Alkali Release from Aggregates in Long-Service Concrete Structures: Laboratory Test Evaluation and ASR Prediction
Abstract
:1. Introduction
2. A Simple Model for Evaluating the Effect of Alkali Release from Aggregates on Deleterious ASR Expansion Development
3. Materials and Methods
3.1. Aggregates Tested
3.2. Leaching Test Procedure
4. Results and Discussion
4.1. Optimization of the Leaching Test for the Evaluation of Alkali Release from Aggregates
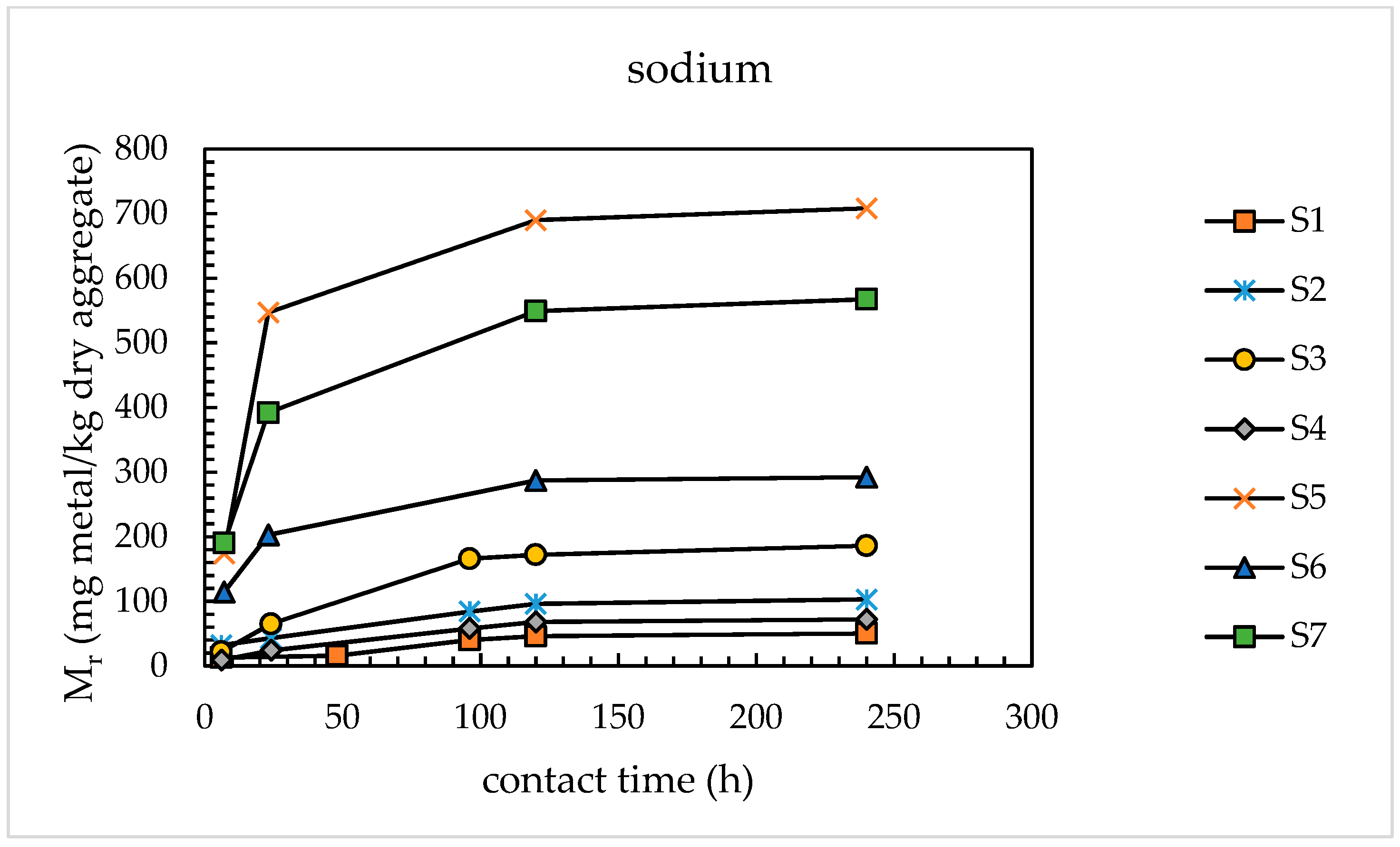
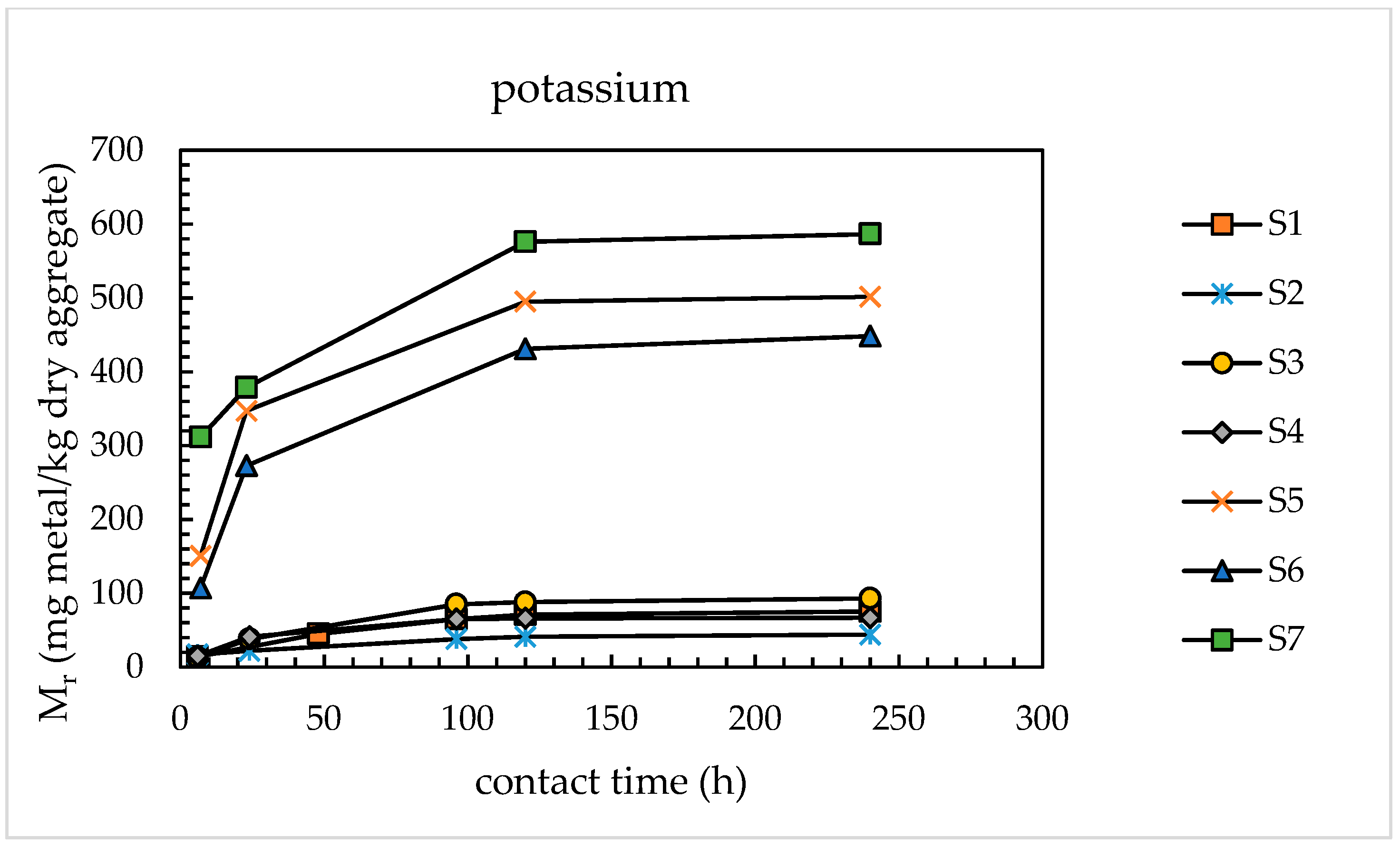
4.1.1. Alkali Releases from Sands
4.1.2. Alkali Releases from Coarse Aggregates
4.2. Application of the Proposed Model for Evaluating the Effect of Alkali Release from Aggregates on Deleterious ASR Development in Long-Service Structures
5. Conclusions
- (1)
- The modified alkali extraction test with saturated calcium hydroxide solution is suitable for maximizing the alkali extraction from concrete aggregates. With respect to the Italian Standard test method UNI 11417-2, the modifications consist of replacing the glass boiler with reflux by a laboratory autoclave operated at 105 °C, reducing the amount of aggregate tested from 400 to 200 g, and prolonging the test duration from 6 to 120 h. No change of the liquid/aggregate ratio (L/S = 0.6 g leachant/g dry aggregate) and the solid lime/aggregate ratio (CH/S = 5 g CH/100 g dry aggregate) was found to be necessary.
