Application of Aluminium Flakes in Fabrication of Open-Cell Aluminium Foams by Space Holder Method
Abstract
:1. Introduction
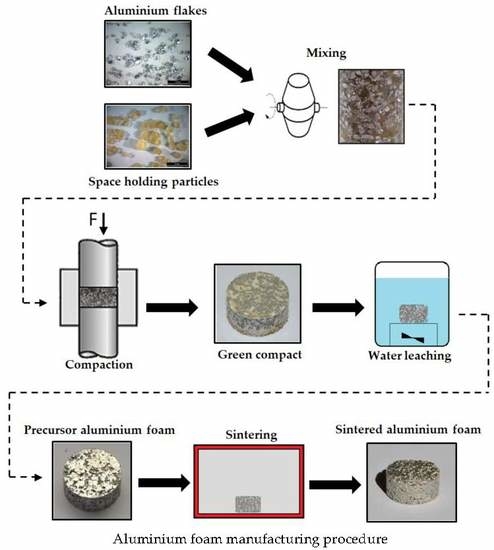
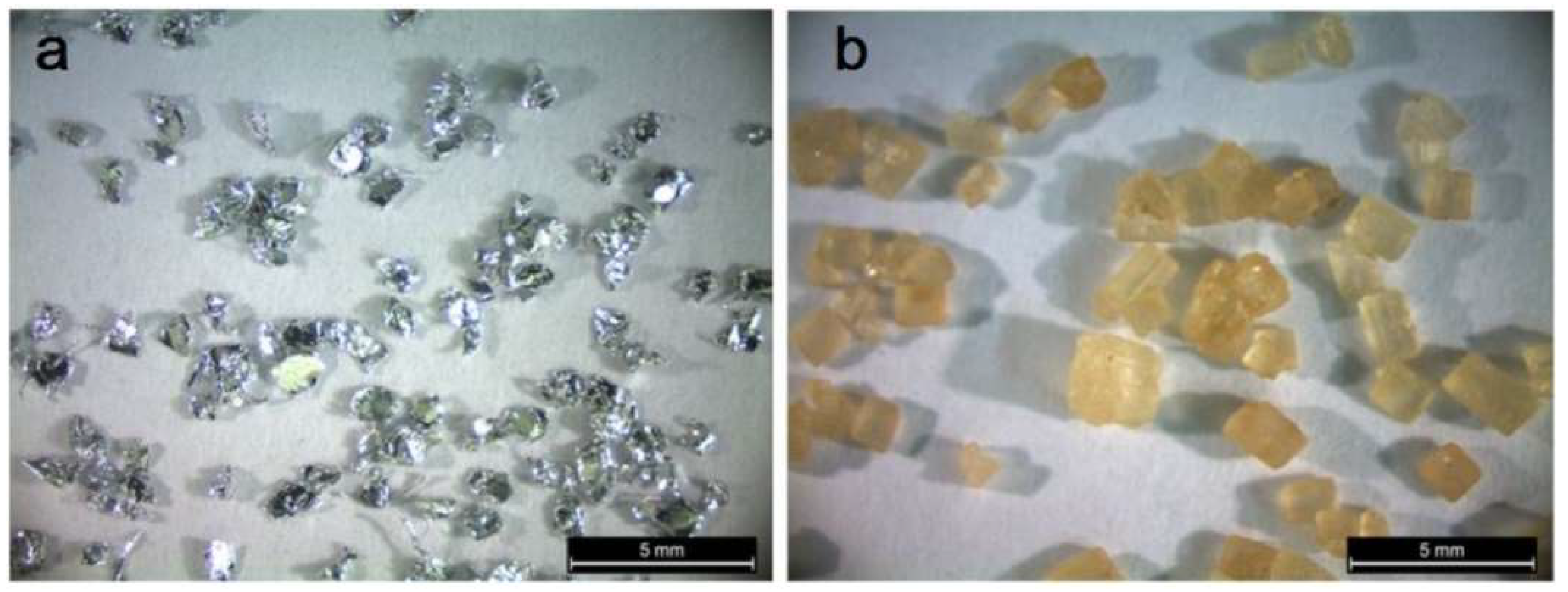
2. Materials and Methods
3. Results
3.1. Compaction
3.2. Dissolution
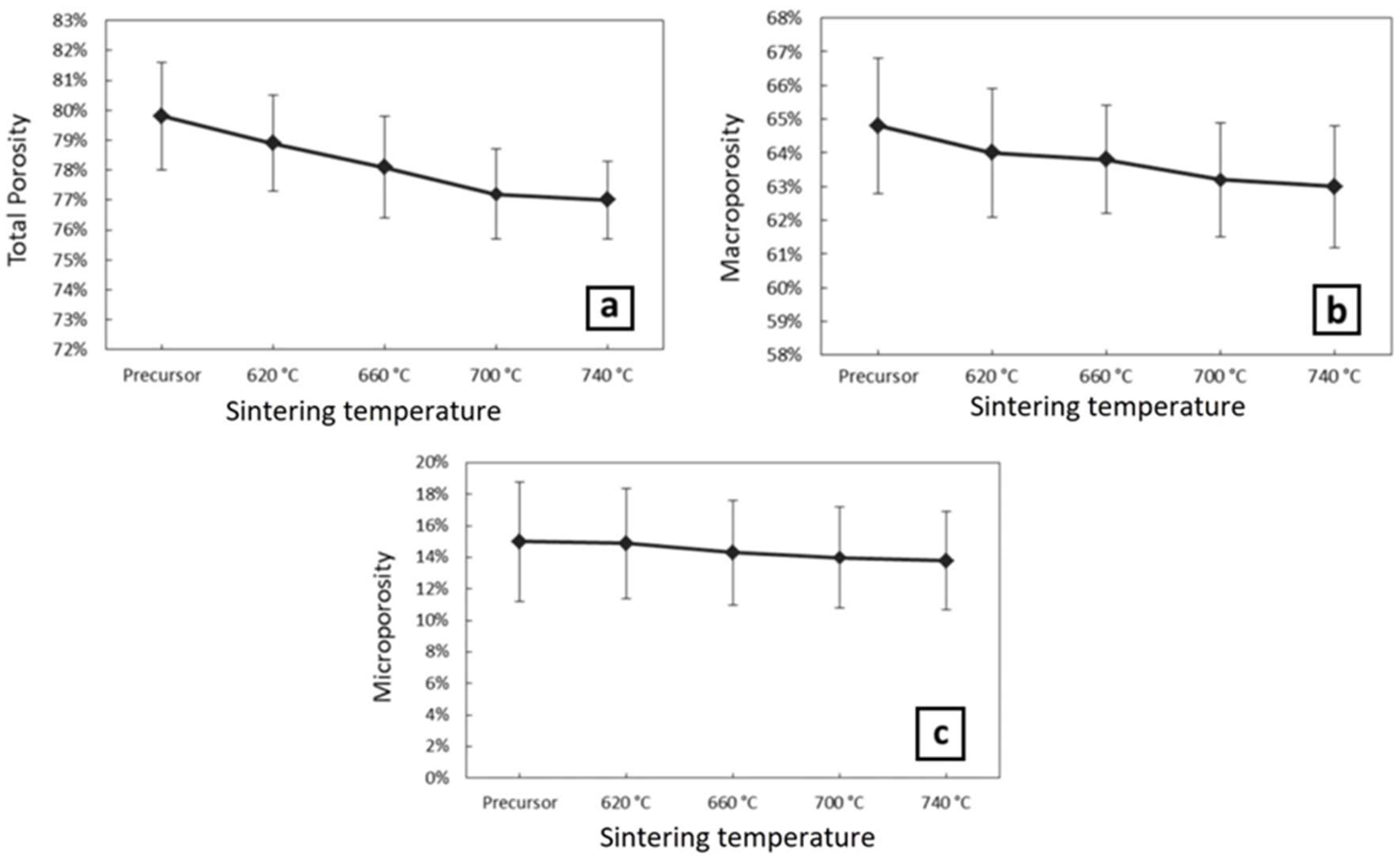
3.3. Sintering
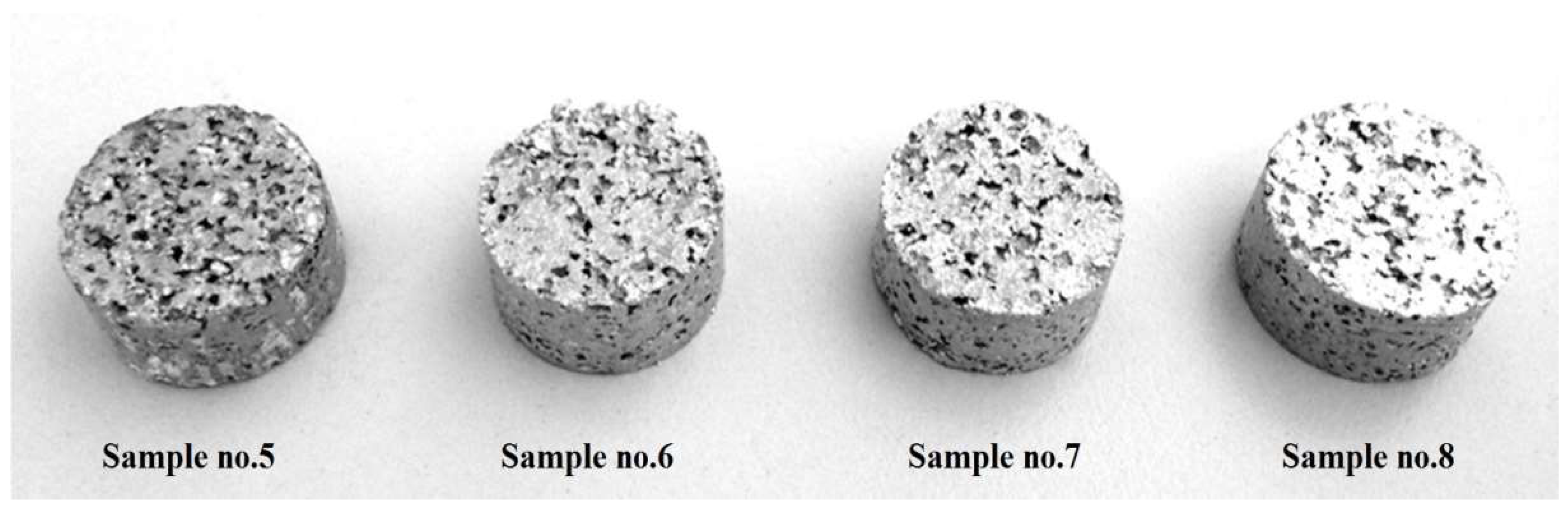
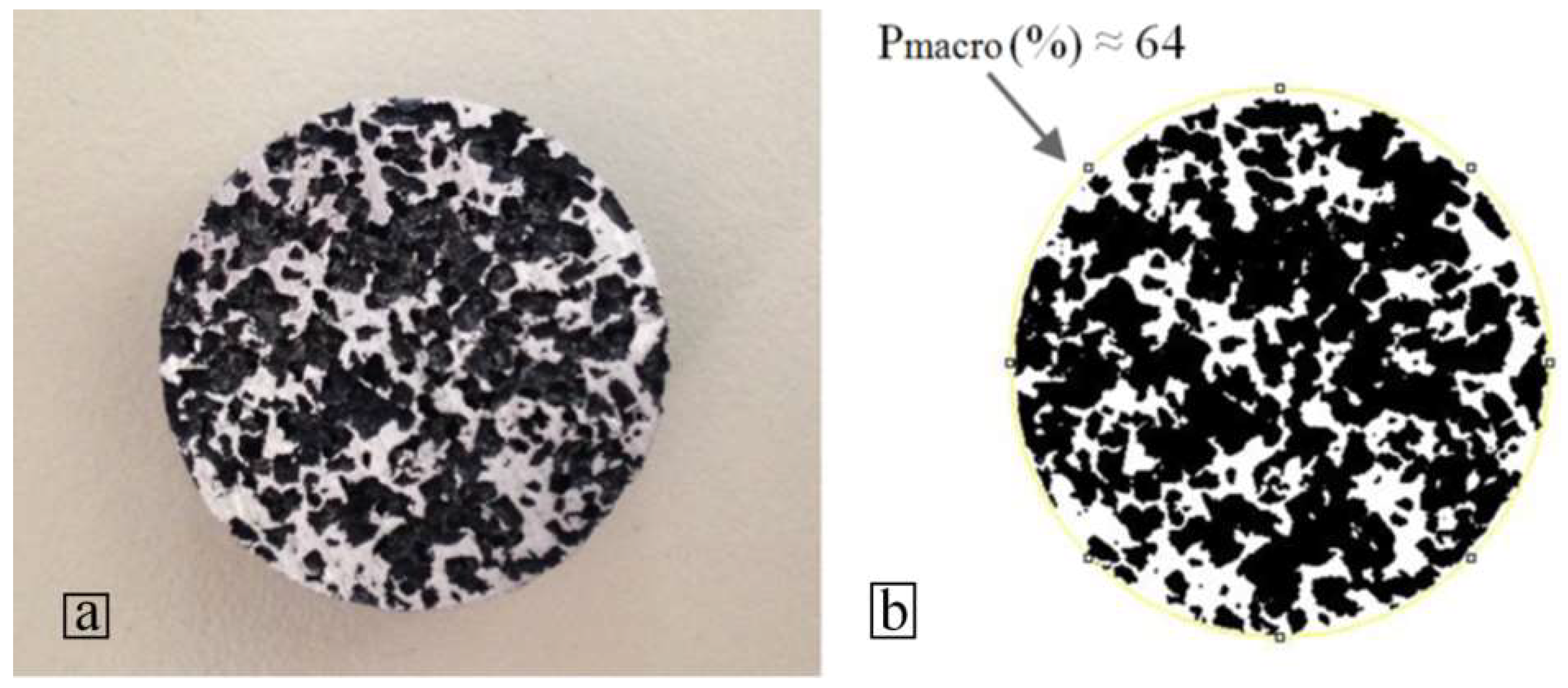
3.4. Evaluation of Specimens after Sintering
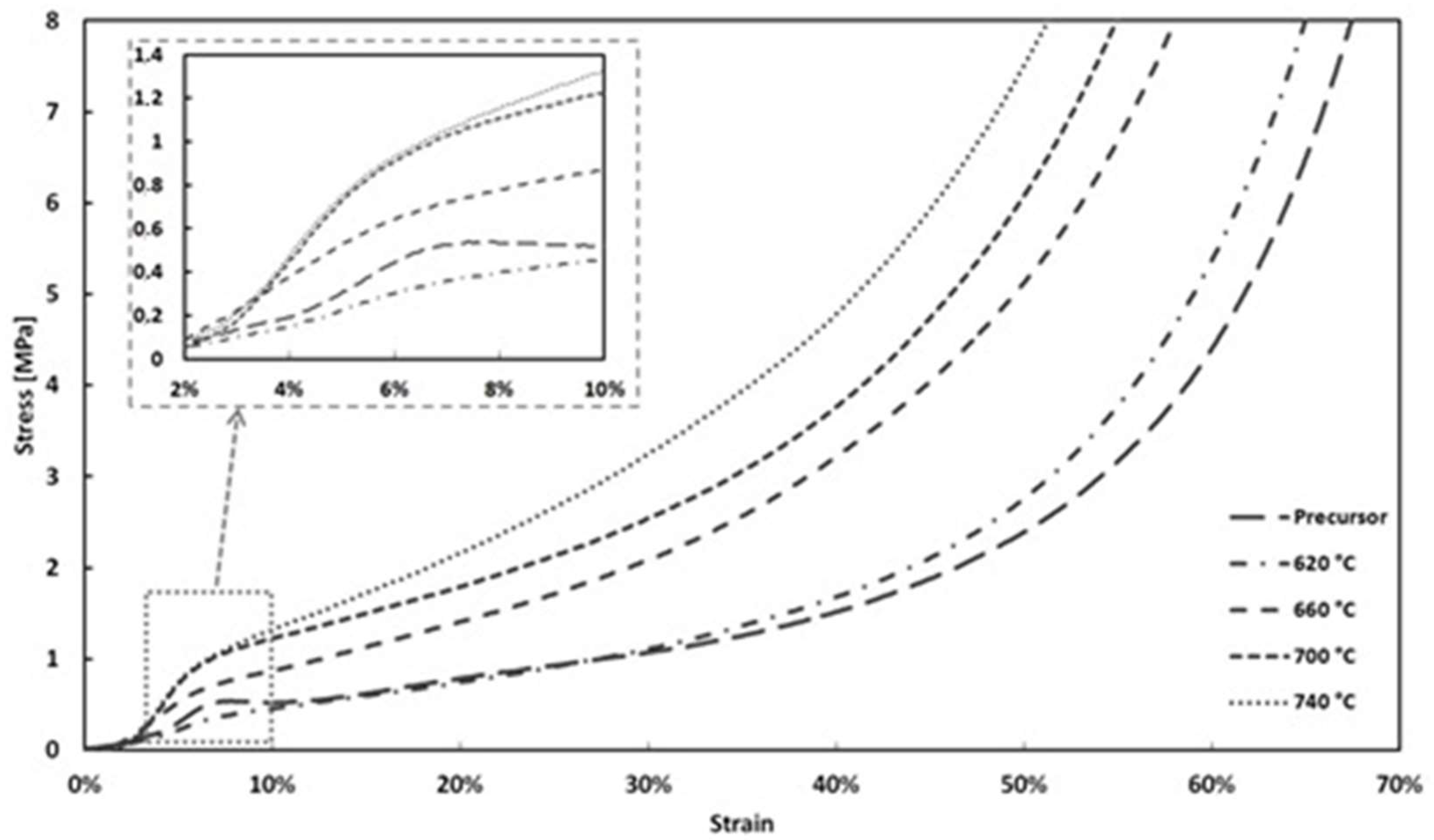
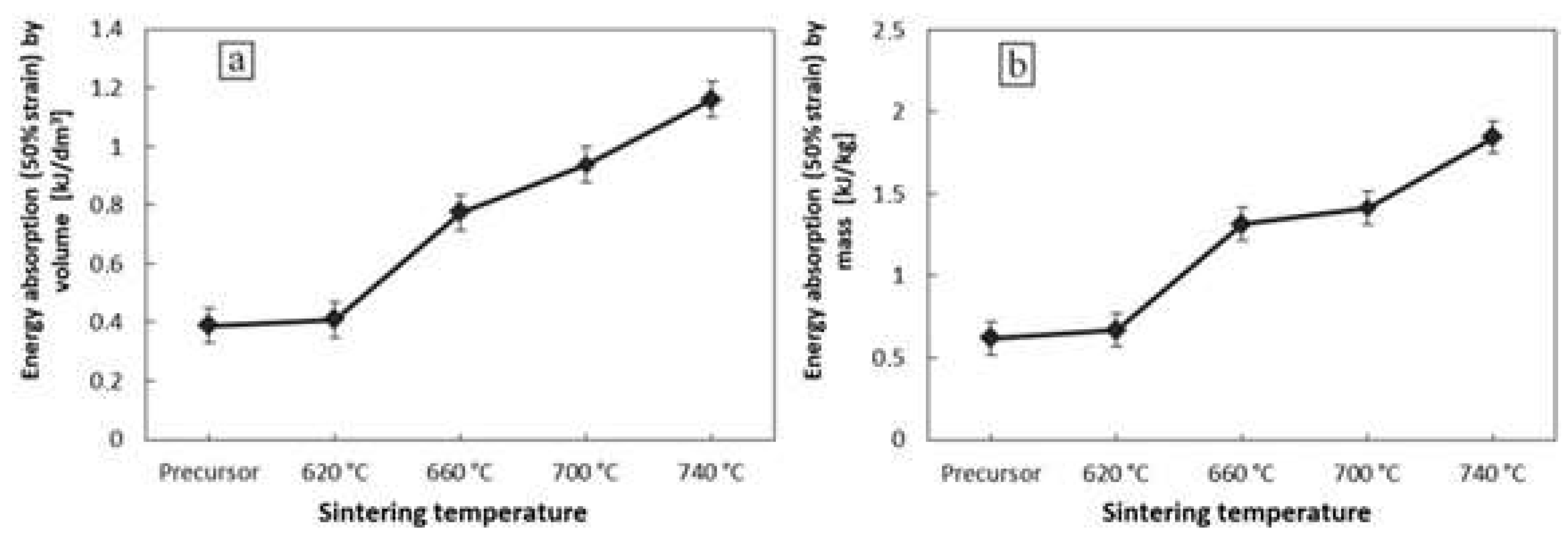
3.5. Mechanical Properties of Foams
3.6. Microstructural Evaluation
4. Discussion
5. Conclusions
- The novel use of aluminum flakes as the main material of this process allows the production of foams with an excellent internal open-cell porous structure at low compaction pressures and with no need for any binding additive.
- The saccharose crystals as a space holder can be easily removed, leaving a porous integral structure. The irregular shape and inhomogeneous morphology of the pore structure are attributed to the space holder mean size, geometry, and volume fraction.
