Radial Compressive Property and the Proof-of-Concept Study for Realizing Self-expansion of 3D Printing Polylactic Acid Vascular Stents with Negative Poisson’s Ratio Structure
Abstract
:1. Introduction
2. Experiment and Methods
2.1. Establishment of Vascular Stent Model with NPR Structure
- L represents the length of the support strut;
- m represents the length of the re-entrant strut;
- h represents the height of the unit;
- t represents the width of the strut;
- d represents the width of the link;
- φ represents the angle between the support strut and the axial direction;
- θ represents the angle between the re-entrant strut and the axial direction;
- D represents the outer diameter of the vascular stent;
- H represents the total length of vascular stent;
- T represents the thickness of the stent.
2.2. Experimental Facility and Materials
2.3. 3D Printing of PLA Vascular Stents
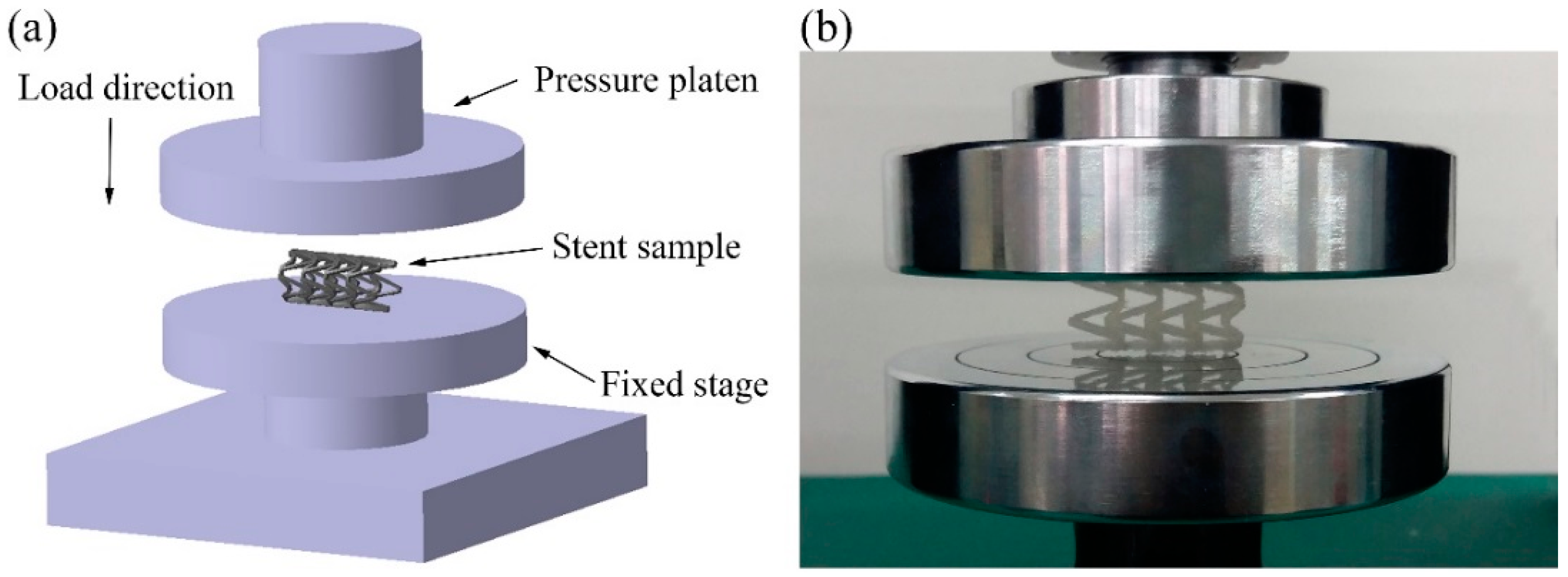
2.4. Compression Experimental Process of PLA Vascular Stents
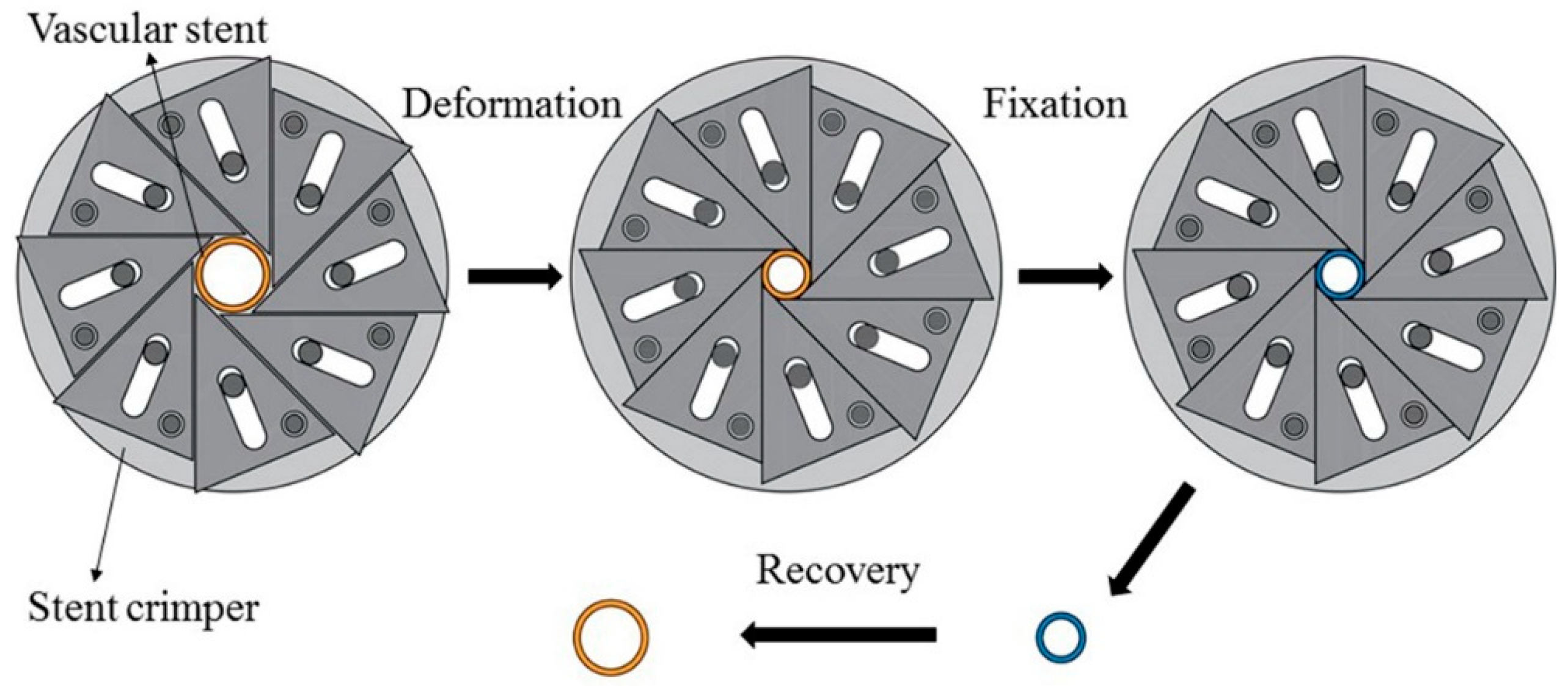
2.5. SME Experimental Process of PLA Vascular Stents
3. Results and Discussion
3.1. Compression Experimental Results
3.2. SME Experimental Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef] [PubMed]
