Hydrolytic Hydrogen Production on Al–Sn–Zn Alloys Processed by High-Pressure Torsion
Abstract
1. Introduction
2. Materials and Methods
3. Results
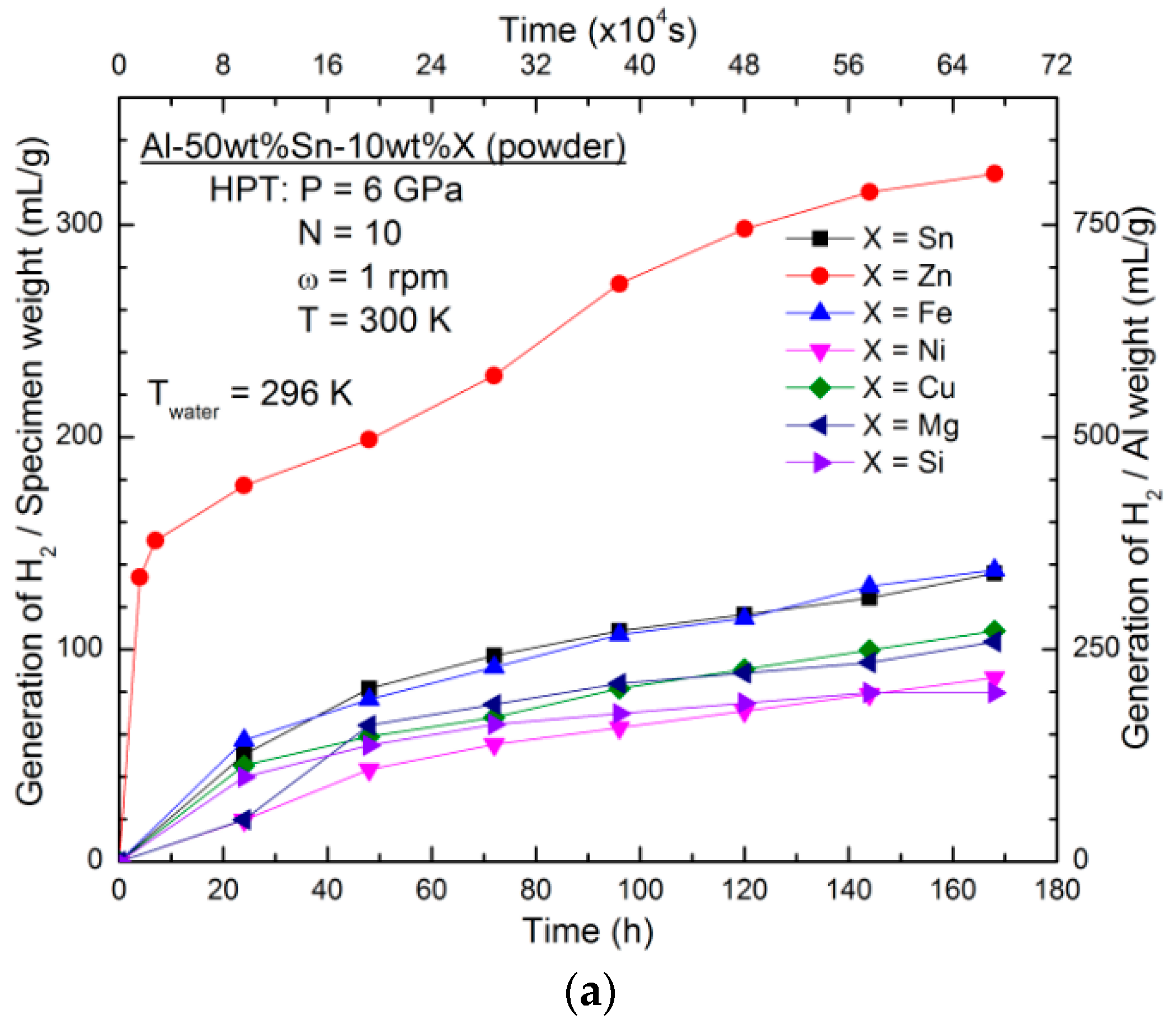
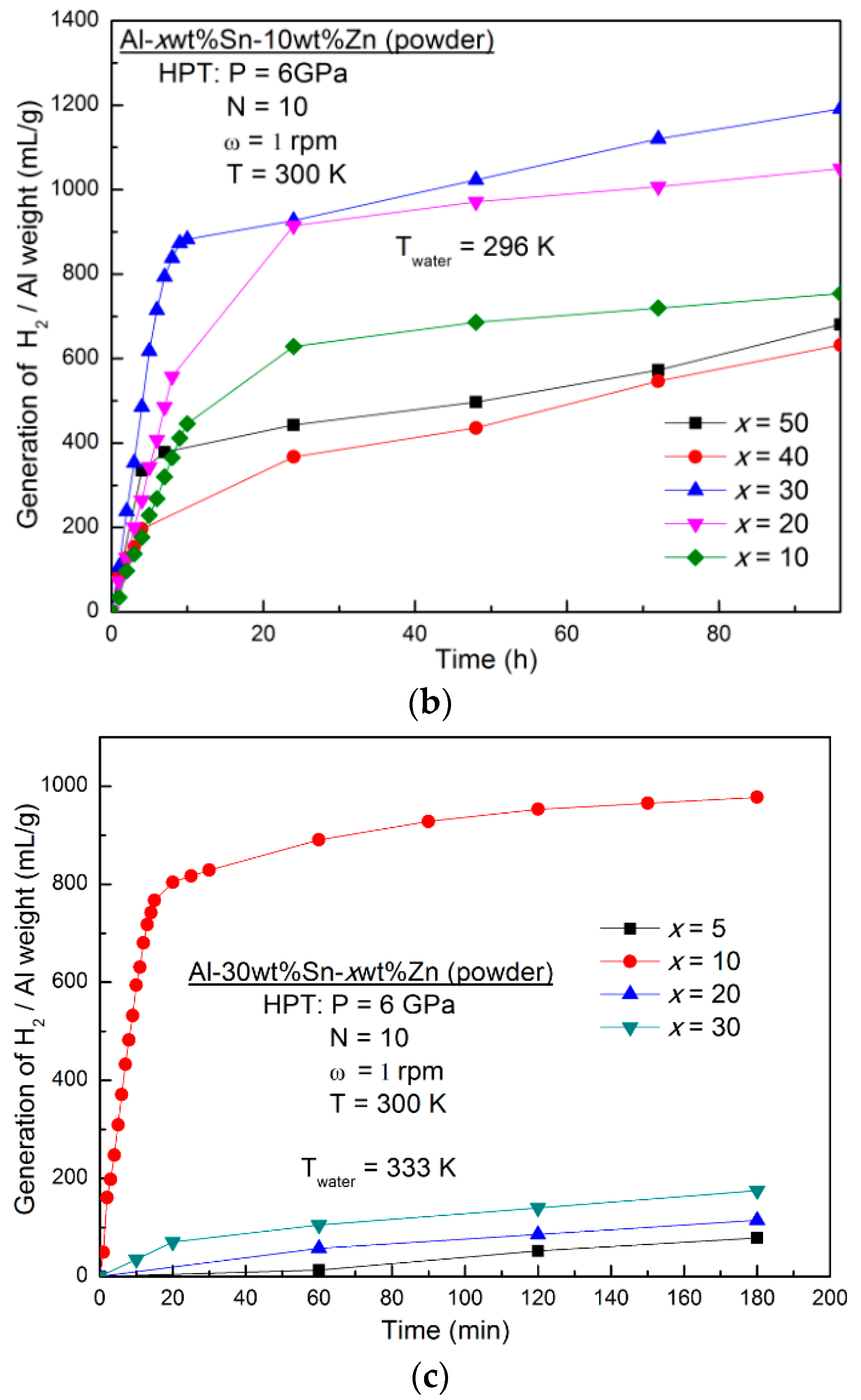
3.1. Effect of Alloying Elements
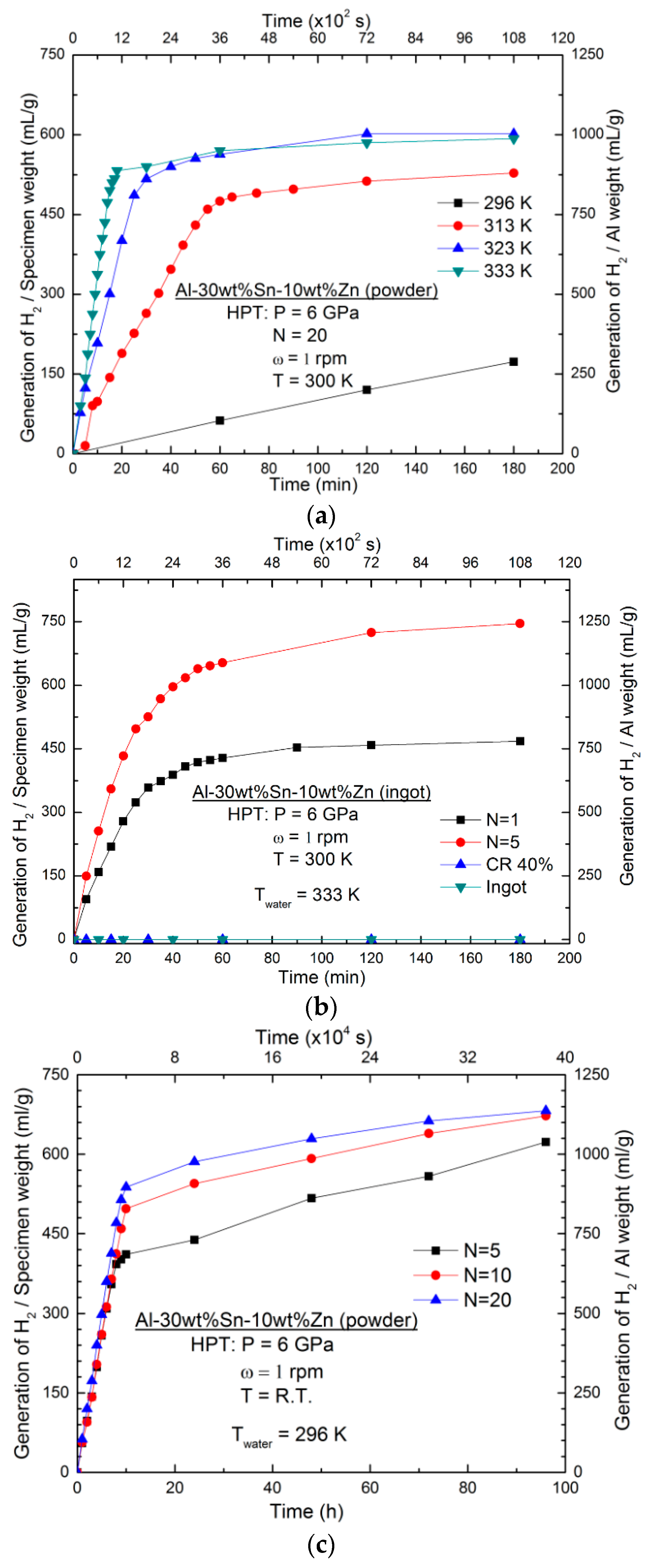
3.2. Effect of Water Temperature
3.3. Effect of Different Processing Routes
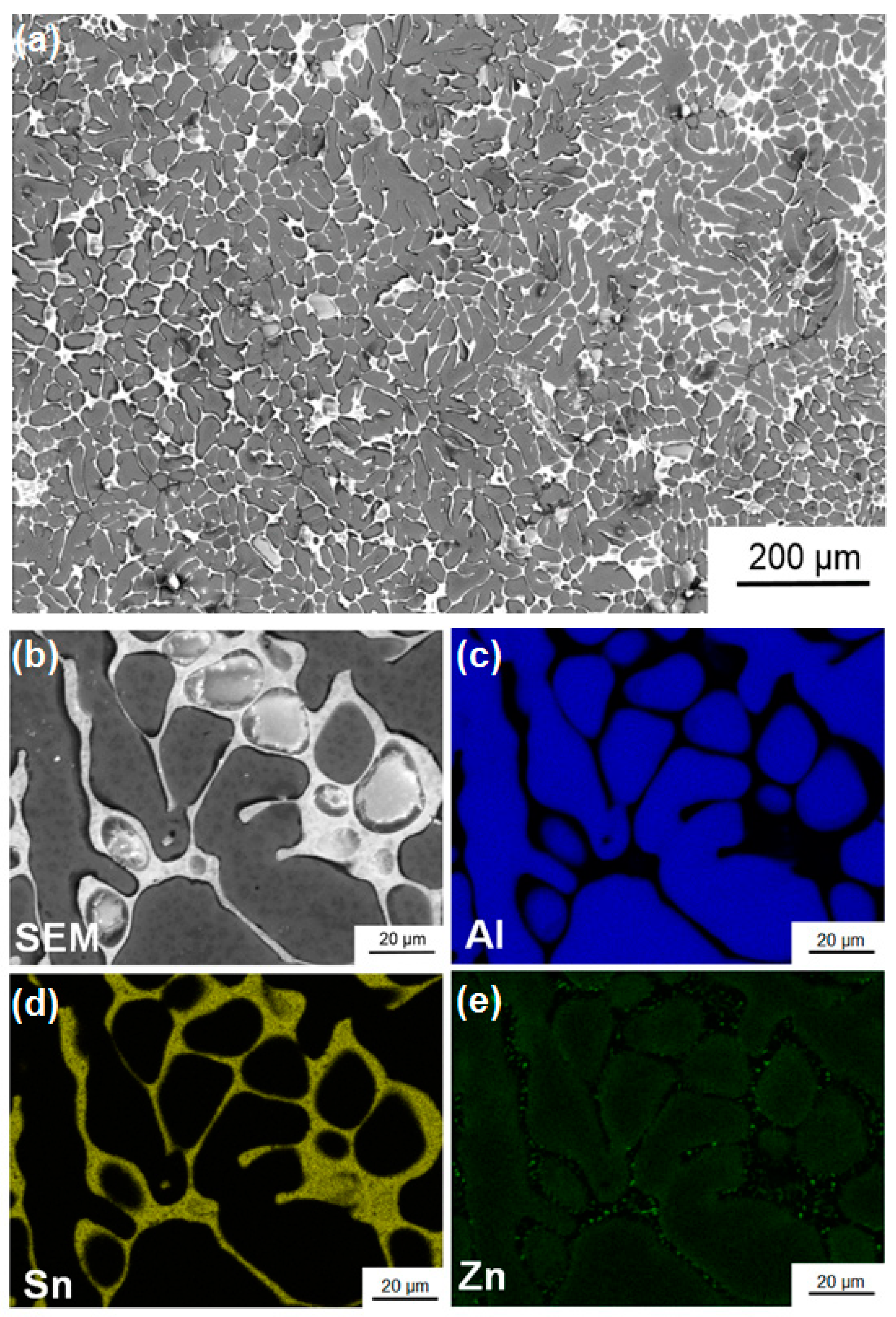
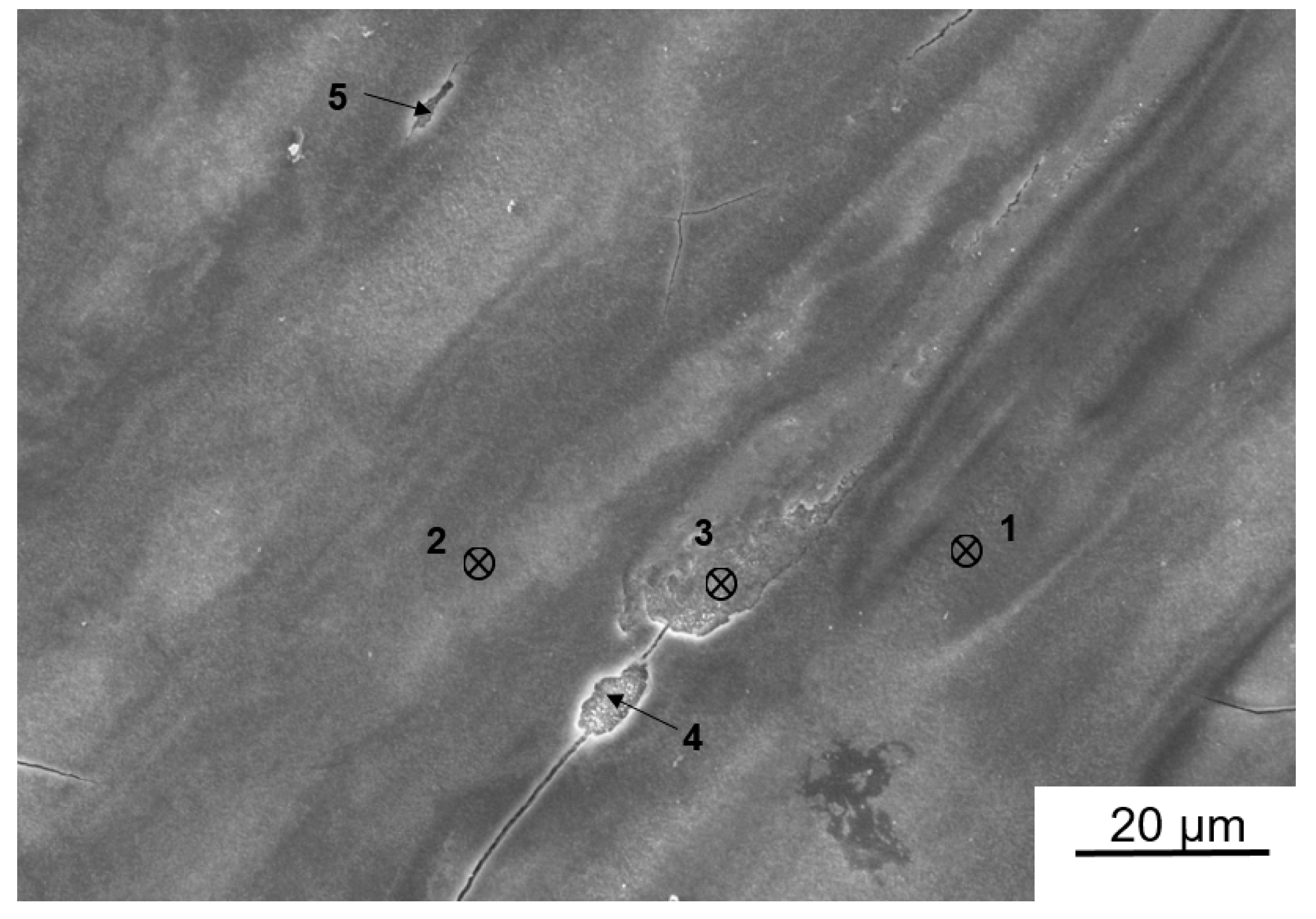
3.4. Characterization of Ingot Processed by HPT
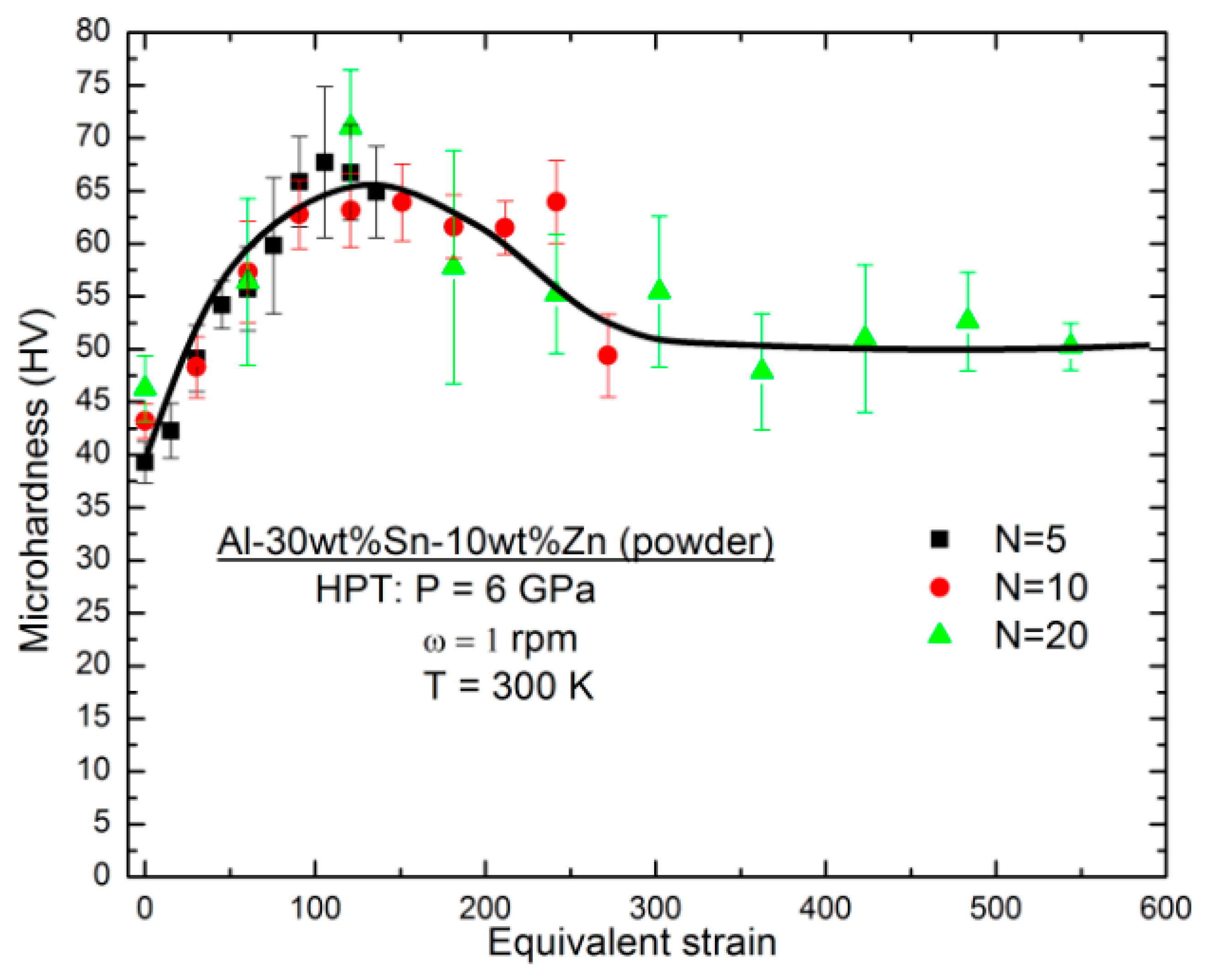
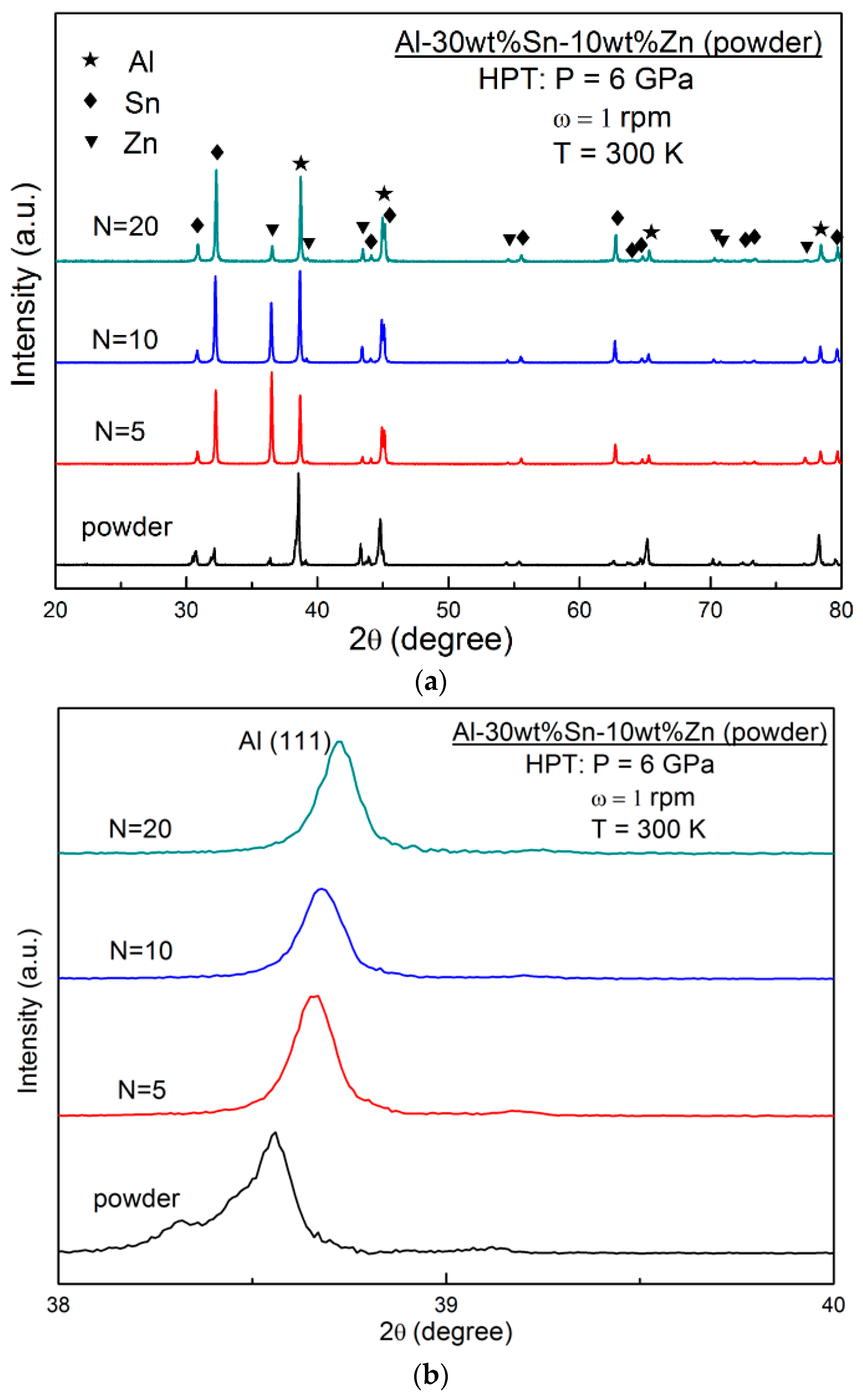
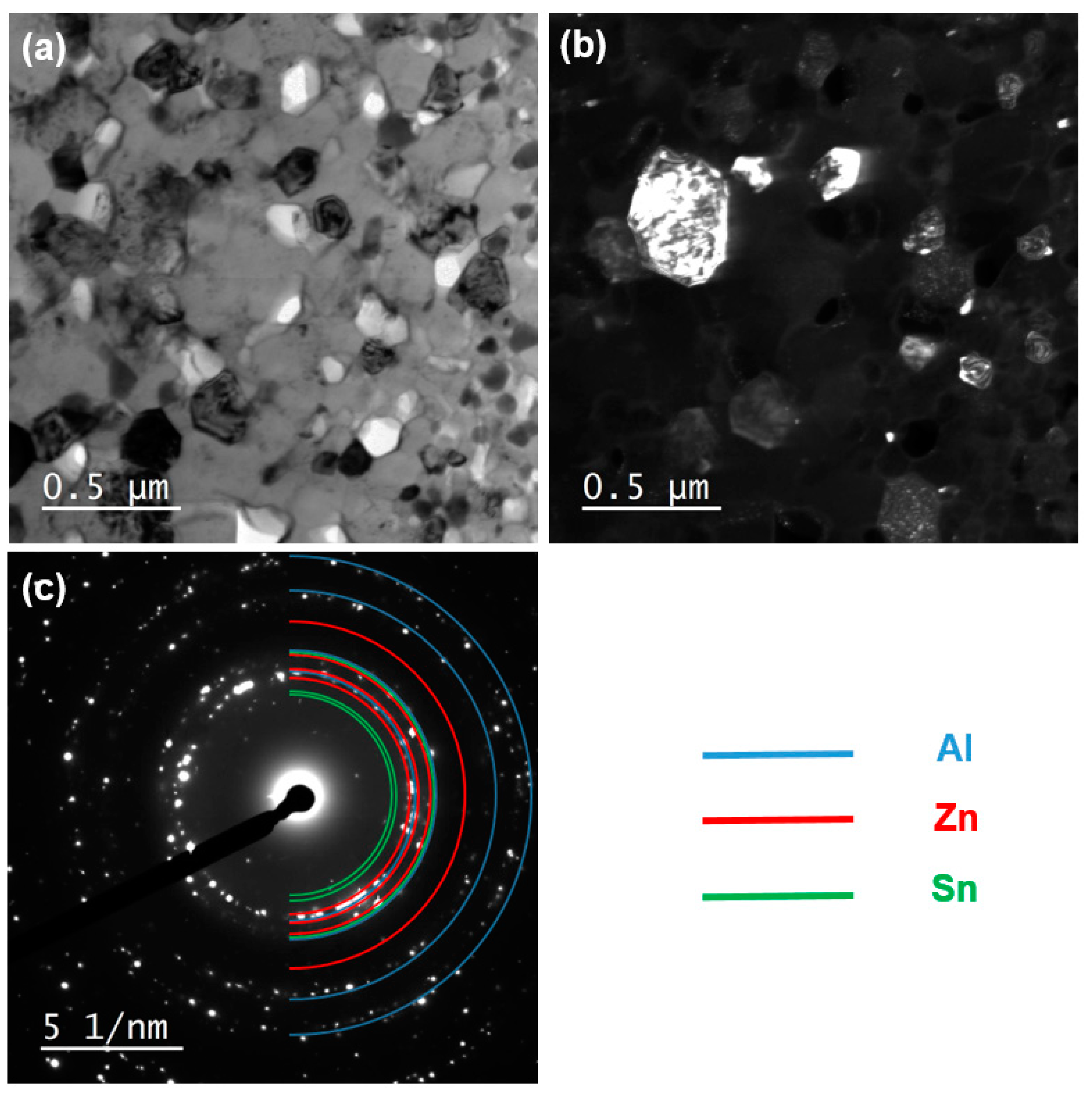
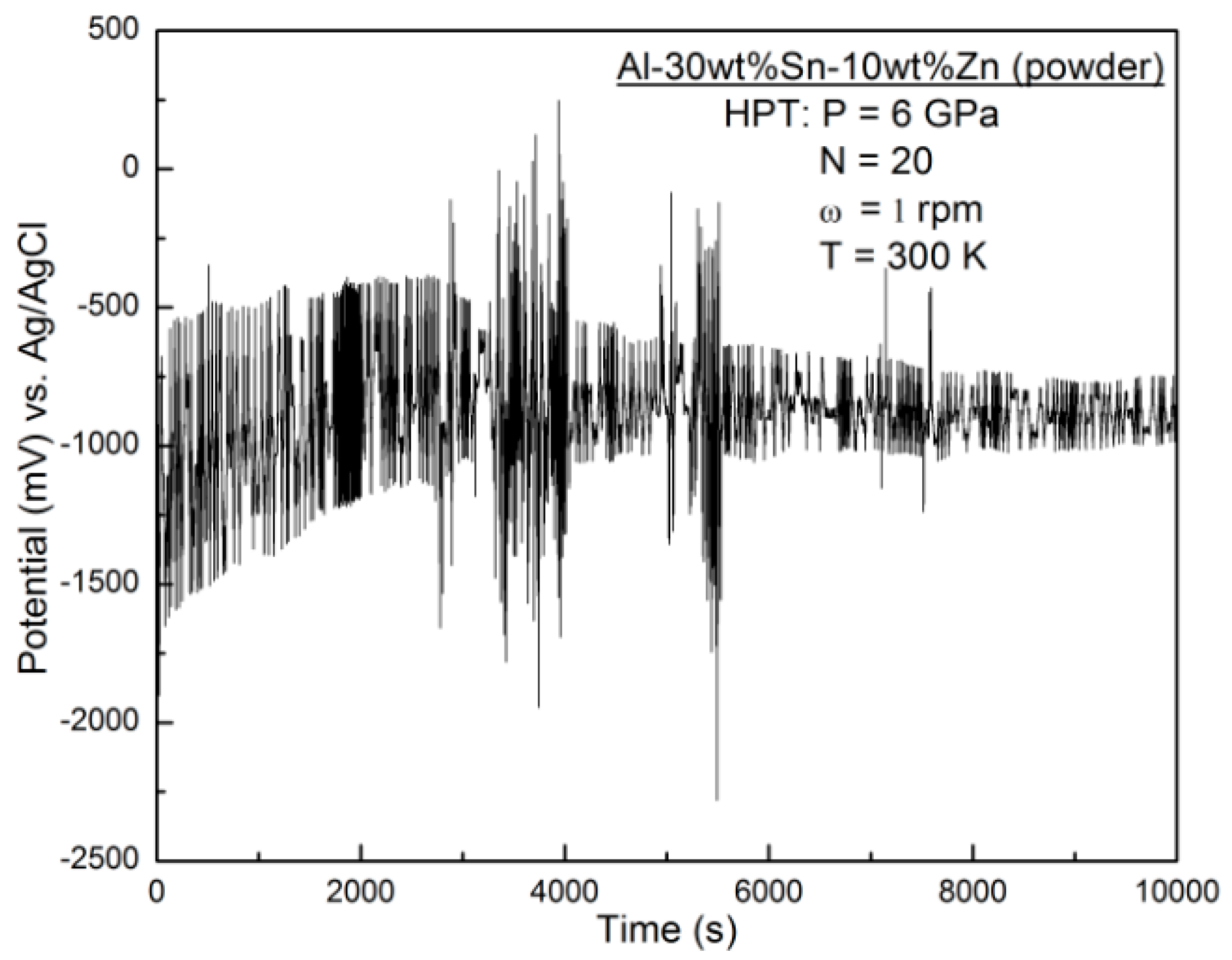
3.5. Characterization of Elemental Powders Processed by HPT
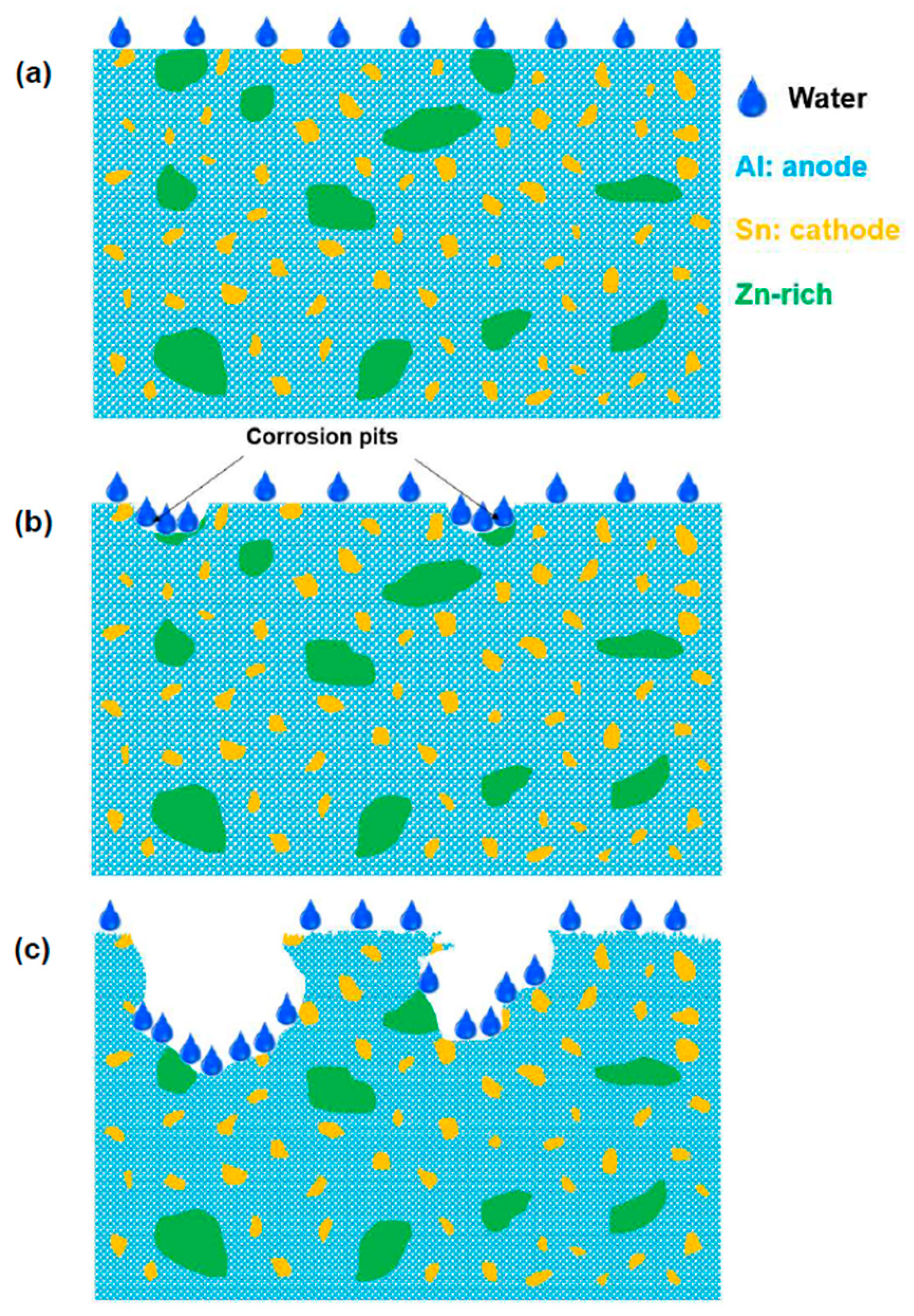
4. Discussion
5. Conclusions
- Different elements such as Zn, Fe, Ni, Cu, Mg, and Si were added to the Al–Sn alloys by HPT processing to enhance the hydrogen generation rate in contact with water. The addition of only Zn to the Al–Sn alloys improved the hydrogen generation rate and yield because of the enhancement of the pitting corrosion mechanism combined with the formation of nanogalvanic cells.
