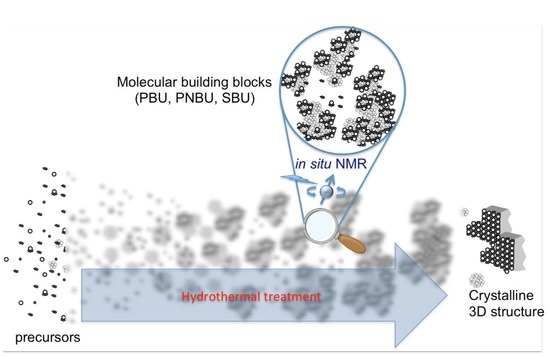
Nuclear Magnetic Resonance Spectroscopy for In Situ Monitoring of Porous Materials Formation under Hydrothermal Conditions
Abstract
:1. Introduction
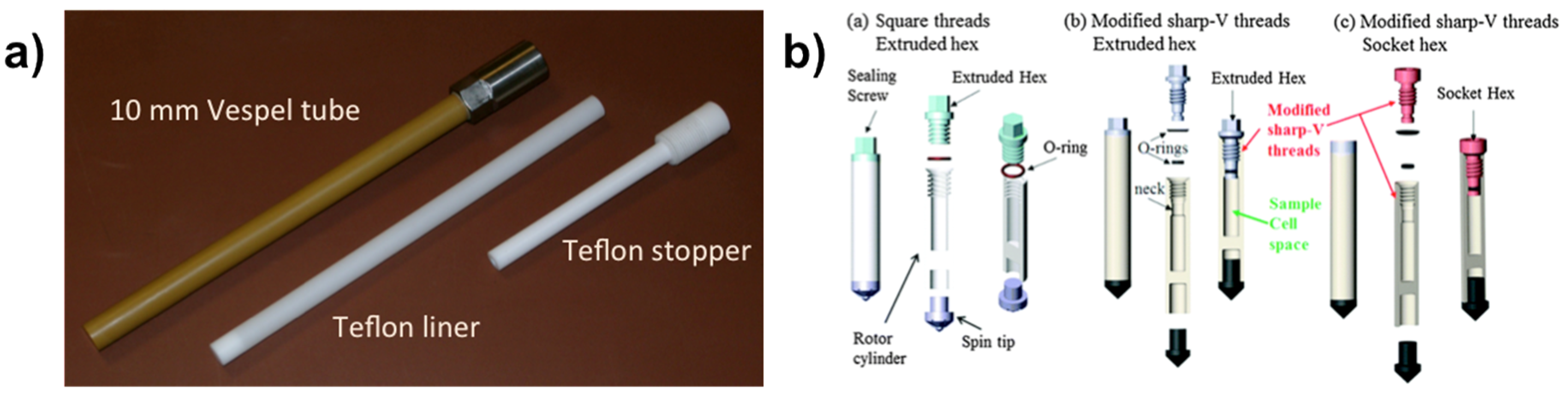
2. Some Experimental Aspects of NMR under Hydrothermal Conditions
2.1. NMR Cells and Devices for High Temperature and High Pressure
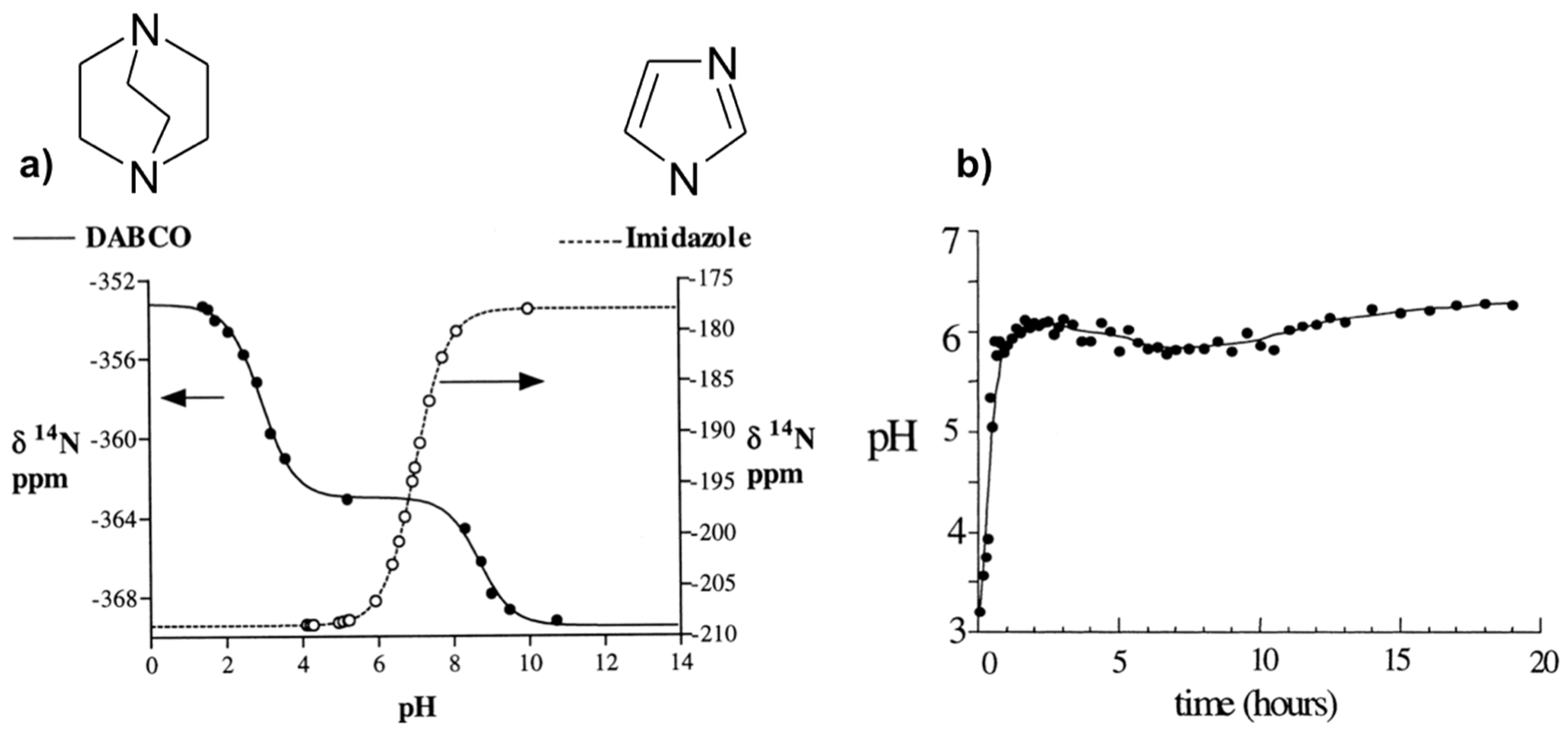
2.2. Measuring pH In Situ by NMR Method
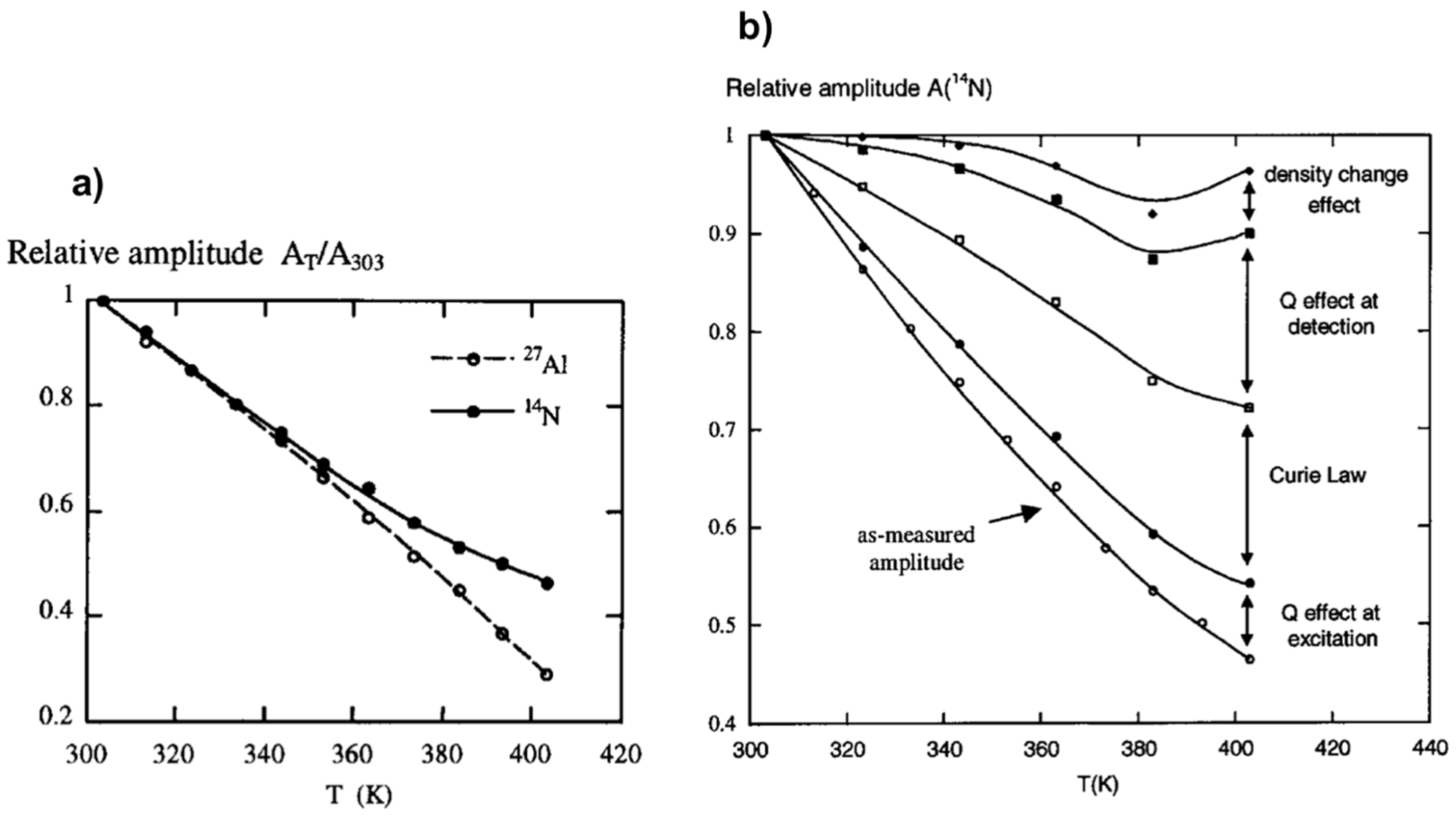
2.3. Quantification by NMR at Variable High Temperature
3. Examples of In Situ NMR Studies on Crystallization of Microporous Materials
3.1. Zeolites
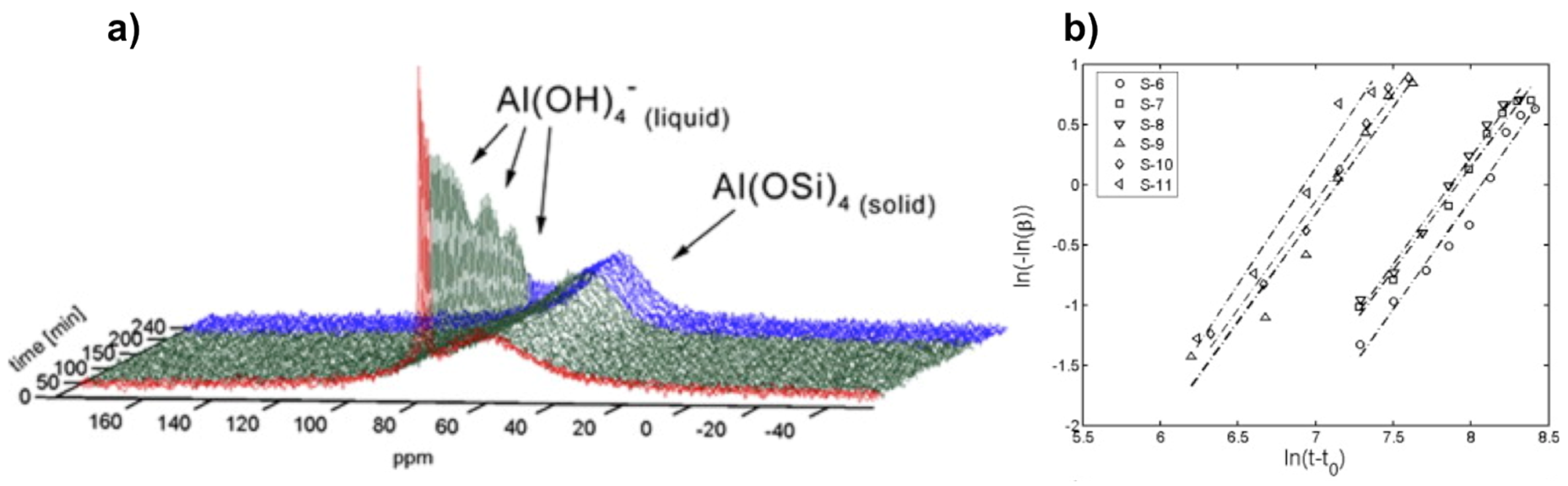
3.1.1. First In Situ MAS NMR Study
3.1.2. Liquid State In Situ NMR Study
3.2. Aluminophosphate Zeotypes
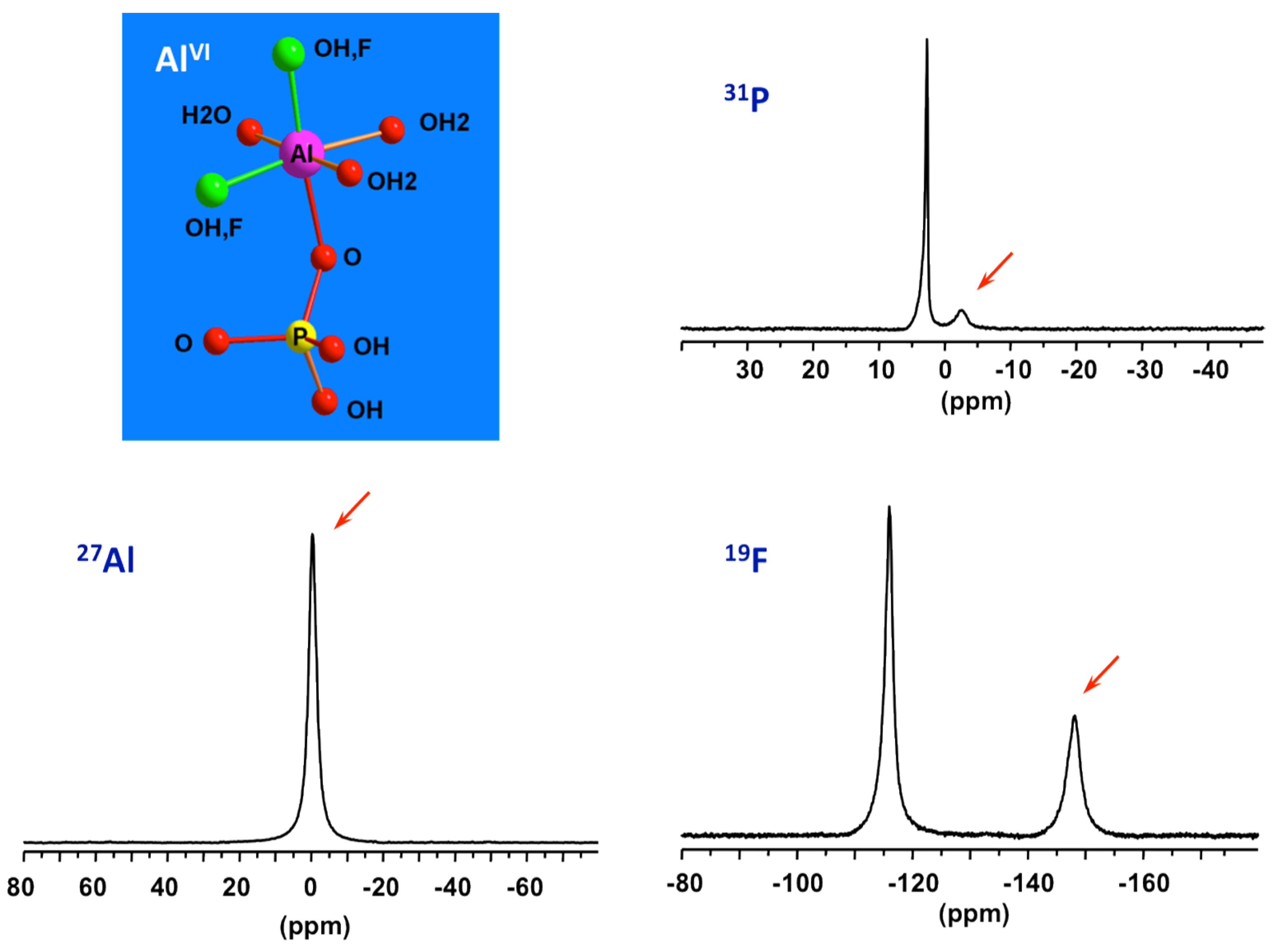
3.2.1. Identifying the Primary Building Units (PBUs)
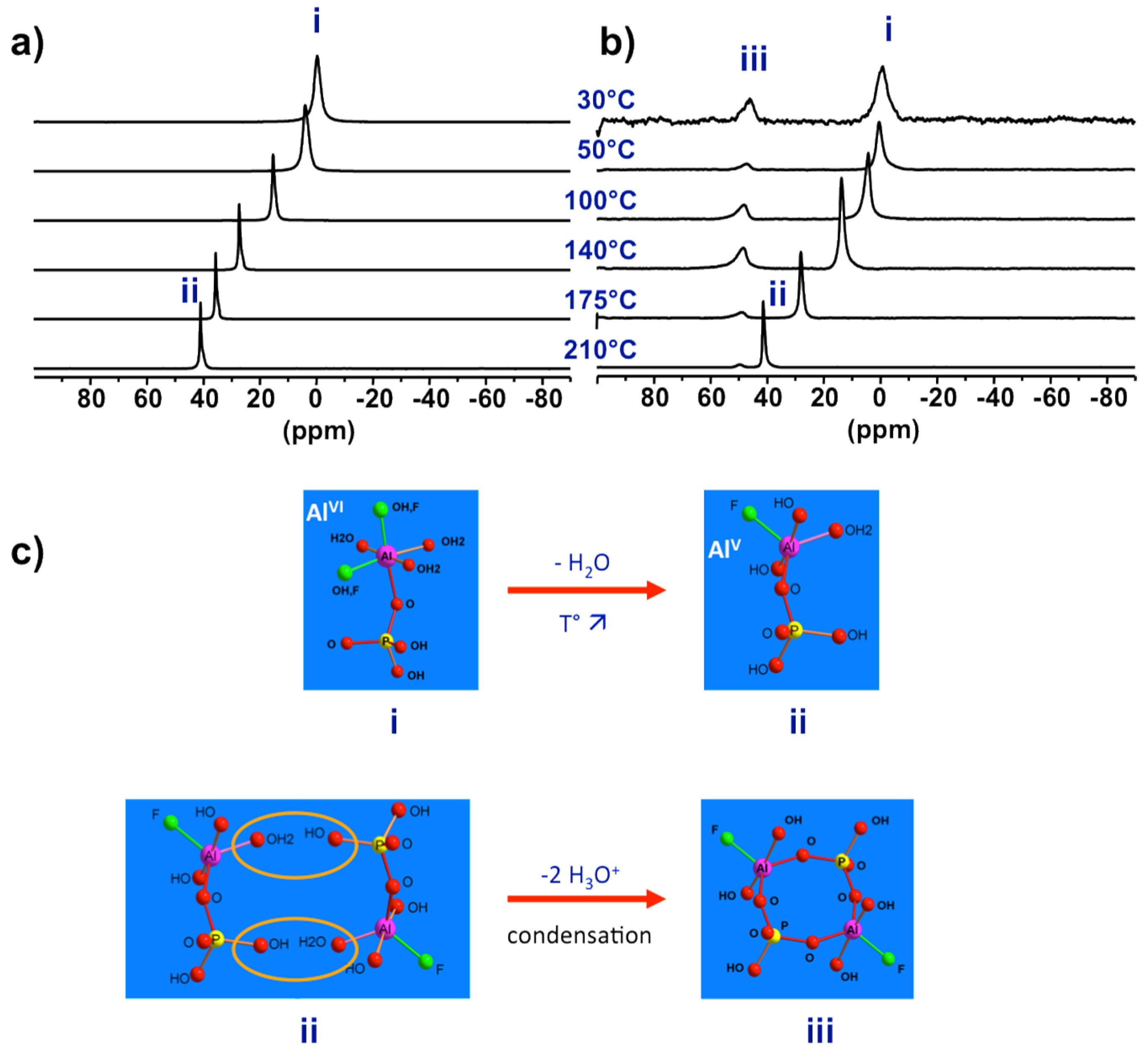
3.2.2. Tracking the Prenucleation Building Units (PNBUs)
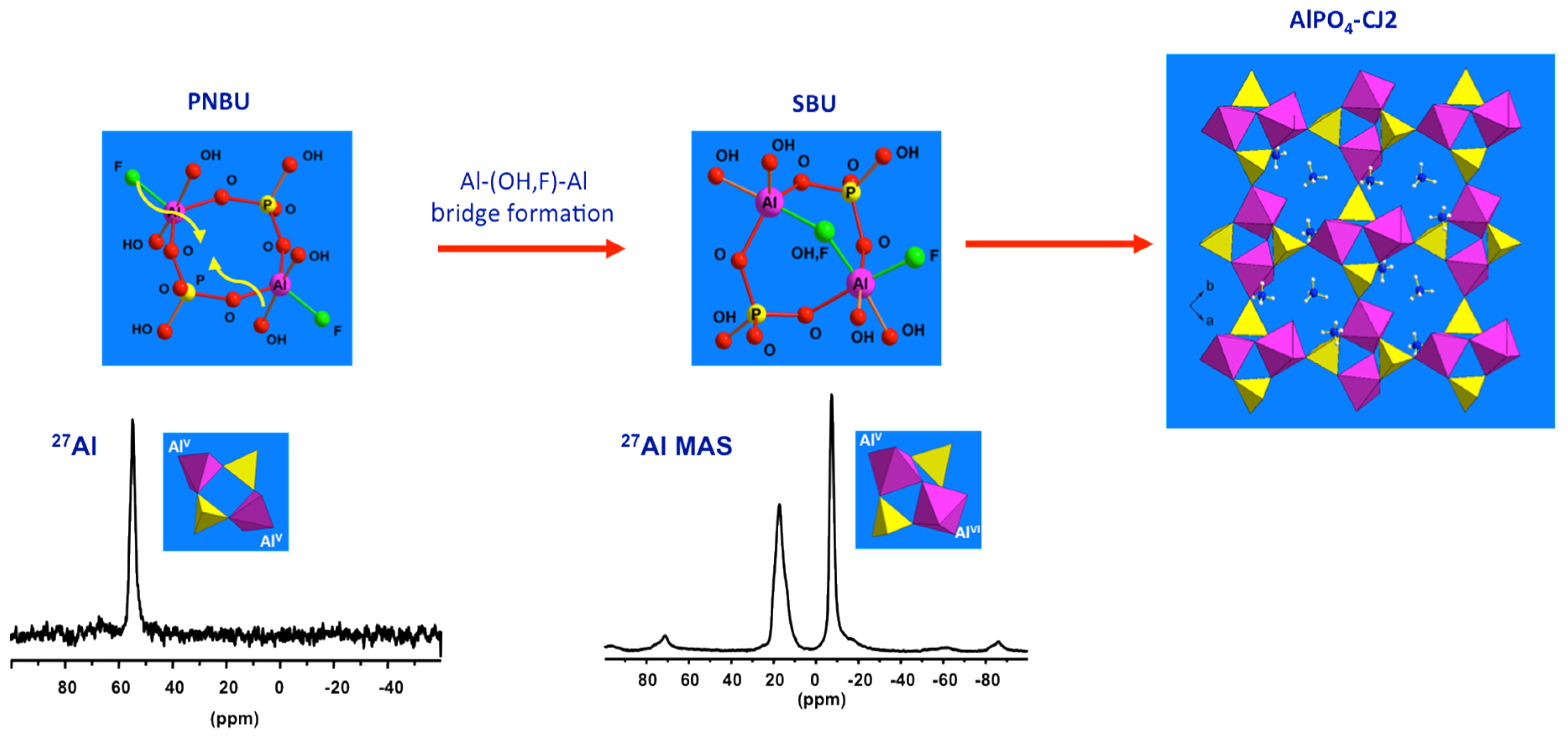
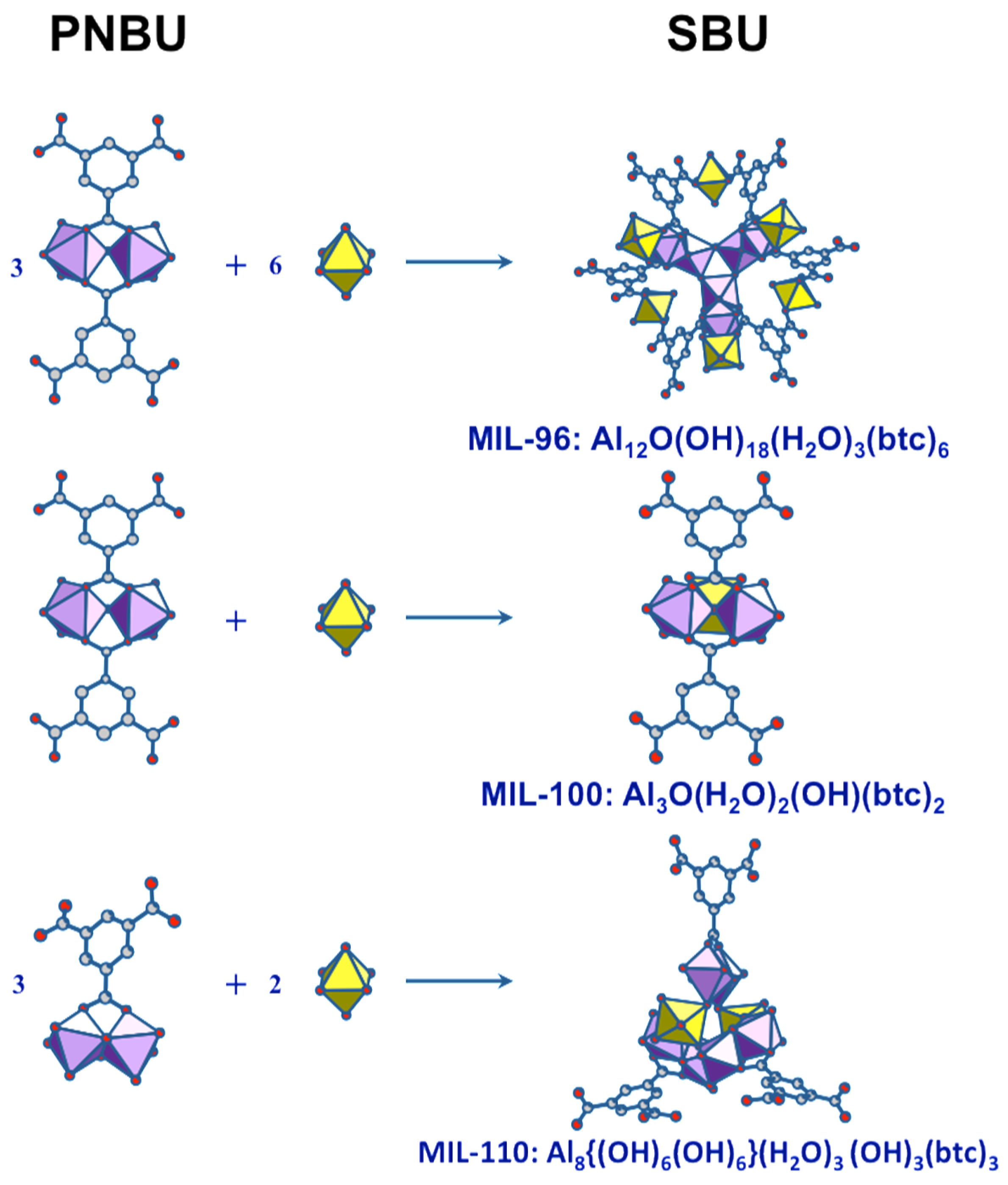
3.2.3. Structural Relationship between PNBUs and SBUs
3.2.4. Recent MAS In Situ Investigation on AlPO4-5
3.3. Aluminum Carboxylate MOFs
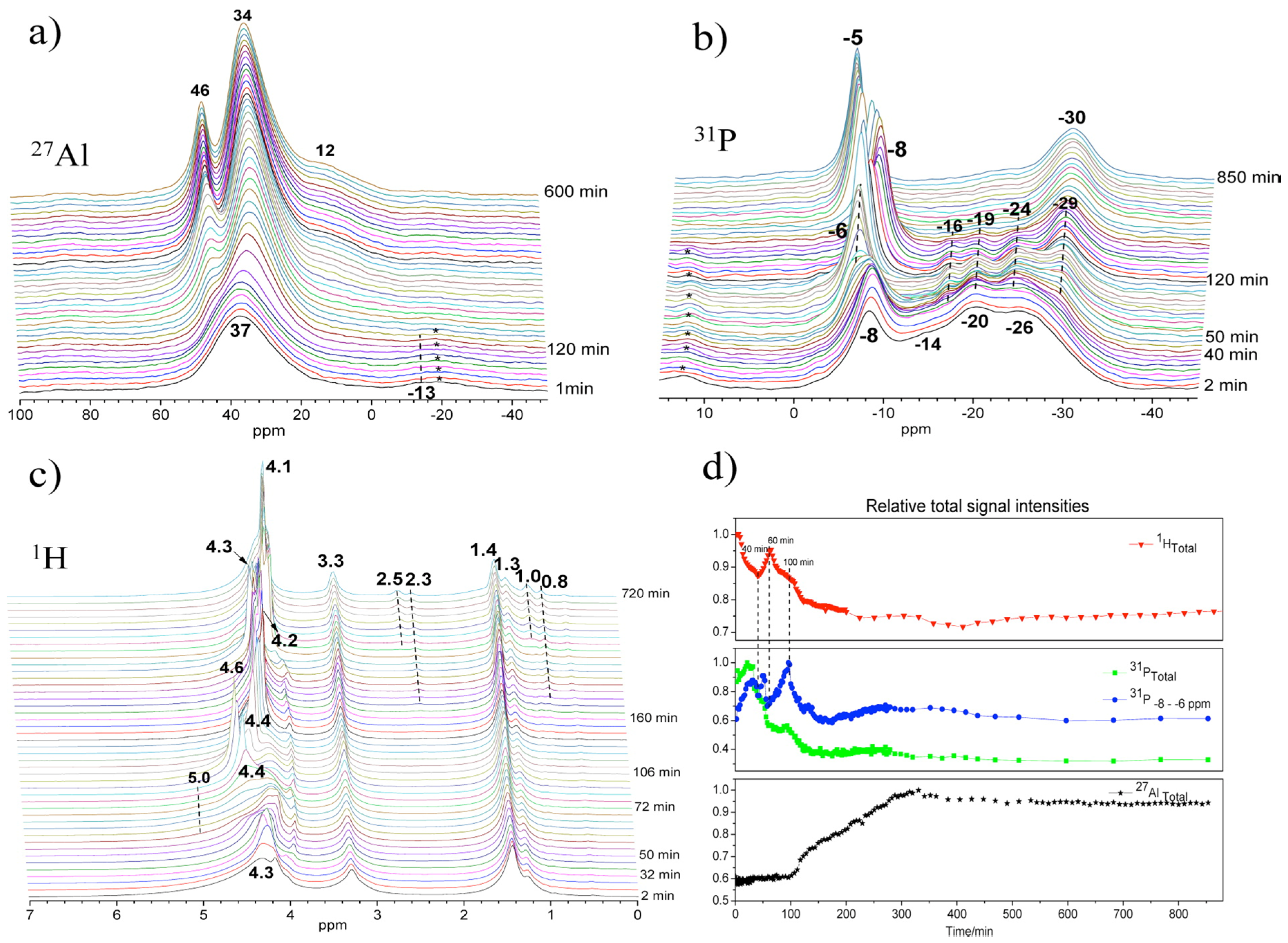
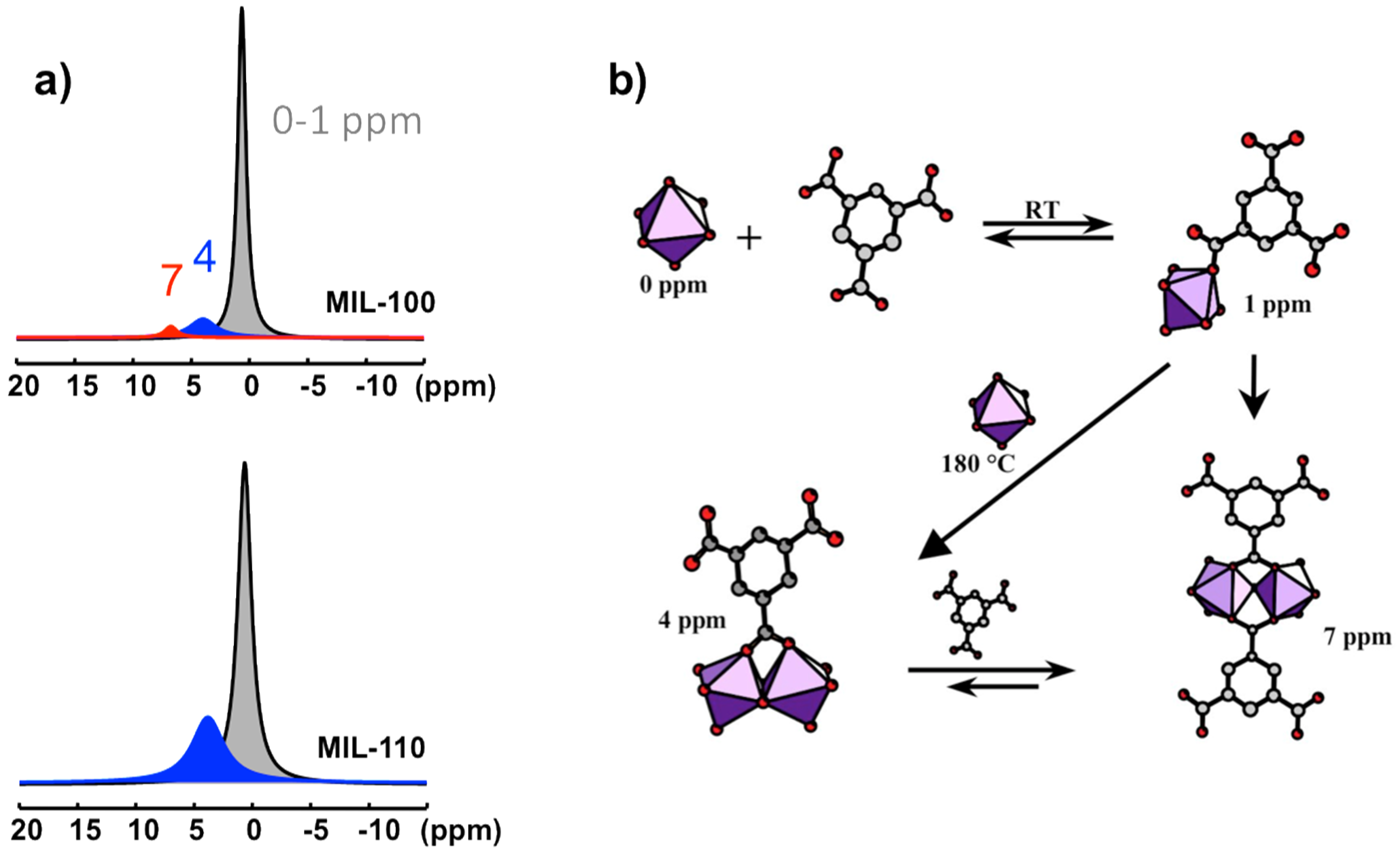
3.3.1. Identifying PNBUs in Growth Solution of Al-Trimesate Based MOFs
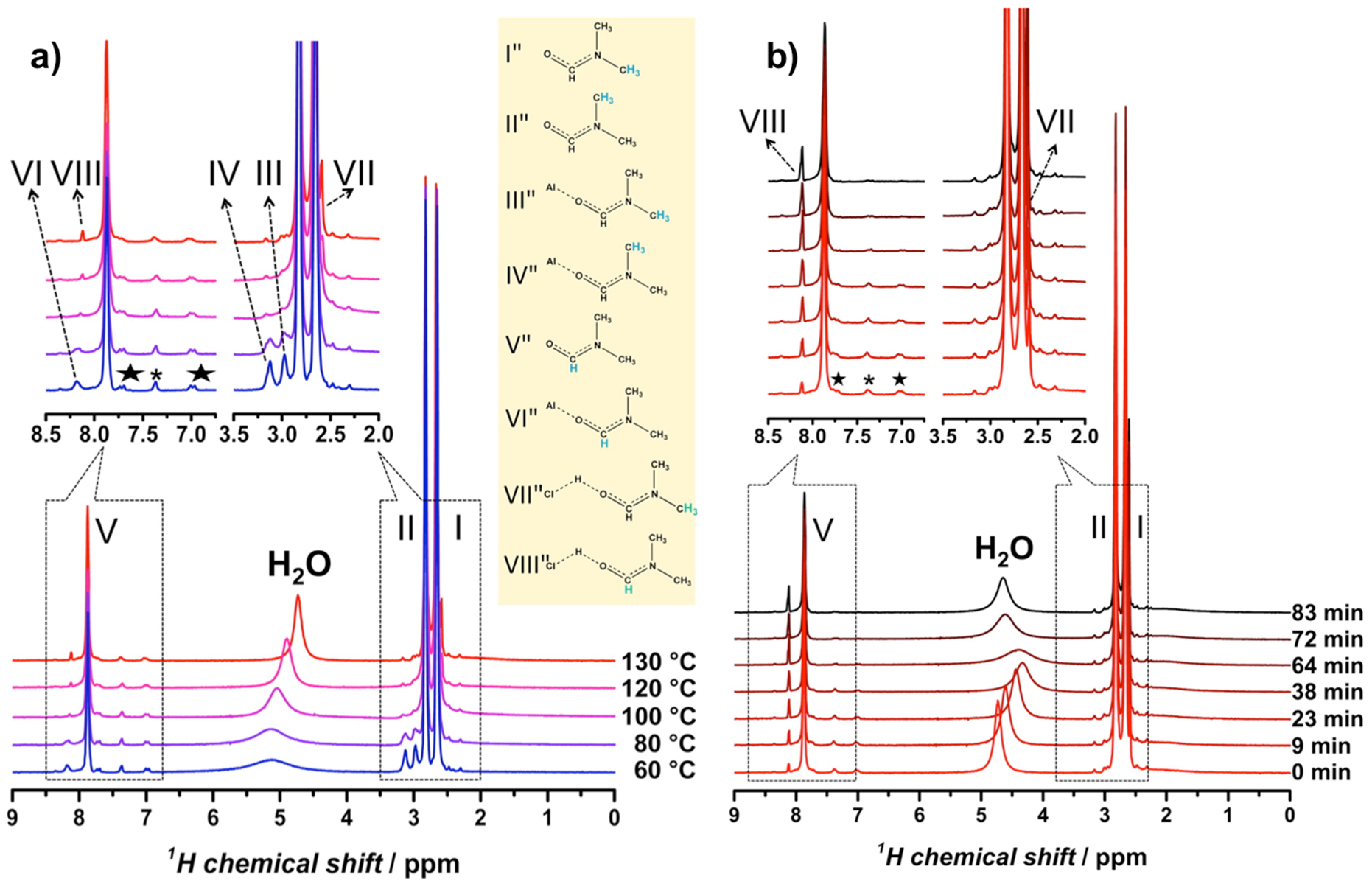
3.3.2. The Role of N,N-Dimethylformamide Solvent on the Synthesis of NH2-MIL-101
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Loiseau, T.; Ferey, G. Crystalline oxyfluorinated open-framework compounds: Silicates, metal phosphates, metal fluorides and metal-organic frameworks (MOF). J. Fluor. Chem. 2007, 128, 413–422. [Google Scholar] [CrossRef]
- Alhamami, M.; Doan, H.; Cheng, C.H. A Review on Breathing Behaviors of Metal-Organic-Frameworks (MOFs) for Gas Adsorption. Materials 2014, 7, 3198–3250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ennaert, T.; Van Aelst, J.; Dijkmans, J.; De Clercq, R.; Schutyser, W.; Dusselier, M.; Verboekend, D.; Sels, B.F. Potential and challenges of zeolite chemistry in the catalytic conversion of biomass. Chem. Soc. Rev. 2016, 45, 584–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gkaniatsou, E.; Sicard, C.; Ricoux, R.; Mahy, J.P.; Steunou, N.; Serre, C. Metal-organic frameworks: A novel host platform for enzymatic catalysis and detection. Mater. Horiz. 2017, 4, 55–63. [Google Scholar] [CrossRef]