- The mechanical properties, i.e., stress at the macroscopic yield point, plateau stress, energy absorption, and the efficiency of energy absorption, can be easily modified/improved by variation of the foam sintering temperature.
- The green compact with no sintering process presented relatively high mechanical properties due to the complex mechanical bonding of the flakes at the green density.
- The procedure introduced high reproducibility of the results with a simple, low cost, and environmentally friendly method.
Author Contributions
Funding
Conflicts of Interest
References
- Ashby, M.F.; Evans, A.; Fleck, N.A.; Gibson, L.J.; Hutchinson, J.W.; Wadley, H.N.G. Metal Foams: A Design Guide; Butterworth-Heinemann: Burlington, MA, USA, 2000. [Google Scholar]
- Gibson, L.G.; Ashby, M.F. Cellular Solids, Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- García-Moreno, F. Commercial Applications of Metal Foams: Their Properties and Production. Materials 2016, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Papantoniou, I.G.; Kyriakopoulou, H.P.; Pantelis, D.I.; Manolakos, D.E. Fabrication of MWCNT-reinforced Al composite local foams using friction stir processing route. Int. J. Adv. Manuf. Technol. 2018, 53, 3817–3835. [Google Scholar] [CrossRef]
- Duarte, I.; Ferreira, J.M.F. Composite and Nanocomposite Metal Foams. Materials 2016, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.; Vinod-Kumar, G.S.; Garcıa-Moreno, F.; Banhart, J. Stability of various particle-stabilised aluminium alloys foams made by gas injection. J. Mater. Sci. 2017, 52, 6401–6414. [Google Scholar] [CrossRef] [Green Version]
- Papantoniou, I.G.; Kyriakopoulou, H.P.; Pantelis, D.I.; Athanasiou-Ioannou, A.; Manolakos, D.E. Manufacturing process of AA5083/nano-γAl2O3 localized composite metal foam fabricated by friction stir processing route (FSP) and microstructural characterization. J. Mater. Sci. 2018, 53, 3817–3835. [Google Scholar] [CrossRef]
- Orbulov, I.N.; Májlinger, K. Description of the compressive response of metal matrix syntactic foams. Mater. Des. 2013, 49, 1–9. [Google Scholar] [CrossRef]
- Fiedler, T.; Taherishargh, M.; Krstulović-Opara, L.; Vesenjak, M. A Dynamic compressive loading of expanded perlite/aluminum syntactic foam. Mater. Sci. Eng. A 2015, 626, 296–304. [Google Scholar] [CrossRef]