- The changes in the amounts of Sn and Zn changed the hydrogen generation behaviour and the best reaction rate and yield were achieved for the Al-30wt %Sn-10wt %Zn alloy.
- The Al-30wt %Sn-10wt %Zn alloy was processed by ingot casting, cold rolling, and HPT processing. While the as-cast ingot and cold-rolled samples did not exhibit hydrogen generation in water, the HPT-processed samples showed high hydrogen generation rate and yield and the hydrogen production performance was enhanced by increasing the shear strain.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Mohammad, M.; Narayanan, B.; Sopian, K. Renewable hydrogen economy in Asia-Opportunities and challenges: An overview. Renew. Sustain. Energy Rev. 2014, 30, 743–757. [Google Scholar] [CrossRef]
- Hwang, J.J. Review on development and demonstration of hydrogen fuel cell scooters. Renew. Sustain. Energy Rev. 2012, 16, 3803–3815. [Google Scholar] [CrossRef]
- Züttel, A.; Borgschulte, A.; Schlapbach, L. Hydrogen as a Future Energy Carrier; Wiley-VCH Press: Weinheim, Germany, 2008. [Google Scholar]
- Minić, D. Hydrogen Energy-Challenges and Perspectives; InTech Press: Rijeka, Croatia, 2012. [Google Scholar]
- Wang, H.Z.; Leung, D.Y.C.; Leung, M.K.H.; Ni, M. A review on hydrogen production using aluminum and aluminumalloys. Renew. Sustain. Energy Rev. 2009, 13, 845–853. [Google Scholar] [CrossRef]