- Janiak, C.; Henninger, S.K. Porous Coordination Polymers as Novel Sorption Materials for Heat Transformation Processes. Chimia 2013, 67, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E.; Wheatley, P.S. Gas storage in nanoporous materials. Angew. Chem. Int. Ed. 2008, 47, 4966–4981. [Google Scholar] [CrossRef] [PubMed]
- Cantu, D.C.; McGrail, B.P.; Glezakou, V.A. Formation Mechanism of the Secondary Building Unit in a Chromium Terephthalate Metal-Organic Framework. Chem. Mater. 2014, 26, 6401–6409. [Google Scholar] [CrossRef]
- Ferey, G.; Haouas, M.; Loiseau, T.; Taulelle, F. Nanoporous Solids: How Do They Form? An In Situ Approach. Chem. Mater. 2014, 26, 299–309. [Google Scholar] [CrossRef]
- Cooper, E.R.; Andrews, C.D.; Wheatley, P.S.; Webb, P.B.; Wormald, P.; Morris, R.E. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature 2004, 430, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Ferey, G. Microporous solids: From organically templated inorganic skeletons to hybrid frameworks … ecumenism in chemistry. Chem. Mater. 2001, 13, 3084–3098. [Google Scholar] [CrossRef]
- Catlow, C.R.A.; Coombes, D.S.; Pereira, J.C.G. Computer modeling of nucleation, growth, and templating in hydrothermal synthesis. Chem. Mater. 1998, 10, 3249–3265. [Google Scholar] [CrossRef]
- Gies, H.; Marler, B. The structure-controlling role of organic templates for the synthesis of porosils in the system SiO2/template/H2O. Zeolites 1992, 12, 42–49. [Google Scholar] [CrossRef]
- Ilyushin, G.D.; Blatov, V.A. Modeling of self-organization processes in crystal-forming systems: Templated precursor nanoclusters T48 and the self-assembly of crystal structures of 15-crown-5, Na-FAU, 18-crown-6, Na-EMT, and Ca,Ba-TSC zeolites. Russ. J. Inorg. Chem. 2015, 60, 469–482. [Google Scholar] [CrossRef]
- Jin, L.; Auerbach, S.M.; Monson, P.A. Modeling Nanoparticle Formation during Early Stages of Zeolite Growth: A Low-Coordination Lattice Model of Template Penetration. J. Phys. Chem. C 2010, 114, 14393–14401. [Google Scholar] [CrossRef] [Green Version]