- Al-Sahlani, K.; Taherishargh, M.; Kisi, E.; Fiedler, T. Controlled Shrinkage of Expanded Glass Particles in Metal Syntactic Foams. Materials 2017, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhao, Y.; Dong, A.; Li, Y. Preparation and Properties of C/C Hollow Spheres and the Energy Absorption Capacity of the Corresponding Aluminum Syntactic Foams. Materials 2018, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Jamal, N.A.; Tan, A.W.; Yusof, F.; Katsuyoshi, K.; Hisashi, I.; Singh, S.; Anuar, H. Fabrication and Compressive Properties of Low to Medium Porosity Closed-Cell Porous Aluminum Using PMMA Space Holder Technique. Materials 2016, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Linul, E.; Movahedi, N.; Marsavina, L. On the Lateral Compressive Behavior of Empty and Ex-Situ Aluminum Foam-Filled Tubes at High Temperature. Materials 2018, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, N.; Stergioudi, F.; Tsouknidas, A. Deformation and energy absorption properties of powder-metallurgy produced Al foams. Mater. Sci. Eng. A 2011, 528, 7222–7227. [Google Scholar] [CrossRef]
- Laughlin, D.; Hono, K. Porous Metal, Physical Metallurgy, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Papadopoulos, D.P.; Omar, H.; Stergioudi, F.; Tsipas, S.A.; Lefakis, H.; Michailidis, N. A novel method for producing Al-foams and evaluation of their compression behavior. J. Porous Mater. 2011, 17, 773–777. [Google Scholar] [CrossRef]