- Elitzur, S.; Rosenband, V.; Gany, A. Study of hydrogen production and storage based on aluminum-water reaction. Int. J. Hydrogen Energy 2014, 39, 6328–6334. [Google Scholar] [CrossRef]
- Huang, X.; Gao, T.; Pan, X.; Wei, D.; Lv, C.; Qin, L.; Huang, Y. A review: Feasibility of hydrogen generation from the reaction between aluminum and water for fuel cell applications. J. Power Sources 2013, 229, 133–140. [Google Scholar] [CrossRef]
- Fan, M.; Xu, F.; Sun, L. Studies on hydrogen generation characteristics of hydrolysis of the ball milling Al-based materials in pure water. Int. J. Hydrogen Energy 2007, 32, 2809–2815. [Google Scholar] [CrossRef]
- Ziebarth, J.T.; Woodall, J.M.; Kramer, R.A.; Choi, G. Liquid phase-enabled reaction of Al-Ga and Al-Ga-In–Snalloys with water. Int. J. Hydrogen Energy 2011, 36, 5271–5279. [Google Scholar] [CrossRef]
- Wang, H.; Chang, Y.; Dong, S.; Lei, Z.; Zhu, Q.; Luo, P.; Xie, Z. Investigation on hydrogen production using multicomponent aluminum alloys at mild conditions and its mechanism. Int. J. Hydrogen Energy 2013, 38, 1236–1243. [Google Scholar] [CrossRef]
