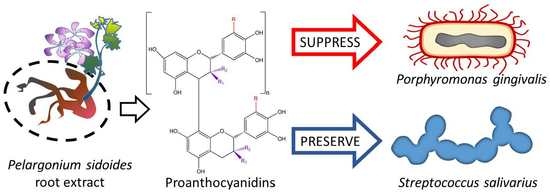
Efficacy of Proanthocyanidins from Pelargonium sidoides Root Extract in Reducing P. gingivalis Viability While Preserving Oral Commensal S. salivarius
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Purification of PACN
Acid/n-Butanol Hydrolysis of Extracts
2.3. Qualitative and Quantitative Analysis
2.3.1. HPLC Analysis
2.3.2. UPLC-ESI-MS Conditions
2.3.3. UPLC-MS/MS Conditions
2.4. Determination of Antioxidant Activity
2.5. Methods and Procedures Implicated for Antibacterial Activity Evaluation
2.5.1. Pelargonium sidoides Root Extract (PSRE) Preparation
2.5.2. Proanthocyanidins from Extract (PACN)
2.5.3. Bacteria and Growth Conditions
2.5.4. PSRE and PACN Antibacterial Activity
- (i)
- PSRE water solution: 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08 and 0.09 g/mL
- (ii)
- PACN water solution: 0.01, 0.03, 0.05, 0.07 and 0.09 mg/mL
2.5.5. Statistical Analysis
3. Results
3.1. Composition of Phenolic Compounds of PACN from PSRE
3.2. Determination of the Mean Degree of Polymerization of PACN
3.3. Antioxidative Activity of PCANs and PSRE
3.4. Antibacterial Activity of PSRE and PACNs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PSRE | Pelargonium sidoides root extract |
| PACN | Proanthocyanidins |
| AF | Acetone fraction |
| LPS | Lipopolysaccharide |
| MRM | Multiple reaction monitoring |
| UPLC | Ultra-performance liquid chromatography |
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. P T 2015, 40, 277–283. [Google Scholar] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Ojcius, D.M.; Yilmaz, Ö. The Oral Microbiota: Living with a Permanent Guest. DNA Cell Biol. 2009, 28, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordell, G.A.; Colvard, M.D. Natural Products and Traditional Medicine: Turning on a Paradigm. J. Nat. Prod. 2012, 75, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.W. Alternative natural sources for a new generation of antibacterial agents. Int. J. Antimicrob. Agents 2013, 42, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Brendler, T.; van Week, B.-E. A historical, scientific and commercial perspective on the medicinal use of Pelargonium sidoides (Geraniaceae). J. Ethnopharmacol. 2008, 119, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Lewu, F.B.; Grierson, D.S.; Afolayan, A.J. Extracts from Pelargonium sidoides. Inhibit the Growth of Bacteria and Fungi. Pharm. Biol. 2006, 44, 279–282. [Google Scholar] [CrossRef]
- Lewu, F.B.; Grierson, D.S.; Afolayan, A.J. The leaves of Pelargonium sidoides may substitute for its roots in the treatment of bacterial infections. Biol. Conserv. 2006, 128, 582–584. [Google Scholar] [CrossRef]
- Kayser, O.; Kolodziej, H. Antibacterial Activity of Extracts and Constituents of Pelargonium sidoides and Pelargonium reniforme. Planta Med. 1997, 63, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Bereznoy, V.V.; Riley, D.S.; Wassmer, G.; Heger, M. Efficacy of extract of Pelargonium sidoides in children with acute non-group A beta-hemolytic streptococcus tonsillopharyngitis: A randomized, double-blind, placebo-controlled trial. Altern. Ther. Health Med. 2003, 9, 68–79. [Google Scholar] [PubMed]
- Bachert, C.; Schapowal, A.; Funk, P.; Kieser, M. Treatment of acute rhinosinusitis with the preparation from Pelargonium sidoides EPs 7630: A randomized, double-blind, placebo-controlled trial. Rhinology 2009, 47, 51–58. [Google Scholar] [PubMed]
- Matthys, H.; Eisebitt, R.; Seith, B.; Heger, M. Efficacy and safety of an extract of Pelargonium sidoides (EPs 7630) in adults with acute bronchitis: A randomised, double-blind, placebo-controlled trial. Phytomedicine 2003, 10, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Jain, S.; Bhardwaj, A.; Nagpal, R.; Puniya, M.; Tomar, R.; Singh, V.; Parkash, O.; Prasad, G.B.K.S.; Marotta, F.; et al. Biological and Medicinal Properties of Grapes and Their Bioactive Constituents: An Update. J. Med. Food 2009, 12, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Palaska, I.; Papathanasiou, E.; Theoharides, C.T. Use of polyphenols in periodontal inflammation. Eur. J. Pharmacol. 2013, 720, 77–83. [Google Scholar] [CrossRef] [PubMed]