- (2)
- With the use of the modified extraction test, the amounts of alkaline metals released from the sands investigated were found to vary from 46 to 690 mg/kg dry aggregate for sodium and from 71 to 576 mg/kg dry aggregate for potassium, corresponding to percentage releases of 0.93–2.84% for Na and 0.41–3.35% for K. These results are congruent with those available in the literature for concrete aggregates subjected to leaching tests using sodium hydroxide and potassium hydroxide as leaching media.
- (3)
- For coarse aggregates, much lower releases of alkaline metals were found (6–11 mg/kg dry aggregate for both sodium and potassium), due to their lower specific surface area, compared to the respective sands. However, these very low releases may not be neglected in calculating the alkali release from combined aggregates (sand + coarse aggregate), in consideration of the very high content of coarse aggregates in concrete mixes. As demonstrated in this study, if the alkali release from sand is only known, it is possible to estimate the alkali release from coarse aggregate in terms of an equivalent sand content of 23 wt %.
- (4)
- A simple model is proposed to predict the potential effect of alkali release from aggregates on deleterious ASR expansion development in long-service concrete structures. This model is based on the knowledge of four key parameters relevant to the components of the concrete mix, such as the initial alkali content of the mix used for the structure construction (), the efficacy parameter () related to cement, the Threshold Alkali Level () of the aggregate, and the long-term alkali contribution by this aggregate to concrete mix (), the last being estimated from the results of laboratory optimized extraction tests (maximum alkali release).
- (5)
- Application of the above model to a typical dam concrete mix leads to ASR expansion predictions that are congruent with both the field experience and the ASR prevention criteria recommended by European Technical Report CEN/TR 16349:2012 and by RILEM Specifications, thus indicating the suitability of the proposed model.
Author Contributions
Funding
Conflicts of Interest
References
- Thomas, M.D.A.; Fournier, B.; Folliard, K.J. Alkali-Aggregate Reactivity (AAR) Facts Book; FHWA-HIF-13-019; Federal Highway Administration, U.S. Department of Transportation: Washington, DC, USA, 2013; p. 221. Available online: https://www.fhwa.dot.gov/pavement/concrete/asr/pubs/hif13019.pdf (accessed on 26 June 2018).
- Sims, I.; Poole, A.B. Alkali-Aggregate Reaction in Concrete: A World Review; CRC Press: London, UK, 2017. [Google Scholar]
- Powers, T.C.; Steinour, H.H. An interpretation of some published research on the alkali-aggregate reaction. II. A hypothesis concerning safe and unsafe reactions with reactive silica in concrete. Am. Concr. Inst. J. Proc. 1955, 26, 497–516. [Google Scholar]
- Chatterji, S.; Jensen, A.; Thaulow, N.; Christensen, P. Studies of alkali-silica reaction: Part 3. Mechanisms by which NaCl and Ca(OH)2 affect the reaction. Cem. Concr. Res. 1986, 16, 245–254. [Google Scholar] [CrossRef]
- Wang, H.; Gillott, J.E. Mechanism of alkali-silica reaction and the significance of calcium hydroxide. Cem. Concr. Res. 1991, 21, 647–654. [Google Scholar] [CrossRef]
- Dent-Glasser, R.S. Osmotic pressure and the swelling gels. Cem. Concr. Res. 1979, 9, 515–517. [Google Scholar] [CrossRef]
- Struble, L.; Diamond, S. Unstable swelling behavior of alkali-silica gels. Cem. Concr. Res. 1981, 11, 611–617. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ogawa, S.; Tanaka, Y.; Maekawa, K. Scale-dependent ASR expansion of concrete and its prediction coupled with silica gel generation and migration. J. Adv. Concr. Technol. 2016, 14, 444–463. [Google Scholar] [CrossRef]
- Bérubé, M.A.; Duchesne, J.; Rivest, M. Alkali contribution by aggregates to concrete. In Proceedings of the 10th International Conference on AAR, Melbourne, Australia, 18–23 August 1996; pp. 899–906. [Google Scholar]