- Welt, F.G.; Rogers, C. Inflammation and restenosis in the stent era. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.A. Restenosis following implantation of bare metal coronary stents: Pathophysiology and pathways involved in the vascular response to injury. Adv. Drug Deliv. Rev. 2006, 58, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Nakamura, M.; Palmaz, J.C.; Schwartz, R.S. Role of stent design and coatings on restenosis and thrombosis. Adv. Drug Deliv. Rev. 2006, 58, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.-X.; Yang, D.-Z.; Wu, J.-Z. Nanoparticle drug-and gene-eluting stents for the prevention and treatment of coronary restenosis. Theranostics 2014, 4, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Gibula, P.; Goosen, M.F. Role of polymers in improving the results of stenting in coronary arteries. Biomaterials 1996, 17, 685–694. [Google Scholar] [CrossRef]
- Griffiths, H.; Peeters, P.; Verbist, J.; Bosiers, M.; Deloose, K.; Heublein, B.; Rohde, R.; Kasese, V.; Ilsley, C.; Di Mario, C. Future devices: Bioabsorbable stents. Br. J. Cardiol. (Acute Interv. Cardiol.) 2004, 11, AIC 80–AIC 84. [Google Scholar]
- Mani, G.; Feldman, M.D.; Patel, D.; Agrawal, C.M. Coronary stents: A materials perspective. Biomaterials 2007, 28, 1689–1710. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly (lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Sobota, M.; Jurczyk, S.; Kwiecień, M.; Smola-Dmochowska, A.; Musioł, M.; Domański, M.; Janeczek, H.; Kawalec, M.; Kurcok, P. Crystallinity as a tunable switch of poly(l-lactide) shape memory effects. J. Mech. Behav. Biomed. Mater. 2016, 66, 144. [Google Scholar] [CrossRef] [PubMed]