- Bakan, H.I. A novel water leaching and sintering process for manufacturing highly porous stainless steel. Scr. Mater. 2006, 55, 203–206. [Google Scholar] [CrossRef]
- El-Hadek, M.A.; Kaytbay, S. Mechanical and physical characterization of copper foam. Int. J. Mech. Mater. Des. 2008, 4, 63–69. [Google Scholar] [CrossRef]
- Nabawy, A.M.; Khalil, K.A.; Al-Ahmari, A.M.; Sherif, E.-S.M. Melt Processing and Characterization of Al-SiC Nanocomposite, Al, and Mg Foam Materials. Metals 2016, 6, 110. [Google Scholar] [CrossRef]
- Bafti, H.; Habibolahzadeh, A. Production of aluminum foam by spherical carbamide space holder technique-processing parameters. Mater. Des. 2010, 31, 4122–4129. [Google Scholar] [CrossRef]
- Hu, L.; Ngai, T.; Peng, H.; Li, L.; Zhou, F.; Peng, Z. Microstructure and Properties of Porous High-N Ni-Free Austenitic Stainless Steel Fabricated by Powder Metallurgical Route. Materials 2018, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, J.; Adamek, G.; Dewidar, M. Titanium foam made with saccharose as a space holder. J. Porous Mater. 2013, 20, 1137–1141. [Google Scholar] [CrossRef] [Green Version]