- Verstraelen, T.; Szyja, B.M.; Lesthaeghe, D.; Declerck, R.; Van Speybroeck, V.; Waroquier, M.; Jansen, A.P.J.; Aerts, A.; Follens, L.R.A.; Martens, J.A.; et al. Multi-level Modeling of Silica-Template Interactions During Initial Stages of Zeolite Synthesis. Top. Catal. 2009, 52, 1261–1271. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Microporous Mesoporous Mater. 2005, 82, 1–78. [Google Scholar] [CrossRef]
- Wiebcke, M. Structural links between zeolite-type and clathrate hydrate-type materials. J. Chem. Soc. Chem. Commun. 1991, 1507–1508. [Google Scholar] [CrossRef]
- Epping, J.D.; Chmelka, B.F. Nucleation and growth of zeolites and inorganic mesoporous solids: Molecular insights from magnetic resonance spectroscopy. Curr. Opin. Colloid Interface Sci. 2006, 11, 81–117. [Google Scholar] [CrossRef]
- Fan, F.T.; Feng, Z.C.; Li, C. UV Raman spectroscopic study on the synthesis mechanism and assembly of molecular sieves. Chem. Soc. Rev. 2010, 39, 4794–4801. [Google Scholar] [CrossRef] [PubMed]
- Mazaj, M.; Kaucic, V.; Logar, N.Z. Chemistry of Metal-organic Frameworks Monitored by Advanced X-ray Diffraction and Scattering Techniques. Acta Chim. Slov. 2016, 63, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Reinsch, H.; Stock, N. Synthesis of MOFs: A personal view on rationalisation, application and exploration. Dalton Trans. 2017, 46, 8339–8349. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.I.; Norquist, A.; Smith, R.I.; O’Hare, D. Recent results from the in situ study of hydrothermal crystallisations using time-resolved X-ray and neutron diffraction methods. Faraday Discuss. 2003, 122, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Embrechts, H.; Kriesten, M.; Hoffmann, K.; Peukert, W.; Hartmann, M.; Distaso, M. Elucidation of the Formation Mechanism of Metal-Organic Frameworks via in-Situ Raman and FTIR Spectroscopy under Solvothermal Conditions. J. Phys. Chem. C 2018, 122, 12267–12278. [Google Scholar] [CrossRef]
- Wragg, D.S.; Morris, R.E. Synthesis and structure determination from an extremely small single crystal of a new layered gallium phosphate. J. Phys. Chem. Solids 2001, 62, 1493–1497. [Google Scholar] [CrossRef]