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape memory polymers: Past, present and future developments. Prog. Polym. Sci. 2015, 49, 3–33. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2011, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Arora, M.; Kumar, M.R. Poly (lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Vink, E.T.; Rabago, K.R.; Glassner, D.A.; Gruber, P.R. Applications of life cycle assessment to natureworks™ polylactide (PLA) production. Polym. Degrad. Stab. 2003, 80, 403–419. [Google Scholar] [CrossRef]
- Stack, R.; Califf, R.; Phillips, H.; Pryor, D.; Quigley, P.; Bauman, R.; Tcheng, J.; Greenfield, J. Interventional cardiac catheterization at duke medical center-the duke interventional cardiac catheterization program. Am. J. Cardiol. 1988, 62, 3F–24F. [Google Scholar] [PubMed]
- Agrawal, C.; Haas, K.; Leopold, D.; Clark, H. Evaluation of poly (l-lactic acid) as a material for intravascular polymeric stents. Biomaterials 1992, 13, 176–182. [Google Scholar] [CrossRef]
- Tamai, H.; Igaki, K.; Kyo, E.; Kosuga, K.; Kawashima, A.; Matsui, S.; Komori, H.; Tsuji, T.; Motohara, S.; Uehata, H. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation 2000, 102, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Poh, T.L.; Vinalia, T.; Mak, K.H.; Boey, F. Collapse pressures of biodegradable stents. Biomaterials 2003, 24, 2105–2111. [Google Scholar] [CrossRef]
- Wu, W.; Geng, P.; Li, G.; Zhao, D.; Zhang, H.; Zhao, J. Influence of layer thickness and raster angle on the mechanical properties of 3d-printed peek and a comparative mechanical study between PEEK and ABS. Materials 2015, 8, 5834–5846. [Google Scholar] [CrossRef] [PubMed]
- Low, Z.X.; Chua, Y.T.; Ray, B.; Mattia, D.; Metcalfe, I.; Patterson, D.A. Perspective on 3D printing of separation membranes and comparison to related unconventional fabrication techniques. J. Membr. Sci. 2016, 523, 596–613. [Google Scholar] [CrossRef]
- Peltola, S.M.; Melchels, F.P.W.; Grijpma, D.W.; Kellomäki, M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, N.; Whitehead, D.; Boor, A.; Oppenlander, W.; Liu, Z.; Li, L. Picosecond laser micromachining of nitinol and platinum–iridium alloy for coronary stent applications. Appl. Phys. A 2012, 106, 607–617. [Google Scholar] [CrossRef]
- Flege, C.; Vogt, F.; Höges, S.; Jauer, L.; Borinski, M.; Schulte, V.A.; Hoffmann, R.; Poprawe, R.; Meiners, W.; Jobmann, M. Development and characterization of a coronary polylactic acid stent prototype generated by selective laser melting. J. Mater. Sci. Mater. Med. 2013, 24, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Kaesemeyer, W.H.; Sprankle, K.G.; Kremsky, J.N.; Lau, W.; Helmus, M.N.; Ghatnekar, G.S. Bioresorbable polystatin fourth-generation stents. Coron. Artery Dis. 2013, 24, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Su, A.P.; Sang, J.L.; Lim, K.S.; Bae, I.H.; Lee, J.H.; Wan, D.K.; Jeong, M.H.; Park, J.K. In vivo evaluation and characterization of a bio-absorbable drug-coated stent fabricated using a 3D-printing system. Mater. Lett. 2015, 141, 355–358. [Google Scholar]
- Van Lith, R.; Baker, E.; Ware, H.; Yang, J.; Farsheed, A.C.; Sun, C.; Ameer, G. 3D-Printing Strong High-Resolution Antioxidant Bioresorbable Vascular Stents. Adv. Mater. Technol. 2016, 1, 1600138. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, H. A review on auxetic structures and polymeric materials. Sci. Res. Essays 2010, 5, 1052–1063. [Google Scholar]
- Prawoto, Y. Seeing auxetic materials from the mechanics point of view: A structural review on the negative poisson’s ratio. Comput. Mater. Sci. 2012, 58, 140–153. [Google Scholar] [CrossRef]
- Mir, M.; Ali, M.; Ansari, U.; Sami, J. Review of mechanics and applications of auxetic structures. Adv. Mater. Sci. Eng. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Amin, F.; Ali, M.N.; Ansari, U.; Mir, M.; Minhas, M.A.; Shahid, W. Auxetic coronary stent endoprosthesis: Fabrication and structural analysis. J. Appl. Biomater. Funct. Mater. 