- Arifvianto, B.; Leeflang, M.; Zhou, J. A new technique for the characterization of the water leaching behavior of space holding particles in the preparation of biomedical titanium scaffolds. Mater. Lett. 2014, 120, 204–207. [Google Scholar] [CrossRef]
- Surace, R.; De Filippis, L.A.C.; Ludovico, A.D.; Boghetich, G. Influence of processing parameters on aluminium foam produced by space holder technique. Mater. Des. 2009, 30, 1878–1885. [Google Scholar] [CrossRef]
- Torres, Y.; Pavón, J.J.; Rodríguez, J.A. Processing and characterization of porous titanium for implants by using NaCl as space holder. J. Mater. Process. Technol. 2012, 212, 1061–1069. [Google Scholar] [CrossRef]
- Esen, Z.; Bor, S. Processing of titanium foams using magnesium spacer particles. Scr. Mater. 2007, 56, 341–344. [Google Scholar] [CrossRef]
- Barletta, M.; Gisario, A.; Guarino, S.; Rubino, G. Production of open cell aluminum foams by using the dissolution and sintering process. J. Manuf. Sci. Eng. 2009, 131, 041009. [Google Scholar] [CrossRef]
- Gulsoy, H.O.; German, R.M. Sintered foams from precipitation hardened stainless steel powder. Powder Met. 2008, 51, 350–353. [Google Scholar] [CrossRef]