- Belton, D.J.; Deschaume, O.; Perry, C.C. An overview of the fundamentals of the chemistry of silica with relevance to biosilicification and technological advances. FEBS J. 2012, 279, 1710–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, S.C.; Auerbach, S.M.; Monson, P.A. Modeling the Self-Assembly of Silica-Templated Nanoparticles in the Initial Stages of Zeolite Formation. Langmuir 2015, 31, 4940–4949. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Dan, M.; Behera, J.N. Chemical design of materials: A case study of inorganic open-framework materials. Pure Appl. Chem. 2005, 77, 1655–1674. [Google Scholar] [CrossRef]
- Zhao, Z.C.; Xu, S.C.; Hu, M.Y.; Bao, X.H.; Hu, J.Z. In Situ High Temperature High Pressure MAS NMR Study on the Crystallization of AlPO4-5. J. Phys. Chem. C 2016, 120, 1701–1708. [Google Scholar] [CrossRef]
- Loiseau, T.; Walton, R.I.; Francis, R.J.; O’Hare, D.; Ferey, G. Open-framework fluorinated gallium and aluminium phosphates: An in situ study of the hydrothermal synthesis by X-ray diffraction using synchrotron radiation. J. Fluor. Chem. 2000, 101, 181–186. [Google Scholar] [CrossRef]
- Miladinovic, Z.P.; Zakrzewska, J.; Kovacevic, B.T.; Miladinovic, J.M. In situ Al-27 NMR kinetic investigation of zeolite A crystallization. Microporous Mesoporous Mater. 2014, 195, 131–142. [Google Scholar] [CrossRef]
- Heidenreich, N.; Rutt, U.; Koppen, M.; Inge, A.K.; Beier, S.; Dippel, A.C.; Suren, R.; Stock, N. A multi-purpose reaction cell for the investigation of reactions under solvothermal conditions. Rev. Sci. Instrum. 2017, 88, 104102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheetham, A.K.; Kieslich, G.; Yeung, H.H.M. Thermodynamic and Kinetic Effects in the Crystallization of Metal-Organic Frameworks. Acc. Chem. Res. 2018, 51, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Choudhary, M.K.; Rimer, J.D. Transient modes of zeolite surface growth from 3D gel-like islands to 2D single layers. Nat. Commun. 2018, 9, 2129. [Google Scholar] [CrossRef] [PubMed]
- Van Vleet, M.J.; Weng, T.T.; Li, X.Y.; Schmidt, J.R. In Situ, Time-Resolved, and Mechanistic Studies of Metal-Organic Framework Nucleation and Growth. Chem. Rev. 2018, 118, 3681–3721. [Google Scholar] [CrossRef] [PubMed]
- Aerts, A.; Kirschhock, C.E.A.; Martens, J.A. Methods for in situ spectroscopic probing of the synthesis of a zeolite. Chem. Soc. Rev. 2010, 39, 4626–4642. [Google Scholar] [CrossRef] [PubMed]