- Bérubé, M.A.; Duchense, J.; Dorion, J.F.; Rivest, J. Laboratory assessment of alkali contribution by aggregates to concrete and application to concrete structures affected by alkali-silica reactivity. Cem. Concr. Res. 2002, 32, 1215–1227. [Google Scholar] [CrossRef]
- Constantine, D.; Diamond, S. Alkali release from feldspars in pore solutions. Cem. Concr. Res. 2003, 33, 549–554. [Google Scholar] [CrossRef]
- Drolet, C.; Duchesne, J.; Fournier, B. Validation of the alkali contribution by aggregates to the concrete pore solution. Cem. Concr. Res. 2017, 98, 10–23. [Google Scholar] [CrossRef]
- Bérubé, M.A.; Fournier, B. Alkali releasable by aggregates in concrete—Significance and test methods. In Proceedings of the 12th International Conference on Alkali Aggregate Reaction (I), Beijing, China, 15–19 October 2004; International Academic Publishers: Beijing, China; World Publishing Corporation: Cleveland, OH, USA, 2004; Volume 1, pp. 17–30. [Google Scholar]
- U.S. Department of the Interior. Bureau of Reclamation, Aggregate and SCM Alkali Release Dam Safety Technology Development Program; Report DSO-2016-03 (8530-2016-23); Technical Service Center: Denver, CO, USA, September 2016; p. 21. Available online: https://www.usbr.gov/ssle/damsafety/TechDev/DSOTechDev/DSO-2016-03.pdf (accessed on 11 July 2018).
- Charlwood, R.; Scrivener, K. Expanding Concrete in Dams: Long-Term Challenges. In Proceedings of the International Symposium on Dams and Reservoirs under Changing Challenges, 79th ICOLD Annual Meeting, Lucerne, Switzerland, 1 June 2011; pp. 179–186. [Google Scholar]
- Charlwood, R.; Scrivener, K.; Sims, I. Recent developments in the management of chemical expansion of concrete in dams and hydro projects—Part 1: Existing structures. In Proceedings of the Hydro 2012: Innovative Approaches to Global Challenges, Bilbao, Spain, 29–31 October 2012. [Google Scholar]
- Berra, M.; Bertacchi, P. The alkali-aggregate reaction in concrete dams. In International Water Power and Dam Construction; Reed Business Publishing Ltd.: Sutton (Surrey), UK, 1991; Volume 43, pp. 12–16. [Google Scholar]
- Paolina, R.; Appendino, M.; Baldovin, E.; Berra, M.; Bianchini, A.; Carabelli, E.; Posta, U.; Vielmo, I. Deterioration problems for concrete and masonry dams in Italy. In Proceedings of the 17th ICOLD Congress, Vienna, Austria, 17–21 June 1991; pp. 785–815. [Google Scholar]
- Charlwood, R.; Solymar, Z.; Zoltan, V. An International Perspective: AAR in Hydroelectric Projects and Dams. In Proceedings of the International Conference on Alkali Aggregate Reactions in Hydroelectric Projects and Dams, USCOLD, Chattanooga, TN, USA, 22–27 October 1995; pp. 19–55. [Google Scholar]
- Amberg, F. A review of expanding concrete cases and consequences on dam performance. In Proceedings of the Hydro 2012: Innovative Approaches to Global Challenges, Bilbao, Spain, 29–31 October 2012. [Google Scholar]
- Charlwood, R.; Sims, I. A Review of the Effectiveness of Strategies to Manage Expansive Chemical Reactions in Dams and Hydro Projects. In Proceedings of the Dam Swelling Concrete DSC 2017, Chambéry, France, 13–15 June 2017; Sellier, A., Grimal, É., Multon, S., Bourdarot, E., Eds.; Wiley: London, UK, 2017; pp. 3–39. [Google Scholar]
- Kawabata, Y.; Yamada, K.; Igarashi, G.; Sagawa, Y. Effects of soak solution type on alkali release from volcanic aggregates—Is alkali release really responsible for accelerating ASR expansion? J. Adv. Concr. Techol. 2018, 16, 61–74. [Google Scholar] [CrossRef]
- West, G. Alkali-Aggregate Reaction in Concrete Roads and Bridges; Thomas Telford Publications: London, UK, 1996. [Google Scholar]
- Katayama, T.; Tagami, M.; Sarai, Y.; Izumi, S.; Hira, T. Alkali-aggregate reaction under the influence of deicing salts in the Hokuriku district. Jpn. Mater. Charact. 2004, 53, 105–122. [Google Scholar] [CrossRef]
- Giebson, C.; Seyfarth, K.; Ludwig, H.-M. Correlation of ASR performance testing for highway pavement concretes with field performance and investigations into boosting the alkali level. In Proceedings of the 14th International Conference on Alkali Aggregate Reaction, Austin, TX, USA, 20–25 May 2012. [Google Scholar]