- La, V.D.; Howell, A.B.; Grenier, D. Anti-Porphyromonas gingivalis and anti-inflammatory activities of A-type cranberry proanthocyanidins. Antimicrob. Agents Chemother. 2010, 54, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Green, B.; Yao, X.; Ganguly, A.; Xu, C.; Dusevich, V.; Walker, M.P.; Wang, Y. Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. J. Dent. 2010, 38, 908–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major periodontopathic pathogen overview. J. Immunol. Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Wu, X.; Wang, L. Management of peri-implantitis: A systematic review, 2010–2015. Springerplus 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Hellström, J.; Sinkkonen, J.; Karonen, M.; Mattila, P. Isolation and structure elucidation of procyanidin oligomers from Saskatoon berries (Amelanchier alnifolia). J. Agric. Food Chem. 2007, 55, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Schötz, K.; Nöldner, M. Mass spectroscopic characterisation of oligomeric proanthocyanidins derived from an extract of Pelargonium sidoides roots (EPs® 7630) and pharmacological screening in CNS models. Phytomedicine 2007, 14, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1998, 299, 15–27. [Google Scholar]
- Amarowicz, R.; Pegg, R.B. Content of proanthocyanidins in selected plant extracts as determined via n-butanol/HCl hydrolysis and a colorimetric assay or by HPLC—A short report. Pol. J. Food Nutr. Sci. 2006, 15, 319–322. [Google Scholar]
- Fu, Y.; Qiao, L.; Cao, Y.; Zhou, X.; Liu, Y.; Ye, X. Structural Elucidation and Antioxidant Activities of Proanthocyanidins from Chinese Bayberry (Myrica rubra Sieb. et Zucc.) Leaves. PLoS ONE 2014, 9, e096162. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Iswaldi, I.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Zarrouk, M. UPLC-QTOF/MS for a rapid characterisation of phenolic compounds from leaves of Myrtus communis L. Phytochem. Anal. 2014, 25, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Bester, M.; Soundy, P.; Apostolides, Z. Activity-guided isolation and identification of the major antioxidant and anticancer compounds from a commercial Pelargonium sidoides tincture. Med. Chem. Res. 2015, 24. [Google Scholar] [CrossRef]
- Engström, M.T.; Pälijärvi, M.; Fryganas, C.; Grabber, J.H.; Mueller-Harvey, I.; Salminen, J.-P. Rapid Qualitative and Quantitative Analyses of Proanthocyanidin Oligomers and Polymers by UPLC-MS/MS. J. Agric. Food Chem. 2014, 62, 3390–3399. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H.; Burmeister, A.; Trun, W.; Radtke, O.A.; Kiderlen, A.F.; Ito, H.; Hatano, T.; Yoshida, T.; Lai, Y.F. Tannins and related compounds induce nitric oxide synthase and cytokines gene expressions in Leishmania major-infected macrophage-like RAW 264.7 cells. Bioorg. Med. Chem. 2005, 13, 6470–6476. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H. Fascinating metabolic pools of Pelargonium sidoides and Pelargonium reniforme, traditional and phytomedicinal sources of the herbal medicine Umckaloabo®. Phytomedicine 2007, 14, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Janecki, A.; Conrad, A.; Engels, I.; Frank, U.; Kolodziej, H. Evaluation of an aqueous-ethanolic extract from Pelargonium sidoides (EPs® 7630) for its activity against group A-streptococci adhesion to human HEp-2 epithelial cells. J. Ethnopharmacol. 2011, 133, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H. Antimicrobial, antiviral and immunomodulatory activity studies of Pelargonium sidoides (EPs® 7630) in the context of health promotion. Pharmaceuticals 2011, 4, 1295–1314. [Google Scholar] [CrossRef] [PubMed]
- Schoetz, K.; Erdelmeier, C.; Germer, S.; Hauer, H. A Detailed View on the Constituents of EPs® 7630. Planta Med. 2008, 74, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Plumb, G.W.; de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C.; Williamson, G. Antioxidant properties of gallocatechin and prodelphinidins from pomegranate peel. Redox Rep. 2002, 7, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial Activity of 10 Different Plant Polyphenols against Bacteria Causing Food-Borne Disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Goncalves, C.; Dinis, T.; Batista, M. Antioxidant properties of proanthocyanidins of bark decoction: A mechanism for anti-inflammatory activity. Phytochemistry 2005, 66, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Pinent, M.; Castell-Auví, A.; Genovese, M.I.; Serrano, J.; Casanova, A.; Blay, M.; Ardévol, A. Antioxidant effects of proanthocyanidin-rich natural extracts from grape seed and cupuassu on gastrointestinal mucosa. J. Sci. Food Agric. 