2015, 13, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Takahata, K.; Gianchandani, Y.B. A planar approach for manufacturing cardiac stents: Design, fabrication, and mechanical evaluation. J. Micro. Syst. 2004, 13, 933–939. [Google Scholar] [CrossRef]
- He, Y.; Xue, G.H.; Fu, J.Z. Fabrication of low cost soft tissue prostheses with the desktop 3D printer. Sci. Rep. 2014, 4, 6973. [Google Scholar] [CrossRef] [PubMed] [Green Version]


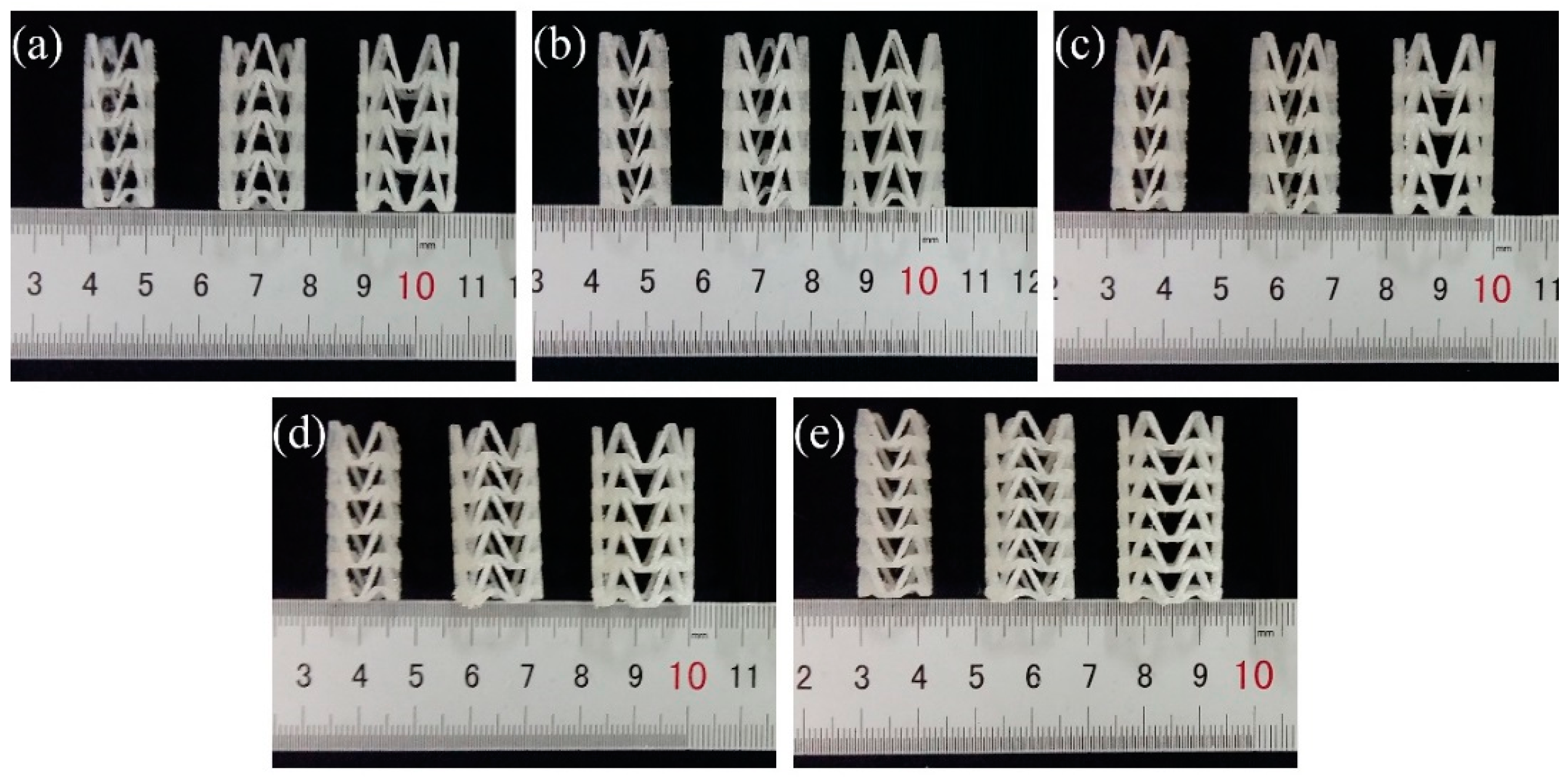

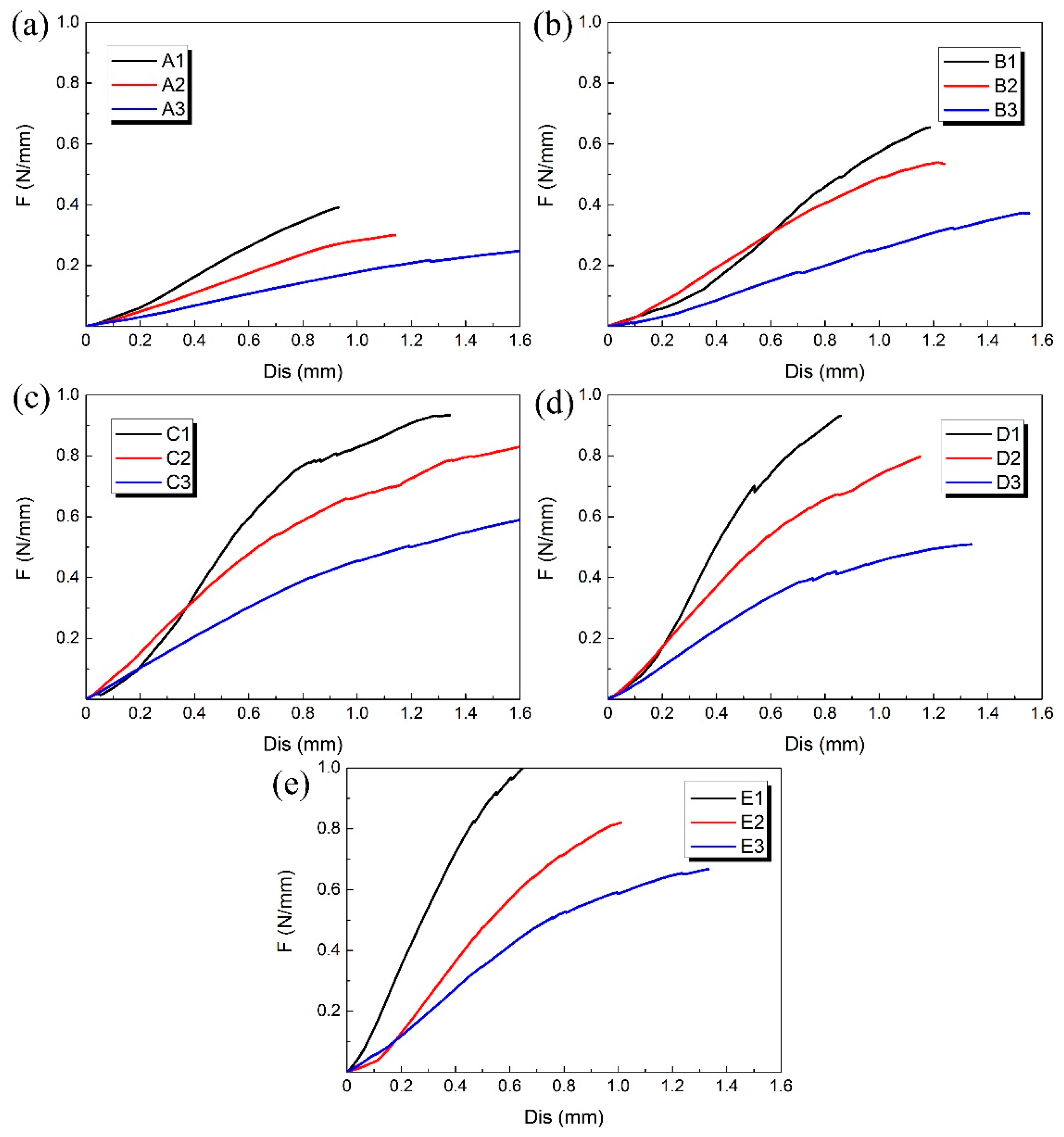
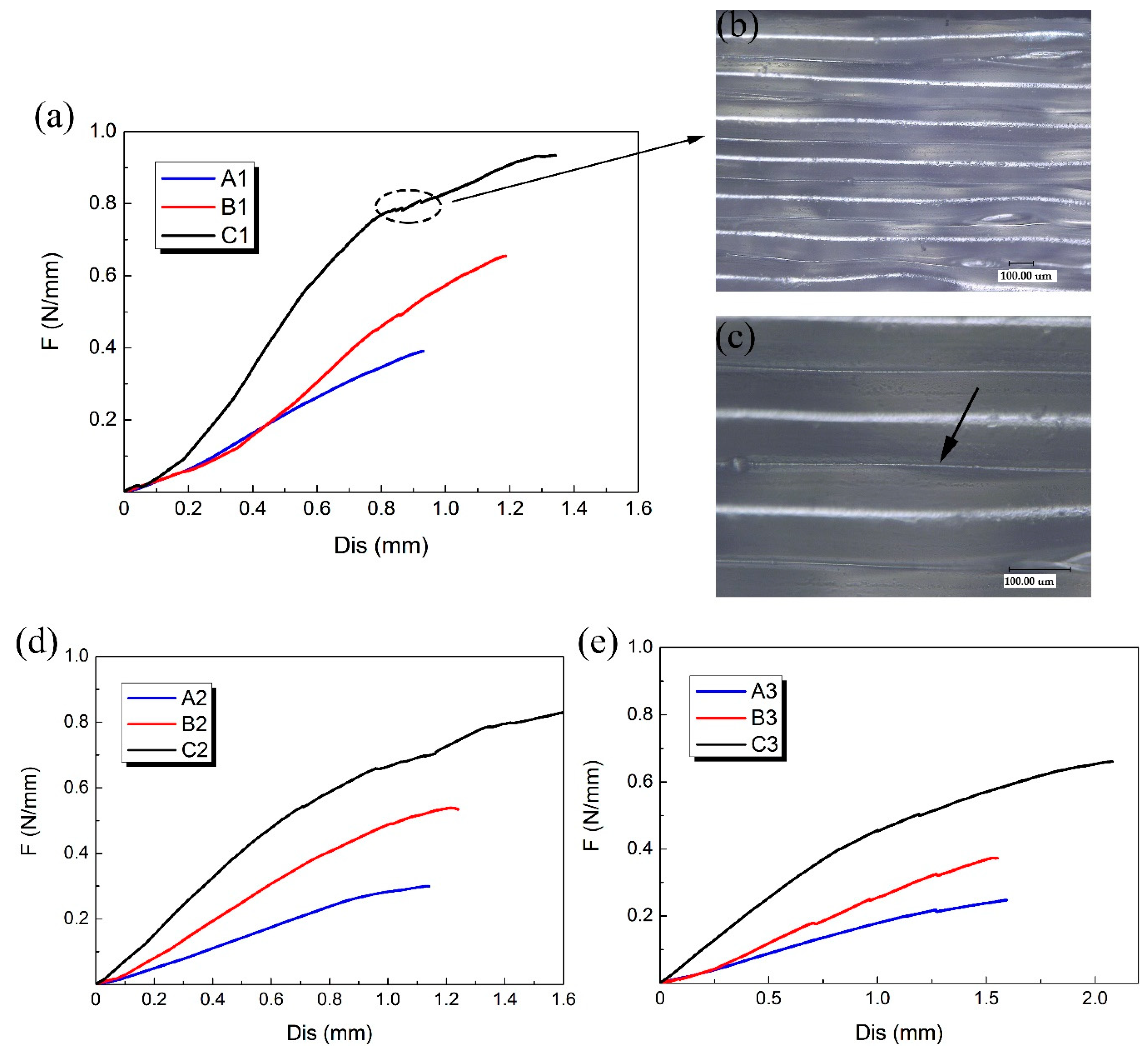
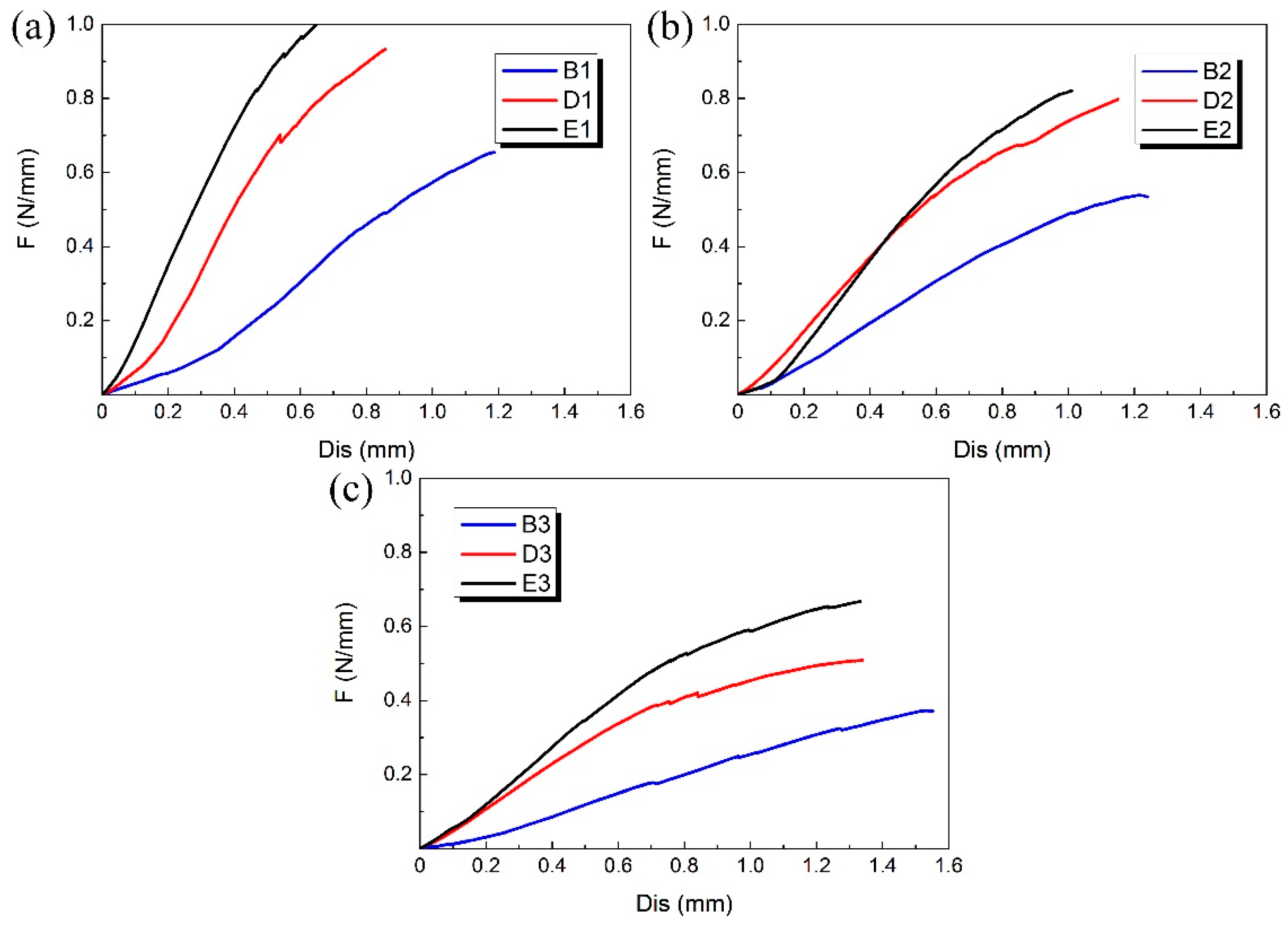
| Group | Number | D/mm | H/mm | T/mm | L/mm | m/mm | h/mm | t/mm | d/mm | φ/° | θ/° |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | A1 | 12 | 30.92 | 1.2 | 9.39 | 3.21 | 9.57 | 0.75 | 1.5 | 20 | 40 |
| A2 | 15 | 30.92 | 1.2 | 9.39 | 3.21 | 9.57 | 0.75 | 1.5 | 20 | 40 | |
| A3 | 18 | 30.92 | 1.2 | 9.39 | 3.21 | 9.57 | 0.75 | 1.5 | 20 | 40 | |
| B | B1 | 12 | 30.92 | 1.5 | 9.39 | 3.21 | 9.57 | 0.75 | 1.5 | 20 | 40 |
| B2 | 15 | 30.92 | 1.5 | 9.39 | 3.21 | 9.57 | 0.75 | 1.5 | 20 | 40 | |
| B3 | 18 | 30.92 | 1.5 | 9.39 | 3.21 | 9.57 | 0.75 | 1.5 | 20 | 40 | |
| C | C1 | 12 | 30.92 | 1.8 | 9.39 | 3.21 | 9.57 | 0.75 | 1.5 | 20 | 40 |
| C2 | 15 | 30.92 | 1.8 | 9.39 | 3.21 | 9.57 | 0.75 | 1.5 | 20 | 40 | |