- Heisig, A.; Urbonas, L.; Beddoe, R.E.; Heinz, D. Ingress of NaCl in concrete with alkali reactive aggregate: Effect on silicon solubility. Mater. Struct. 2015, 49, 4291–4303. [Google Scholar] [CrossRef]
- UNI (Ente Nazionale Italiano di Normazione). UNI 11417—Part 2, Durabilità Delle Opere di Calcestruzzo e Degli Elementi Prefabbricati di Calcestruzzo—Istruzioni per Prevenire la Reazione Alcali-Silice; UNI: Milano, Italy, 2014. (In Italian) [Google Scholar]
- LCPC (Laboratoire Central des Ponts et Chaussées). Essai de Granulats—Détermination des Alcalins Solubles dans L’eau de Chaux; Méthode D’essai LPC n. 37; LCPC: Paris, France, 1993. (In French) [Google Scholar]
- Wang, Y.; Deng, M.; Tang, M. Alkali release from aggregate and the effect on AAR expansion. Mater. Struct. 2008, 41, 159–171. [Google Scholar]
- Soares, D.; Santos Silva, A.; Mirão, J.; Ramos, V.; Fernandes, I.; Menéndez, E. Assessment of alkalis released by aggregates. Contribution to the alkalinity increase and AAR development in concrete. In Proceedings of the Second International World Dam Conference, Lisbon, Portugal, 21–24 April 2015; p. 10. [Google Scholar]
- Menendez, E.; Garcia-Rovés, R. Alkali release from aggregates: Contribution to ASR. Proc. ICE Construct. Mater. 2017, 169, 206–214. [Google Scholar] [CrossRef]
- RILEM (International Union of Laboratories and Experts in Construction Materials, Systems and Structures). Recommended Test Method AAR-8, Determination of Alkalis Releasable by Aggregates in Concrete. In Preparation by RILEM TC 258-AAA, Draft. February 2018; in preparation. [Google Scholar]
- Berra, M.; Mangialardi, T.; Paolini, A.E. Rapid evaluation of the threshold alkali level for alkali-reactive siliceous aggregates in concrete. Cem. Concr. Compos. 1999, 21, 325–333. [Google Scholar] [CrossRef]
- Berra, M.; Costa, U.; Mangialardi, T.; Paolini, A.E.; Turriziani, R. A new approach to assessing the performance of ASR inhibitors in concrete. Mater. Struct. 2013, 46, 971–985. [Google Scholar] [CrossRef]
- Costa, U.; Mangialardi, T.; Paolini, A.E. Assessment of blended cements effectiveness against ASR by mortar and concrete expansion tests. J. Adv. Concr. Technol. 2014, 12, 266–278. [Google Scholar] [CrossRef]
- Berra, M.; Costa, U.; Mangialardi, T.; Paolini, A.E. Application of an innovative methodology to assessing the alkali-silica reaction in concrete. Mater. Struct. 2015, 48, 2727–2740. [Google Scholar] [CrossRef]
- Thomas, M.D. The effect of supplementary cementing materials on alkali-silica reaction. A review. Cem. Concr. Res. 2011, 41, 1224–1231. [Google Scholar] [CrossRef]
- Kawabata, Y.; Yamada, K. Evaluation of alkalinity of pore solution based on the phase composition of cement hydrates with supplementary cementitious materials and its relation to suppressing ASR expansion. J. Adv. Concr. Technol. 2015, 13, 538–553. [Google Scholar] [CrossRef]
- Costa, U.; Mangialardi, T.; Paolini, A.E. Briefing: Low-alkali cements as inhibitors of alkali–silica reaction. Proc. ICE Construct. Mater. 2017, 170, 73–76. [Google Scholar] [CrossRef]
- EN (European Standard). EN 197-1, Cement-Part 1: Composition, Specifications and Conformity Criteria for Common Cements; European Committee for Standardization: Brussels, Belgium, 2011. [Google Scholar]
- RILEM (International Union of Laboratories and Experts in Construction Materials, Systems and Structures). Recommended Test Method AAR-1.1 Detection of Potential Alkali-Reactivity—Part 1: Petrographic Examination Method. In RILEM Recommendations for the Prevention of Damage by Alkali-Aggregate Reactions in New Concrete Structures; Rilem State-of-the-Art Report; Springer: Berlin, Germany, 2016. [Google Scholar]
- APHA; AWWA; WEF (American Public Health Association, American Water Works Association, Water Environment Federation). Standard Methods for Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012; 1360p. [Google Scholar]
- Kermit, P.H. Determining Alkali Content in ASR Performance-Tested Concrete. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2017; 72p. Available online: https://brage.bibsys.no/xmlui/bitstream/handle/11250/2452852/16866_FULLTEXT.pdf (accessed on 13 July 2018).