- Depla, A.; Lesthaeghe, D.; van Erp, T.S.; Aerts, A.; Houthoofd, K.; Fan, F.T.; Li, C.; Van Speybroeck, V.; Waroquier, M.; Kirschhock, C.E.A.; et al. Si-29 NMR and UV-Raman Investigation of Initial Oligomerization Reaction Pathways in Acid-Catalyzed Silica Sol-Gel Chemistry. J. Phys. Chem. C 2011, 115, 3562–3571. [Google Scholar] [CrossRef]
- Souleiman, M.; Cambon, O.; Haidoux, A.; Haines, J.; Levelut, C.; Ranieri, V.; Hazemann, J.L. Study of Ga3+-Induced Hydrothermal Crystallization of an alpha-Quartz Type Ga1-xFexPO4 Single Crystal by in Situ X-ray Absorption Spectroscopy (XAS). Inorg. Chem. 2012, 51, 11811–11819. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.L.; Gladysiak, A.; Boyd, P.G.; Ireland, C.P.; Mieville, P.; Tiana, D.; Vlaisavljevich, B.; Schouwink, P.; van Beek, W.; Gagnon, K.J.; et al. Formation pathways of metal-organic frameworks proceeding through partial dissolution of the metastable phase. CrystEngComm 2017, 19, 3407–3413. [Google Scholar] [CrossRef]
- Xia, F.; O’Neill, B.; Ngothai, Y.; Peak, J.; Tenailleau, C.; Etschmann, B.; Qian, G.J.; Brugger, J.; Studer, A.; Olsen, S.; et al. A thermosyphon-driven hydrothermal flow-through cell for in situ and time-resolved neutron diffraction studies. J. Appl. Crystallogr. 2010, 43, 511–519. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, M.G.; Beale, A.M.; Weckhuysen, B.M. The role of synchrotron radiation in examining the self-assembly of crystalline nanoporous framework materials: From zeolites and aluminophosphates to metal organic hybrids. Chem. Soc. Rev. 2010, 39, 4767–4782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Liu, R.G.; Zhang, H.; Shang, Y.S.; Song, Y.; Liu, C.; Wang, T.; Gong, Y.J.; Li, Z.H. Structure evolution of aluminosilicate sol and its structure-directing effect on the synthesis of NaY zeolite. J. Appl. Crystallogr. 2017, 50, 231–239. [Google Scholar] [CrossRef]
- Wagia, R.; Strashnov, I.; Anderson, M.W.; Attfield, M.P. Determination of the Preassembled Nucleating Units That Are Critical for the Crystal Growth of the Metal-Organic Framework CdIF-4. Angew. Chem. Int. Ed. 2016, 55, 9075–9079. [Google Scholar] [CrossRef] [PubMed]