| C3 | 18 | 30.92 | 1.8 | 9.39 | 3.21 | 9.57 | 0.75 | 1.5 | 20 | 40 | |
| D | D1 | 12 | 31.10 | 1.5 | 7.60 | 2.76 | 7.64 | 0.75 | 1.5 | 25 | 50 |
| D2 | 15 | 31.10 | 1.5 | 7.60 | 2.76 | 7.64 | 0.75 | 1.5 | 25 | 50 | |
| D3 | 18 | 31.10 | 1.5 | 7.60 | 2.76 | 7.64 | 0.75 | 1.5 | 25 | 50 | |
| E | E1 | 12 | 31.67 | 1.5 | 6.42 | 2.47 | 6.31 | 0.75 | 1.5 | 30 | 60 |
| E2 | 15 | 31.67 | 1.5 | 6.42 | 2.47 | 6.31 | 0.75 | 1.5 | 30 | 60 | |
| E3 | 18 | 31.67 | 1.5 | 6.42 | 2.47 | 6.31 | 0.75 | 1.5 | 30 | 60 |
| Group | Number | DO/mm | HO/mm | DC/mm | HC/mm | Dr/mm | Hr/mm | RD | RH |
|---|---|---|---|---|---|---|---|---|---|
| A | A1 | 12.20 | 31.04 | 8.60 | 30.11 | 11.75 | 30.42 | 0.9631 | 0.9800 |
| A2 | 15.13 | 31.03 | 10.38 | 29.76 | 14.76 | 30.39 | 0.9755 | 0.9794 | |
| A3 | 17.92 | 31.12 | 12.39 | 29.67 | 17.41 | 30.25 | 0.9715 | 0.9720 | |
| B | B1 | 12.10 | 30.99 | 9.22 | 30.03 | 11.74 | 30.39 | 0.9702 | 0.9806 |
| B2 | 15.16 | 30.94 | 10.89 | 29.59 | 14.54 | 30.29 | 0.9591 | 0.9790 | |
| B3 | 18.10 | 31.08 | 13.31 | 29.83 | 17.41 | 30.27 | 0.9619 | 0.9739 | |
| C | C1 | 12.22 | 30.92 | 9.91 | 29.97 | 11.64 | 30.53 | 0.9525 | 0.9874 |
| C2 | 15.19 | 31.04 | 10.84 | 29.55 | 14.62 | 30.46 | 0.9625 | 0.9813 | |
| C3 | 18.12 | 30.96 | 12.21 | 28.83 | 17.84 | 30.39 | 0.9845 | 0.9816 | |
| D | D1 | 12.09 | 31.26 | 8.62 | 29.19 | 11.73 | 30.40 | 0.9702 | 0.9725 |