- Ilyukhina, A.V.; Ilyukhin, A.S.; Shkolnikov, E.I. Hydrogen generation from water by means of activated aluminium. Int. J. Hydrogen Energy 2012, 37, 16382–16387. [Google Scholar] [CrossRef]
- Mahmoodi, K.; Alinejad, B. Enhancement of hydrogen generation rate in reaction of aluminum with water. Int. J. Hydrogen Energy 2010, 35, 5227–5232. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Islamgaliev, R.K.; Alexandrov, I.V. Bulk nanostructured materials from severe plastic deformation. Progress Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Estrin, Y.; Horita, Z.; Langdon, T.G.; Zehetbauer, M.J.; Zhu, Y.T. Producing bulk ultrafine-grained materials by severe plastic deformation. JOM 2006, 58, 33–39. [Google Scholar] [CrossRef]
- Zhang, F.; Yonemoto, R.; Arita, M.; Horita, Z. Hydrogen generation from pure water using Al–Sn powders consolidated through high-pressure torsion. J. Mater. Res. 2016, 31, 775–782. [Google Scholar] [CrossRef]
- Zhilyaev, A.P.; Nurislamova, G.V.; Kim, B.K.; Baró, M.D.; Szpunard, J.A.; Langdon, T.G. Experimental parameters influencing grain refinement and microstructural evolution during high-pressure torsion. Acta Mater. 2003, 51, 753–765. [Google Scholar] [CrossRef]
- Xu, C.; Horita, Z.; Langdon, T.G. The evolution of homogeneity in processing by high-pressure torsion. Acta Mater. 2007, 55, 203–212. [Google Scholar] [CrossRef]