- Shimizu, T.; Matsuzaki, K.; Nagai, H.; Kanetake, N. Production of high porosity metal foams using EPS beads as space holders. Mater. Sci. Eng. A 2012, 558, 343–348. [Google Scholar] [CrossRef]
- Michailidis, N.; Stergioudi, F. Establishment of process parameters for producing Al-foam by dissolution and powder sintering method. Mater. Des. 2011, 32, 1559–1564. [Google Scholar] [CrossRef]
- Niu, W.; Bai, C.; Qui, G.; Wang, Q.; Wen, L.; Chen, D.; Dong, L. Preparation and characterization of porous titanium using space-hoder technique. Rare Met. 2009, 28, 338–342. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Sun, D.X. A novel sintering-dissolution process for manufacturing al foams. Scr. Mater. 2001, 44, 105–110. [Google Scholar] [CrossRef]





| Samples Set (Three Samples Per Set) | Compaction Pressure [MPa] | Precursor Stability |
|---|---|---|
| 1 | 100 | Unstable (wall delamination) |
| 2 | 150 | Unstable (minor wall delamination) |
| 3 | 200 | Stable |
| 4 | 250 | Unstable (cracks) |
| Samples Set (Three Samples per Set) | Compaction Pressure [mpa] | Sintering Temperature [°C] | Energy Absorption (50% Strain) by Volume [kj/dm3] | Energy Absorption (50% Strain) by Mass [kj/kg] | Porosity [%] |