| D2 | 15.14 | 31.24 | 11.02 | 28.98 | 14.63 | 30.54 | 0.9663 | 0.9776 | |
| D3 | 18.14 | 31.34 | 11.31 | 28.12 | 17.68 | 30.51 | 0.9746 | 0.9735 | |
| E | E1 | 12.13 | 31.82 | 9.21 | 30.66 | 11.71 | 31.07 | 0.9654 | 0.9764 |
| E2 | 15.26 | 31.71 | 10.79 | 29.50 | 14.57 | 30.80 | 0.9548 | 0.9713 | |
| E3 | 18.14 | 31.76 | 13.03 | 29.42 | 17.72 | 30.83 | 0.9768 | 0.9707 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Zhao, J.; Wu, W.; Wang, P.; Wang, B.; Li, G.; Zhang, S. Radial Compressive Property and the Proof-of-Concept Study for Realizing Self-expansion of 3D Printing Polylactic Acid Vascular Stents with Negative Poisson’s Ratio Structure. Materials 2018, 11, 1357. https://doi.org/10.3390/ma11081357
Wu Z, Zhao J, Wu W, Wang P, Wang B, Li G, Zhang S. Radial Compressive Property and the Proof-of-Concept Study for Realizing Self-expansion of 3D Printing Polylactic Acid Vascular Stents with Negative Poisson’s Ratio Structure. Materials. 2018; 11(8):1357. https://doi.org/10.3390/ma11081357
Chicago/Turabian StyleWu, Zichao, Ji Zhao, Wenzheng Wu, Peipei Wang, Bofan Wang, Guiwei Li, and Shuo Zhang. 2018. "Radial Compressive Property and the Proof-of-Concept Study for Realizing Self-expansion of 3D Printing Polylactic Acid Vascular Stents with Negative Poisson’s Ratio Structure" Materials 11, no. 8: 1357. https://doi.org/10.3390/ma11081357