2016, 96, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernández, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masdea, L.; Kulik, E.M.; Hauser-Gerspach, I.; Ramseier, A.M.; Filippi, A.; Waltimo, T. Antimicrobial activity of Streptococcus salivarius K12 on bacteria involved in oral malodour. Arch. Oral Biol. 2012, 57, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Sliepen, I.; Van Damme, J.; Van Essche, M.; Loozen, G.; Quirynen, M.; Teughels, W. Microbial Interactions Influence Inflammatory Host Cell Responses. J. Dent. Res. 2009, 88, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Ulrey, R.K.; Barksdale, S.M.; Zhou, W.; van Hoek, M.L. Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2014, 14, 499. [Google Scholar] [CrossRef] [PubMed]
- Maisuria, V.B.; Los Santos, Y.L.; Tufenkji, N.; Déziel, E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 30169. [Google Scholar] [CrossRef] [PubMed]
- Krachler, A.M.; Orth, K. Targeting the bacteria-host interface: Strategies in anti-adhesion therapy. Virulence 2013, 4, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef]



| A | |||
| PACN | Compound | [M − H]− m/z | Product Ions m/z |
| 6 | Prodelphinidin dimer | 609 | 305, 423, 441, 483, 565, 591 |
| 7 | Prodelphinidin trimer | 913 | 303, 423, 533, 483, 559 |
| 8 | Prodelphinidin tetramer | 1217 | 955, 1133, 1155, 1064, 1144, 1133, 732, 661 |
| 9 | Prodelphinidin pentamer | 1521 | 1421, 1283 |
| 10 | Prodelphinidin hexamer | 1825 | 609 |
| B | |||
| PSRE | Compound | [M − H]− m/z | Product Ions m/z |
| 1 | Epicatechin gallate | 441 | 289 |
| 2 | Epigallocatechin gallate | 457 | 305 |
| 3 | Catechin | 289 | |
| 4 | Epicatechin | 289 | |
| 5 | Epigallocatechin | 305 | 179, 221, 261 |
| 6 | Prodelphinidin dimer | 609 | 305, 423, 441, 483, 565, 591 |
| 7 | Prodelphinidin trimer | 913 | 303, 423, 533, 483, 559 |
| 8 | Prodelphinidin tetramer | 1217 | 955, 1133, 1155, 1064, 1144, 1133, 732, 661 |
| 9 | Prodelphinidin pentamer | 1521 | 1421, 1283 |
| 10 | Prodelphinidin hexamer | 1825 | 609 |
| P. gingivalis Viability | S. salivarius Viability | ||
|---|---|---|---|
| Extract (g/mL) | Viability (% vs. cnt) | Extract (g/mL) | Viability (% vs. cnt) |
| 0.02 | 26.200 | 0.02 | 40.978 |
| 0.03 | 36.927 | 0.03 | 44.290 |
| 0.04 | 35.212 | 0.04 | 41.539 |
| 0.05 | 33.443 | 0.05 | 21.930 |
| 0.06 | 25.911 | 0.06 | 20.081 |
| 0.07 | 21.398 | 0.07 | 23.278 |
| 0.08 | 10.693 | 0.08 | 16.933 |
| 0.09 | 12.674 | 0.09 | 9.717 |
| P. gingivalis Viability | S. salivarius Viability | ||
|---|---|---|---|
| Extract (mg/mL) | Viability (% vs. cnt) | Extract (mg/mL) | Viability (% vs. cnt) |
| 0.01 | 88.919 | 0.01 | 42.345 |
| 0.03 | 93.379 | 0.03 | 39.408 |
| 0.05 | 12.299 | 0.05 | 51.330 |
| 0.07 | 11.436 | 0.07 | 47.193 |
| 0.08 | 9.612 | 0.08 | 47.156 |
| 0.09 | 8.488 | 0.09 | 42.964 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savickiene, N.; Jekabsone, A.; Raudone, L.; Abdelgeliel, A.S.; Cochis, A.; Rimondini, L.; Makarova, E.; Grinberga, S.; Pugovics, O.; Dambrova, M.; et al. Efficacy of Proanthocyanidins from Pelargonium sidoides Root Extract in Reducing P. gingivalis Viability While Preserving Oral Commensal S. salivarius. Materials 2018, 11, 1499. https://doi.org/10.3390/ma11091499
Savickiene N, Jekabsone A, Raudone L, Abdelgeliel AS, Cochis A, Rimondini L, Makarova E, Grinberga S, Pugovics O, Dambrova M, et al. Efficacy of Proanthocyanidins from Pelargonium sidoides Root Extract in Reducing P. gingivalis Viability While Preserving Oral Commensal S. salivarius. Materials. 2018; 11(9):1499. https://doi.org/10.3390/ma11091499
Chicago/Turabian StyleSavickiene, Nijole, Aiste Jekabsone, Lina Raudone, Asmaa S. Abdelgeliel, Andrea Cochis, Lia Rimondini, Elina Makarova, Solveiga Grinberga, Osvalds Pugovics, Maija Dambrova, and et al. 2018. "Efficacy of Proanthocyanidins from Pelargonium sidoides Root Extract in Reducing P. gingivalis Viability While Preserving Oral Commensal S. salivarius" Materials 11, no. 9: 1499. https://doi.org/10.3390/ma11091499