- Berra, M.; Mangialardi, T.; Paolini, A.E. Alkali-silica reactivity criteria for concrete aggregates. Mater. Struct. 2005, 38, 373–380. [Google Scholar] [CrossRef]
- CEN (European Committee for Standardization). CEN/TR 16349:2012, Framework for a Specification on the Avoidance of a Damaging Alkali-Silica Reaction (ASR) in Concrete; Technical Report; CEN: Brussels, Belgium, 2012; 20p. [Google Scholar]
- RILEM (International Union of Laboratories and Experts in Construction Materials, Systems and Structures). Recommended Specification AAR-7.1 International Specification to Minimise Damage from Alkali Reactions in Concrete—Part 1: Alkali-Silica Reaction. In RILEM Recommendations for the Prevention of Damage by Alkali-Aggregate Reactions in New Concrete Structures; Rilem State-of-the-Art Report; Springer: Berlin, Germany, 2016. [Google Scholar]
- Fournier, B.; Nkinamubanzi, P.-C.; Chevrier, R. Comparative field and laboratory investigation on the use of supplementary cementing materials to control alkali-silica reaction in concrete. In Proceedings of the 12th International Conference on Alkali-Aggregate Reaction, Beijing, China, 15–19 October 2004; International Academic Publisher: Beijing, China; World Publishing Corporation: Cleveland, OH, USA, 2004; Volume 1, pp. 528–537. [Google Scholar]




| Sand | Lithological Composition |
|---|---|
| S1 | medium to fine grained sedimentary carbonate rocks including mono- or polycrystalline quartz, rarely showing undulatory extinction angle, flint and chalcedony |
| S2 | sedimentary carbonatic rocks and sandstones with flint as the main alkali reactive phase |
| S3 | arenaceous, quartzitic-feldspatic and epidote rocks, with fine flints (sometimes with a fibrous-radiate texture typical of chalcedony), mono- and polycrystalline quartz and fine-grained quartzites with a marked undulatory extinction angle |
| S4 | similar to sand S3, except for a smaller amount of flint and a remarkable presence of carbonate rocks |
| S5 | cataclasite, a metamorphic rock formed by mechanical fracturing on fault lines. Main constituents of this sand were feldspar particles within a strongly stressed quartz matrix, fractured feldspars, dark minerals and mica |
| S6 | granite, an intrusive igneous rock mainly containing strained and micro-crystalline quartz, K-feldspar, plagioclase and biotite |
| S7 | granodiorite, an intrusive igneous rock similar to sand S6, with a higher content of plagioclase and a lower content of K-feldspar than S6, containing strained and poorly crystalline quartz, biotite and hornblende |
| Aggregate | Na (g/kg) | K (g/kg) | Na2O (g/kg) | K2O (g/kg) | Na2Oeq (g/kg) * |
|---|---|---|---|---|---|
| S1 | 4.97 | 4.98 | 6.70 | 6.00 | 10.70 |
| S2 | 13.50 | 10.04 | 18.20 | 12.10 | 26.20 |
