SrZrO3 solid solution/NaCl-KCl composite electrolyte. Ceram. Int. 2017, 43, 16931–16935. [Google Scholar] [CrossRef]
- Hei, Y.; Huang, J.; Wang, C.; Mao, Z. Novel doped barium cerateecarbonate composite electrolyte material for low temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 14328–14333. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Sheng, L.; Li, H. Gadolinium doped strontium cerate prepared by citric-nitrate auto-combustion process and intermediate temperature electrical properties of its composite electrolyte. Int. J. Electrochem. Sci. 2017, 12, 9689–9696. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, H.; Miao, H.; Sheng, L.; Li, H. Synthesis and conductivity of strontium cerate doped by erbium oxide and its composite electrolyte for intermediate temperature fuel cell. Ceram. Int. 2017, 43, 9317–9321. [Google Scholar] [CrossRef]
- Shi, R.; Liu, J.; Wang, H.; Wu, F.; Miao, H.; Cui, Y. Low temperature synthesis of SrCe0.9Eu0.1O3-α by sol-gel method and SrCe0.9Eu0.1O3-α-NaCl-KCl composite electrolyte for intermediate temperature fuel cells. Int. J. Electrochem. Sci. 2017, 12, 11594–11601. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Djilali, N.; Bahrami, M. Counter-intuitive reduction of thermal contact resistance with porosity: A case study of polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2014, 41, 6833–6841. [Google Scholar] [CrossRef]
- Sadeghifar, H. Reconstruction and analysis of fuel cell gas diffusion layers using fiber spacing rather than pore size data: Questioned validity of widely-used porosity-based thermal conductivity models. J. Power Sources 2016, 307, 673–677. [Google Scholar] [CrossRef]
- Sadeghifar, H. An optimized microstructure to minimizing in-plane and through-plane pressure drops of fibrous materials: Counter-intuitive reduction of gas diffusion layer permeability with porosity. J. Power Sources 2018, 385, 100–113. [Google Scholar] [CrossRef]
- Sadeghifar, H. In-plane and through-plane local and average Nusselt numbers in fibrous porous materials with different fiber layer temperatures: Gas diffusion layers for fuel cells. J. Power Sources 2016, 325, 311–321. [Google Scholar] [CrossRef]
- Miranda, M.I.G.; Bica, C.I.D.; Nachtigall, S.M.B.; Rehman, N.; Rosa, S.M.L. Kinetical thermal degradation study of maize straw and soybean hull celluloses by simultaneous DSC–TGA and MDSC techniques. Thermochim. Acta 2013, 565, 65–71. [Google Scholar] [CrossRef]
- Huang, S.; Sheng, J.J. An innovative method to build a comprehensive kinetic model for air injection using TGA/DSC experiments. Fuel 2017, 210, 98–106. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.-J.; Fu, Y.-P.; Li, C.; Wang, J.-Y.; Lee, S. Effect of potassium substituted for A-site of SrCe0.95Y0.05O3 on microstructure, conductivity and chemical stability. Ceram. Int. 2015, 41, 2948–2954. [Google Scholar] [CrossRef]
- Tsuji, T.; Nagano, T. Electrical conduction in SrCeO3 doped with Eu2O3. Solid State Ion. 2000, 136–137, 179–182. [Google Scholar] [CrossRef]
- Philips, R.J.; Bonanos, N.; Poulsen, F.W.; Ahlgren, E.O. Structural and electrical characterisation of SrCe1−xYxOξ. Solid State Ion. 1999, 125, 389–395. [Google Scholar] [CrossRef]
- Jaiswal, N.; Singh, N.K.; Kumar, D.; Parkash, O. Effect of strontium (Sr) doping on the conductivity of ceria. J. Power Sources 2012, 202, 78–84. [Google Scholar] [CrossRef]
- Tanwar, K.; Jaiswal, N.; Sharma, P.; Kumar, D.; Parkash, O. Structural analysis of Ce0.83Dy0.14Ca0.03O1.90 (CDC) and enhanced electrical conductivity of its composites with alkali carbonates for LT-SOFCs. J. Alloys Compd. 2018, 741, 532–541. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, R.; Chen, W.; Hu, W.; Liu, J.; Wang, H. SrCe0.9Sm0.1O3-α Compounded with NaCl-KCl as a Composite Electrolyte for Intermediate Temperature Fuel Cell. Materials 2018, 11, 1583. https://doi.org/10.3390/ma11091583
Shi R, Chen W, Hu W, Liu J, Wang H. SrCe0.9Sm0.1O3-α Compounded with NaCl-KCl as a Composite Electrolyte for Intermediate Temperature Fuel Cell. Materials. 2018; 11(9):1583. https://doi.org/10.3390/ma11091583
Chicago/Turabian StyleShi, Ruijuan, Wei Chen, Wenli Hu, Junlong Liu, and Hongtao Wang. 2018. "SrCe0.9Sm0.1O3-α Compounded with NaCl-KCl as a Composite Electrolyte for Intermediate Temperature Fuel Cell" Materials 11, no. 9: 1583. https://doi.org/10.3390/ma11091583
APA StyleShi, R., Chen, W., Hu, W., Liu, J., & Wang, H. (2018). SrCe0.9Sm0.1O3-α Compounded with NaCl-KCl as a Composite Electrolyte for Intermediate Temperature Fuel Cell. Materials, 11(9), 1583. https://doi.org/10.3390/ma11091583