- Kawasaki, M.; Langdon, T.G. The significance of strain reversals during processing by high-pressure torsion. Mater. Sci. Eng. A 2008, 498, 341–348. [Google Scholar] [CrossRef]
- Pouryazdan, M.; Kaus, B.; Rack, A.; Ershov, A.; Hahn, H. Mixing instabilities during shearing of metals. Nat. Commun. 2017, 8, 1611. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, Y.B.; Figueiredo, R.B.; Chang, L.; Liao, X.Z.; Kawasaki, M.; Zheng, W.L.; Ringer, S.P.; Langdon, T.G.; Zhu, Y.T. Three-dimensional shear-strain patterns induced by high-pressure torsion and their impact on hardness evolution. Acta Mater. 2011, 59, 3903–3914. [Google Scholar] [CrossRef]
- Kai, M.; Horita, Z.; Langdon, T.G. Developing grain refinement and superplasticity in a magnesium alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2008, 488, 117–124. [Google Scholar] [CrossRef]
- Serre, P.; Figueiredo, R.B.; Gao, N.; Langdon, T.G. Influence of strain rate on the characteristics of a magnesium alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2011, 528, 601–3608. [Google Scholar] [CrossRef]
- Ito, Y.; Horita, Z. Microstructural evolution in pure aluminum processed by high-pressure torsion. Mater. Sci. Eng. A 2009, 503, 32–36. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. Significance of homologous temperature in softening behavior and grain size of pure metals processed by high-pressure torsion. Mater. Sci. Eng. A 2011, 528, 7514–7523. [Google Scholar] [CrossRef]
- Edalati, K.; Yamamoto, A.; Horita, Z.; Ishihara, T. High-pressure torsion of pure magnesium: Evolution of mechanical properties, microstructures and hydrogen storage capacity with equivalent strain. Scr. Mater. 2011, 64, 880–883. [Google Scholar] [CrossRef]
- Mazilkin, A.A.; Straumal, B.B.; Borodachenkova, M.V.; Valiev, R.Z.; Kogtenkova, O.A.; Baretzky, B. Gradual softening of Al–Zn alloys during high-pressure torsion. Mater. Lett. 2008, 84, 63–65. [Google Scholar] [CrossRef]
- Straumal, B.; Kilmametov, A.; Ivanisenko, Y.; Mazilkin, A.; Kogtenkova, O.; Kurmanaeva, L.; Korneva, A.; Zieba, P.; Baretzky, B. Phase transitions induced by severe plastic deformation: Steady-state and equifinality. Int. J. Mater. Res. 2015, 106, 657–664. [Google Scholar] [CrossRef]
- Yi, Y.; Huo, J.; Wang, W. Electrochemical Properties of Al–based Solid Solutions Alloyed by Element Mg, Ga, Zn and Mn under the Guide of First Principles. Fuel Cells 2017, 17, 723–729. [Google Scholar] [CrossRef]