| S3 | 5.94 | 5.97 | 8.00 | 7.20 | 12.70 |
| S4 | 7.27 | 9.63 | 9.80 | 11.60 | 17.50 |
| S5 | 24.26 | 14.77 | 32.70 | 17.80 | 44.40 |
| S6 | 22.18 | 43.07 | 29.90 | 51.90 | 64.10 |
| S7 | 21.59 | 35.93 | 29.10 | 43.30 | 57.60 |
| C1 | 13.00 | 10.00 | 17.52 | 12.05 | 25.47 |
| C2 | 5.00 | 5.10 | 6.74 | 6.15 | 10.79 |
| Grain Size | Weight Percent |
|---|---|
| 4–2 mm | 10 |
| 2–1 mm | 20 |
| 1 mm–500 m | 20 |
| 500–250 m | 25 |
| 250–125 m | 15 |
| <125 m | 10 |
| Grain Size (mm) | Weight Percent | |
|---|---|---|
| C1 | C2 | |
| 12–16 | 18 | 22 |
| 16–20 | 27 | 34 |
| 20–24 | 35 | 28 |
| 24–32 | 20 | 16 |
| Sand | Percentage Release (wt %) | ||||||
|---|---|---|---|---|---|---|---|
| Na | K | Na | K | Na2O | K2O | Na2Oeq | |
| S1 | 46 | 71 | 0.93 | 1.43 | 62 | 86 | 118 |
| S2 | 96 | 41 | 0.71 | 0.41 | 129 | 49 | 162 |
| S3 | 172 | 88 | 2.90 | 1.47 | 232 | 106 | 302 |
| S4 | 68 | 66 | 0.94 | 0.69 | 92 | 80 | 144 |
| S5 | 690 | 495 | 2.84 | 3.35 | 930 | 597 | 1323 |
| S6 | 287 | 431 | 1.29 | 1.00 | 387 | 519 | 729 |
| S7 | 549 | 576 | 2.54 | 1.60 | 740 | 694 | 1198 |
| Coarse Aggregate | Metal Release (wt %) | ||||||
|---|---|---|---|---|---|---|---|
| Na | K | Na | K | Na2O | K2O | Na2Oeq | |
| C1 | 6 | 11 | 0.05 | 0.11 | 8 | 13 | 17 |
| C2 | 11 | 8 | 0.22 | 0.16 | 15 | 10 | 21 |
| Combined Aggregate | Release (mg Na/kg Combined Aggregate) | Release (mg K/kg Combined Aggregate) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S1-C1 | 0.35 | 20 | 19.8 | 0.43 | 24.2 | 32 | 30.6 | 0.43 | 28.8 |
| S2-C2 | 0.35 | 41 | 41.3 | 0.42 | 21.3 | 20 | 17.7 | 0.48 | 36.2 |
| S1-C1 | 0.45 | 24 | 25.4 | 0.52 | 15.9 | 38 | 39.3 | 0.54 | 18.9 |
| S2-C2 | 0.45 | 49 | 53.1 | 0.51 | 14.0 | 23 | 22.7 | 0.56 | 23.8 |
| Aggregate | (kg Na2Oeq/m3) | (kg Na2Oeq/m3) | (kg Na2Oeq/m3) |
|---|---|---|---|
| 1 | 3.9 | 0.10 | 2.10 |
| 2 | 8.2 | 0.14 | 2.14 |
| 3 | 7.2 | 0.26 | 2.26 |
| 4 | 6.0 | 0.12 | 2.12 |
| 5 | 2.8 | 1.12 | 3.12 |
| 6 | n.a. | 0.62 | - |
| 7 | n.a. | 1.01 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berra, M.; Mangialardi, T.; Paolini, A.E. Alkali Release from Aggregates in Long-Service Concrete Structures: Laboratory Test Evaluation and ASR Prediction. Materials 2018, 11, 1393. https://doi.org/10.3390/ma11081393
Berra M, Mangialardi T, Paolini AE. Alkali Release from Aggregates in Long-Service Concrete Structures: Laboratory Test Evaluation and ASR Prediction. Materials. 2018; 11(8):1393. https://doi.org/10.3390/ma11081393
Chicago/Turabian StyleBerra, Mario, Teresa Mangialardi, and Antonio Evangelista Paolini. 2018. "Alkali Release from Aggregates in Long-Service Concrete Structures: Laboratory Test Evaluation and ASR Prediction" Materials 11, no. 8: 1393. https://doi.org/10.3390/ma11081393