- Brabants, G.; Lieben, S.; Breynaert, E.; Reichel, E.K.; Taulelle, F.; Martens, J.A.; Jakoby, B.; Kirschhock, C.E.A. Monitoring early zeolite formation via in situ electrochemical impedance spectroscopy. Chem. Commun. 2016, 52, 5478–5481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.H.; Deng, F. Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2013; Volume 78. [Google Scholar]
- Brunner, E. Solid-state NMR—A powerful tool for the investigation of surface hydroxyl-groups in zeolites and their interaction with adsorbed probe molecules. J. Mol. Struct. 1995, 355, 61–85. [Google Scholar] [CrossRef]
- Koller, H.; Weiss, M. Solid State NMR; Chan, J.C.C., Ed.; Springer: Berlin, Germany, 2012. [Google Scholar]
- Zhang, L.; Ren, Y.H.; Yue, B.; He, H.Y. Recent development in in situ NMR study on heterogeneous catalysis: Mechanisms of light alkane functionalisation. Chem. Commun. 2012, 48, 2370–2384. [Google Scholar] [CrossRef] [PubMed]
- Gerardin, C.; In, M.; Allouche, L.; Haouas, M.; Taulelle, F. In situ pH probing of hydrothermal solutions by NMR. Chem. Mater. 1999, 11, 1285–1292. [Google Scholar] [CrossRef]
- Haouas, M.; Gerardin, C.; Taulelle, F.; Estournes, C.; Loiseau, T.; Ferey, G. In situ NMR study of hydrothermal synthesis of a template-mediated microporous aluminophosphate material: AlPO4-CJ2. J. Chim. Phys. Chim. Biol. 1998, 95, 302–309. [Google Scholar] [CrossRef]
- Taulelle, F.; Haouas, M.; Gerardin, C.; Estournes, C.; Loiseau, T.; Ferey, G. NMR of microporous compounds—From in situ reactions to solid paving. Colloid Surf. A Physicochem. Eng. Asp. 1999, 158, 299–311. [Google Scholar] [CrossRef]
- Hu, J.Z.; Hu, M.Y.; Zhao, Z.C.; Xu, S.C.; Vjunov, A.; Shi, H.; Camaioni, D.M.; Peden, C.H.F.; Lercher, J.A. Sealed rotors for in situ high temperature high pressure MAS NMR. Chem. Commun. 2015, 51, 13458–13461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.C.; Zhao, Z.C.; Hu, M.Y.; Han, X.W.; Hu, J.Z.; Bao, X.H. Investigation of water assisted phase transformation process from AlPO4-5 to AlPO4-tridymite. Microporous Mesoporous Mater. 2016, 223, 241–246. [Google Scholar] [CrossRef]
- In-gerardin, C.; In, M.; Taulelle, F. In-situ NMR measurements under hydrothermal conditions—Study of the formation of polymeric Al hydrolysis species. J. Chim. Phys. Chim. Biol. 1995, 92, 1877–1880. [Google Scholar] [CrossRef]