- Khireche, S.; Boughrara, D.; Kadri, A.; Hamadou, L.; Benbrahim, N. Corrosion mechanism of Al, Al-Zn and Al-Zn-Sn alloys in 3 wt. % NaCl solution. Corros. Sci. 2014, 87, 504–516. [Google Scholar] [CrossRef]
- Richardson, J.A.; Wood, G.C. A study of the pitting corrosion of Al by scanning electron microscopy. Corros. Sci. 1970, 10, 313–323. [Google Scholar] [CrossRef]
- Breslin, C.B.; Friery, L.P.; Carroll, W.M. The electrochemical behaviour of Al-Zn-In and Al-Zn-Hg alloys in aqueous halide solutions. Corros. Sci. 1994, 36, 85–97. [Google Scholar] [CrossRef]
- El Shayeb, H.A.; El Wahab, F.M.A.; El Abedin, S.Z. Electrochemical behaviour of Al, Al-Sn, Al-Zn and Al-Zn-Sn alloys in chloride solutions containing stannous ions. Corros. Sci. 2001, 43, 655–669. [Google Scholar] [CrossRef]
- El Shayeb, H.A.; El Wahab, F.M.A.; El Abedin, S.Z. Electrochemical behaviour of Al, A-Sn, Al-Zn and Al-Zn-Sn alloys in chloride solutions containing indium ions. J. Appl. Electrochem. 1999, 29, 473–480. [Google Scholar] [CrossRef]
- Wang, X.; Guo, M.; Zhang, J.; Zhuang, L. Effect of Zn addition on the microstructure, texture evolution and mechanical properties of Al-Mg-Si-Cu alloys. Mater. Sci. Eng. A 2016, 677, 522–533. [Google Scholar] [CrossRef]