|---|---|---|---|---|---|
| 3 | 200 | No sintering | 0.39 | 0.62 | 79.8 ± 1.8% |
| 5 | 200 | 620 | 0.41 | 0.67 | 78.9 ± 1.6% |
| 6 | 200 | 660 | 0.78 | 1.32 | 78.1 ± 1.7% |
| 7 | 200 | 700 | 0.94 | 1.41 | 77.2 ± 1.5% |
| 8 | 200 | 740 | 1.16 | 1.85 | 76.8 ± 1.3% |
| 9 | 200 | 780 | Precursor foams sintered at 780 °C collapsed in the oven | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papantoniou, I.G.; Markopoulos, A.P.; Pantelis, D.I.; Manolakos, D.E. Application of Aluminium Flakes in Fabrication of Open-Cell Aluminium Foams by Space Holder Method. Materials 2018, 11, 1420. https://doi.org/10.3390/ma11081420
Papantoniou IG, Markopoulos AP, Pantelis DI, Manolakos DE. Application of Aluminium Flakes in Fabrication of Open-Cell Aluminium Foams by Space Holder Method. Materials. 2018; 11(8):1420. https://doi.org/10.3390/ma11081420
Chicago/Turabian StylePapantoniou, Ioannis G., Angelos P. Markopoulos, Dimitrios I. Pantelis, and Dimitrios E. Manolakos. 2018. "Application of Aluminium Flakes in Fabrication of Open-Cell Aluminium Foams by Space Holder Method" Materials 11, no. 8: 1420. https://doi.org/10.3390/ma11081420