- Haouas, M.; Taulelle, F.; Martineau, C. Recent advances in application of Al-27 NMR spectroscopy to materials science. Prog. Nucl. Magn. Reson. Spectrosc. 2016, 94–95, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Gerardin, C.; Haouas, M.; Lorentz, F.; Taulelle, F. NMR quantification in hydrothermal in situ syntheses. Magn. Reson. Chem. 2000, 38, 429–435. [Google Scholar] [CrossRef]
- Shi, J.M.; Anderson, M.W.; Carr, S.W. Direct observation of zeolite a synthesis by in situ solid-state NMR. Chem. Mater. 1996, 8, 369–375. [Google Scholar] [CrossRef]
- Miladinovic, Z.; Zakrzewska, J.; Kovacevic, B.; Bacic, G. Monitoring of crystallization processes during synthesis of zeolite A by in situ (27)A1 NMR spectroscopy. Mater. Chem. Phys. 2007, 104, 384–389. [Google Scholar] [CrossRef]
- Ferey, G. Oxyfluorinated microporous compounds ULM-n—Chemical-parameters, structures and proposed mechanism for their molecular tectonics. J. Fluor. Chem. 1995, 72, 187–193. [Google Scholar] [CrossRef]
- Ferey, G. The new porous solids: Miracles in the holes. Actual Chim. 2007, 304, III–XV. [Google Scholar]
- Surble, S.; Millange, F.; Serre, C.; Ferey, G.; Walton, R.I. An EXAFS study of the formation of a nanoporous metal-organic framework: Evidence for the retention of secondary building units during synthesis. Chem. Commun. 2006, 1518–1520. [Google Scholar] [CrossRef] [PubMed]
- Taulelle, F.; Loiseau, T.; Maquet, J.; Livage, J.; Ferey, G. Oxyfluorinated microporous compounds. 2. Solid-state NMR of (NH4)0.88(H3O)0.12AlPO4(OH)0.33F0.67. J. Solid State Chem. 1993, 105, 191–196. [Google Scholar] [CrossRef]
- Taulelle, F.; Pruski, M.; Amoureux, J.P.; Lang, D.; Bailly, A.; Huguenard, C.; Haouas, M.; Gerardin, C.; Loiseau, T.; Ferey, G. Isomerization of the prenucleation building unit during crystallization of ALPO4-CJ2: An MQMAS, CP-MQMAS, and HETCOR NMR study. J. Am. Chem. Soc. 1999, 121, 12148–12153. [Google Scholar] [CrossRef]
- Goesten, M.G.; Magusin, P.; Pidko, E.A.; Mezari, B.; Hensen, E.J.M.; Kapteijn, F.; Gascon, J. Molecular Promoting of Aluminum Metal-Organic Framework Topology MIL-101 by N,N-Dimethylformamide. Inorg. Chem. 2014, 53, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Haouas, M.; Volkringer, C.; Loiseau, T.; Ferey, G.; Taulelle, F. In Situ NMR, Ex Situ XRD and SEM Study of the Hydrothermal Crystallization of Nanoporous Aluminum Trimesates MIL-96, MIL-100, and MIL-110. Chem. Mater. 2012, 24, 2462–2471. [Google Scholar] [CrossRef]
- Loiseau, T.; Lecroq, L.; Volkringer, C.; Marrot, J.; Ferey, G.; Haouas, M.; Taulelle, F.; Bourrelly, S.; Llewellyn, P.L.; Latroche, M. MIL-96, a porous aluminum trimesate 3D structure constructed from a hexagonal network of 18-membered rings and mu(3)-oxo-centered trinuclear units. J. Am. Chem. Soc. 2006, 128, 10223–10230. [Google Scholar] [CrossRef] [PubMed]
- Volkringer, C.; Popov, D.; Loiseau, T.; Ferey, G.; Burghammer, M.; Riekel, C.; Haouas, M.; Taulclle, F. Synthesis, Single-Crystal X-ray Microdiffraction, and NMR Characterizations of the Giant Pore Metal-Organic Framework Aluminum Trimesate MIL-100. Chem. Mater. 2009, 21, 5695–5697. [Google Scholar] [CrossRef]
- Volkringer, C.; Popov, D.; Loiseau, T.; Guillou, N.; Ferey, G.; Haouas, M.; Taulelle, F.; Mellot-Draznieks, C.; Burghammer, M.; Riekel, C. A microdiffraction set-up for nanoporous metal-organic-framework-type solids. Nat. Mater. 2007, 6, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Stavitski, E.; Goesten, M.; Juan-Alcaniz, J.; Martinez-Joaristi, A.; Serra-Crespo, P.; Petukhov, A.V.; Gascon, J.; Kapteijn, F. Kinetic Control of Metal-Organic Framework Crystallization Investigated by Time-Resolved In Situ X-Ray Scattering. Angew. Chem. Int. Ed. 2011, 50, 9624–9628. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haouas, M. Nuclear Magnetic Resonance Spectroscopy for In Situ Monitoring of Porous Materials Formation under Hydrothermal Conditions. Materials 2018, 11, 1416. https://doi.org/10.3390/ma11081416
Haouas M. Nuclear Magnetic Resonance Spectroscopy for In Situ Monitoring of Porous Materials Formation under Hydrothermal Conditions. Materials. 2018; 11(8):1416. https://doi.org/10.3390/ma11081416
Chicago/Turabian StyleHaouas, Mohamed. 2018. "Nuclear Magnetic Resonance Spectroscopy for In Situ Monitoring of Porous Materials Formation under Hydrothermal Conditions" Materials 11, no. 8: 1416. https://doi.org/10.3390/ma11081416