- Shin, S.; Lim, K.; Park, I. Effects of high Zn content on the microstructure and mechanical properties of Al-Zn-Cu gravity-cast alloys. Mater. Sci. Eng. A 2017, 679, 340–349. [Google Scholar] [CrossRef]
- Wu, Y.; Du, J.; Choy, K.; Hench, L.L.; Guo, J. Formation of interconnected microstructural ZnAl2O4 films prepared by sol-gel method. Thin Solid Films 2005, 472, 150–156. [Google Scholar] [CrossRef]
- Strohmeier, B.R.; Hercules, D.M. Surface spectroscopic characterization of the interaction between zinc ions and γ-alumina. J. Catal. 1984, 86, 266–279. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, X.; Zhao, Q.; Liu, S.; Hu, X.; Chen, G. Facile solution synthesis and characterization of porous cubic-shaped superstructure of ZnAl2O4. Mater. Lett. 2011, 65, 194–197. [Google Scholar] [CrossRef]
- Zhang, F.; Edalati, K.; Arita, M.; Horita, Z. Fast hydrolysis and hydrogen generation on Al-Bi alloys and Al-Bi-C composites synthesized by high-pressure torsion. Int. J. Hydrogen Energy 2017, 42, 29121–29130. [Google Scholar] [CrossRef]
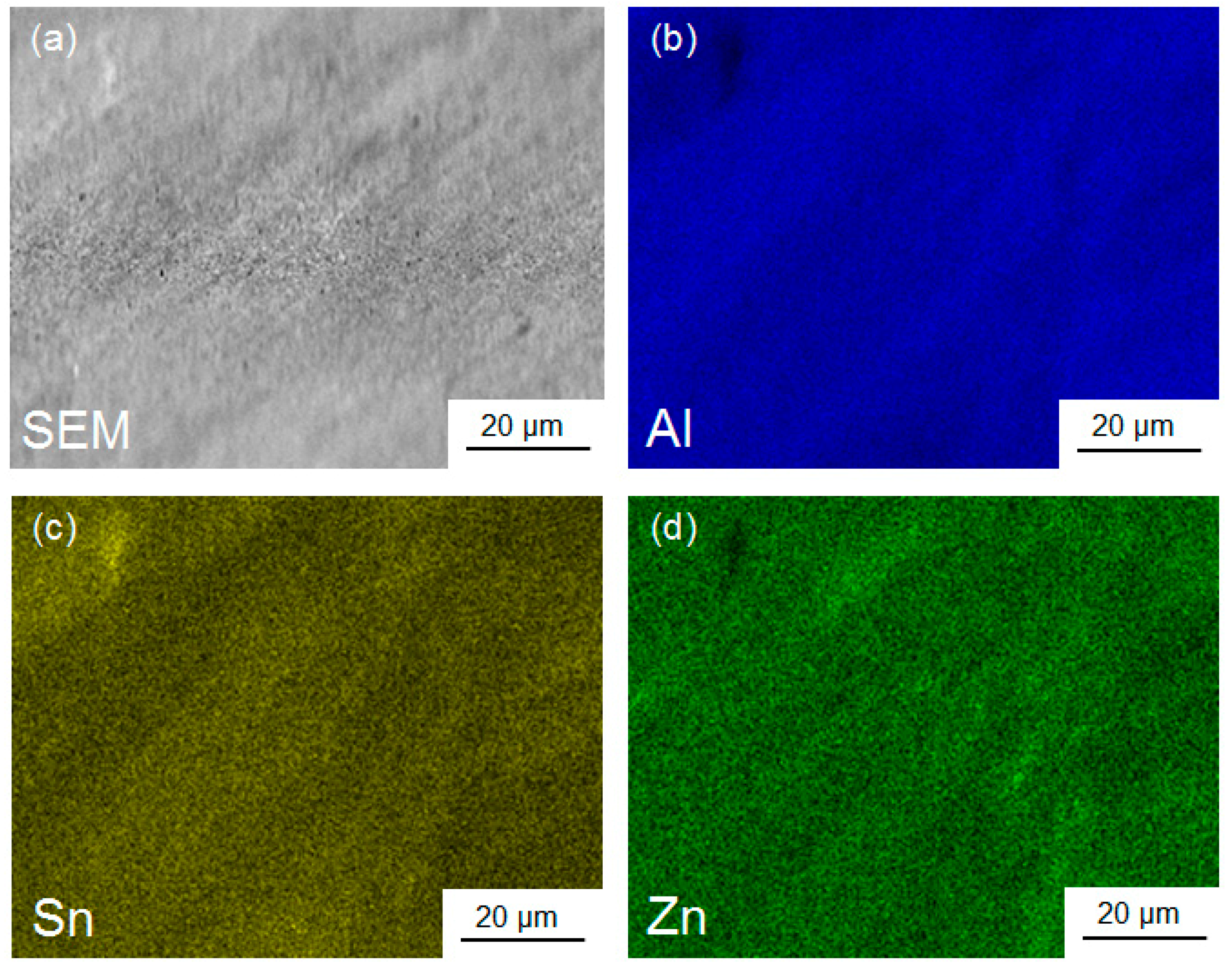

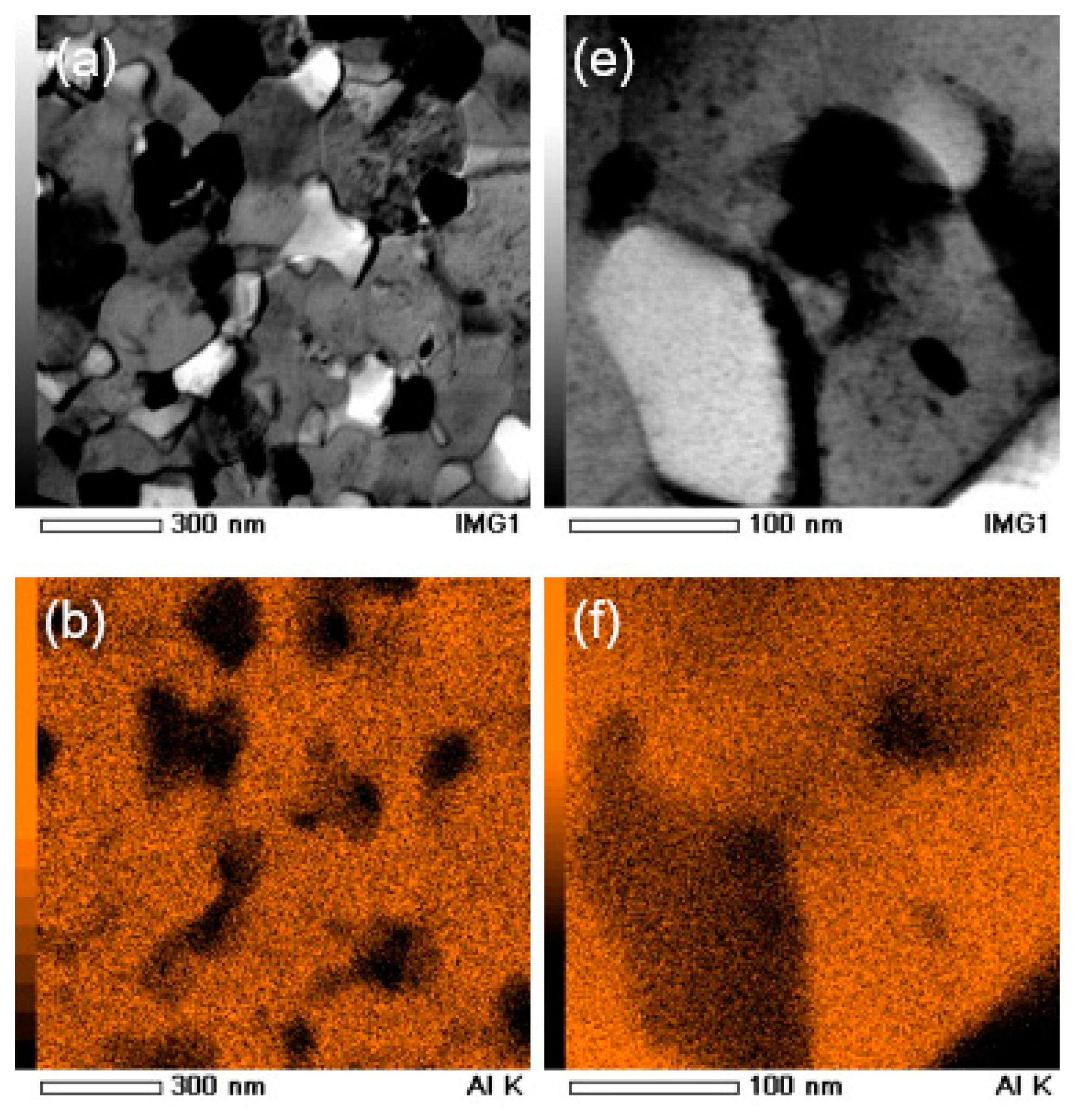
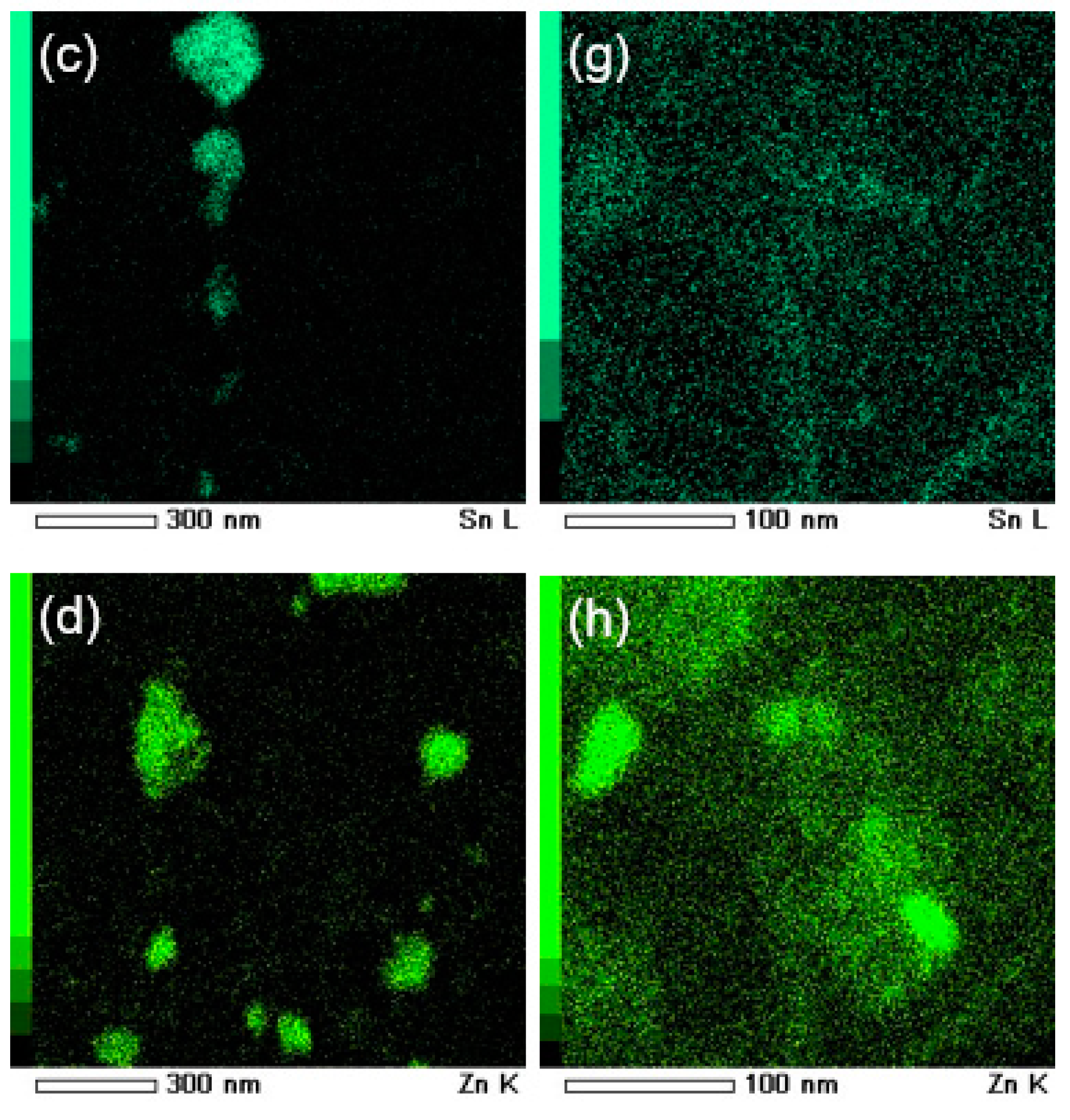
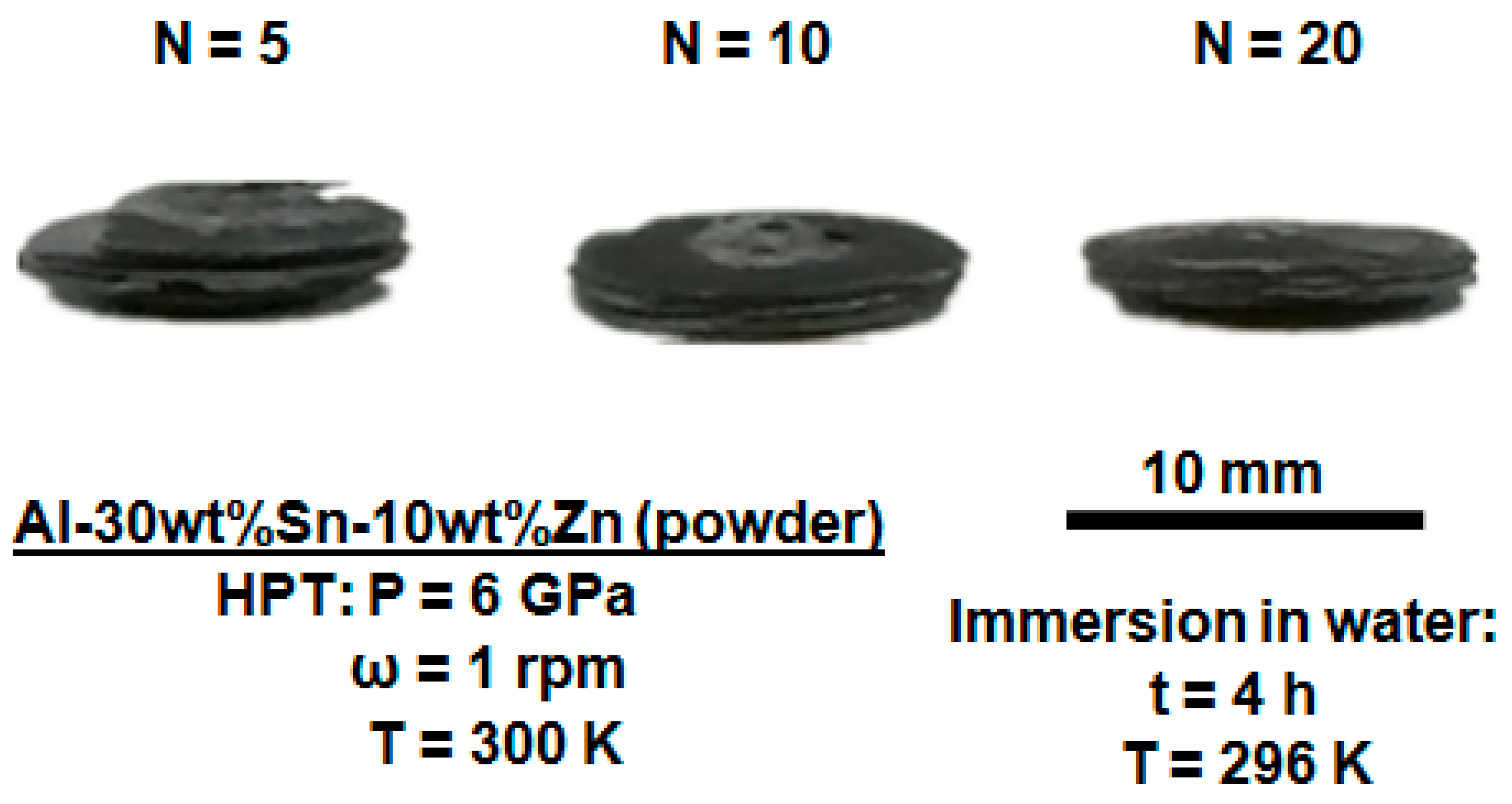
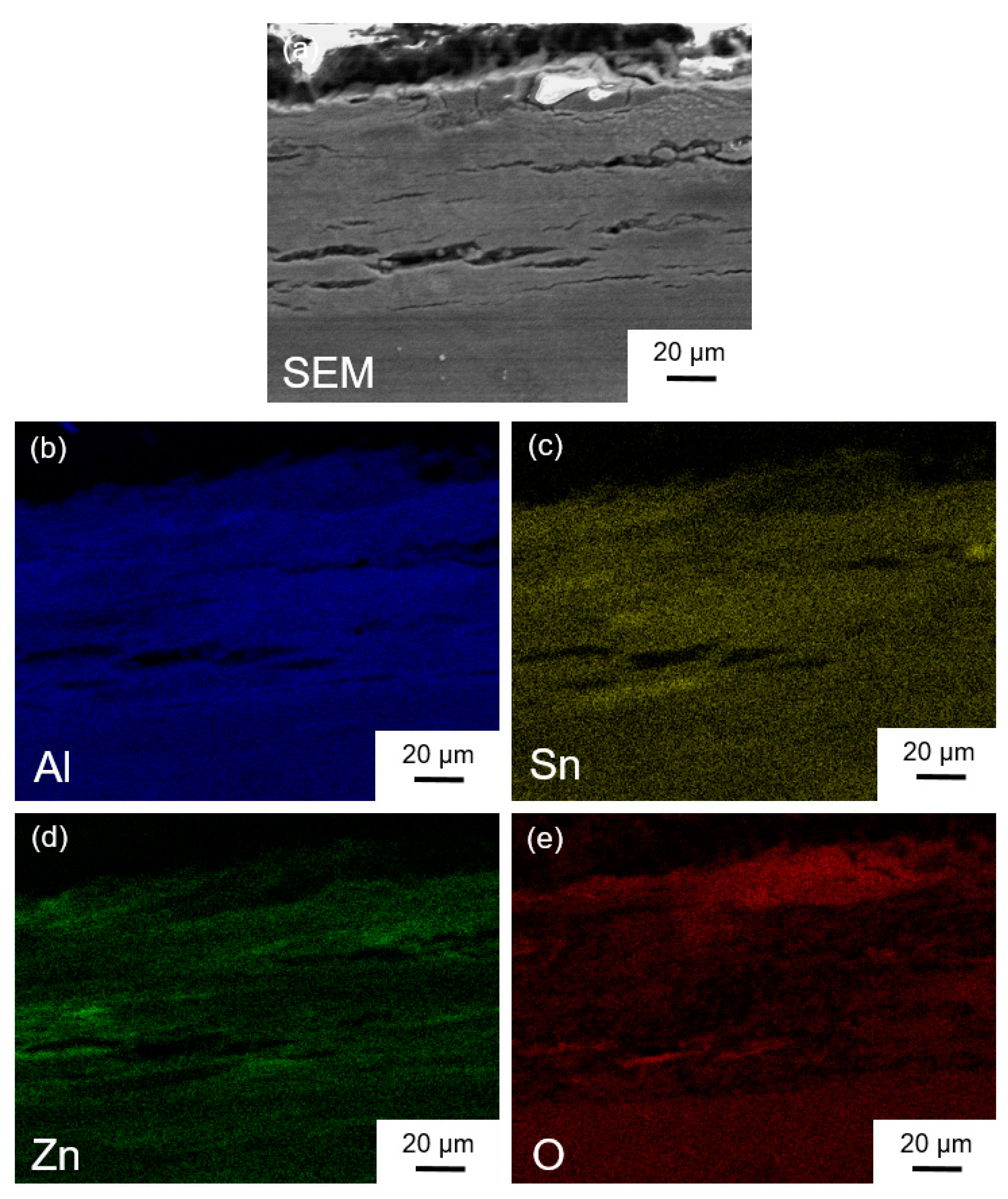
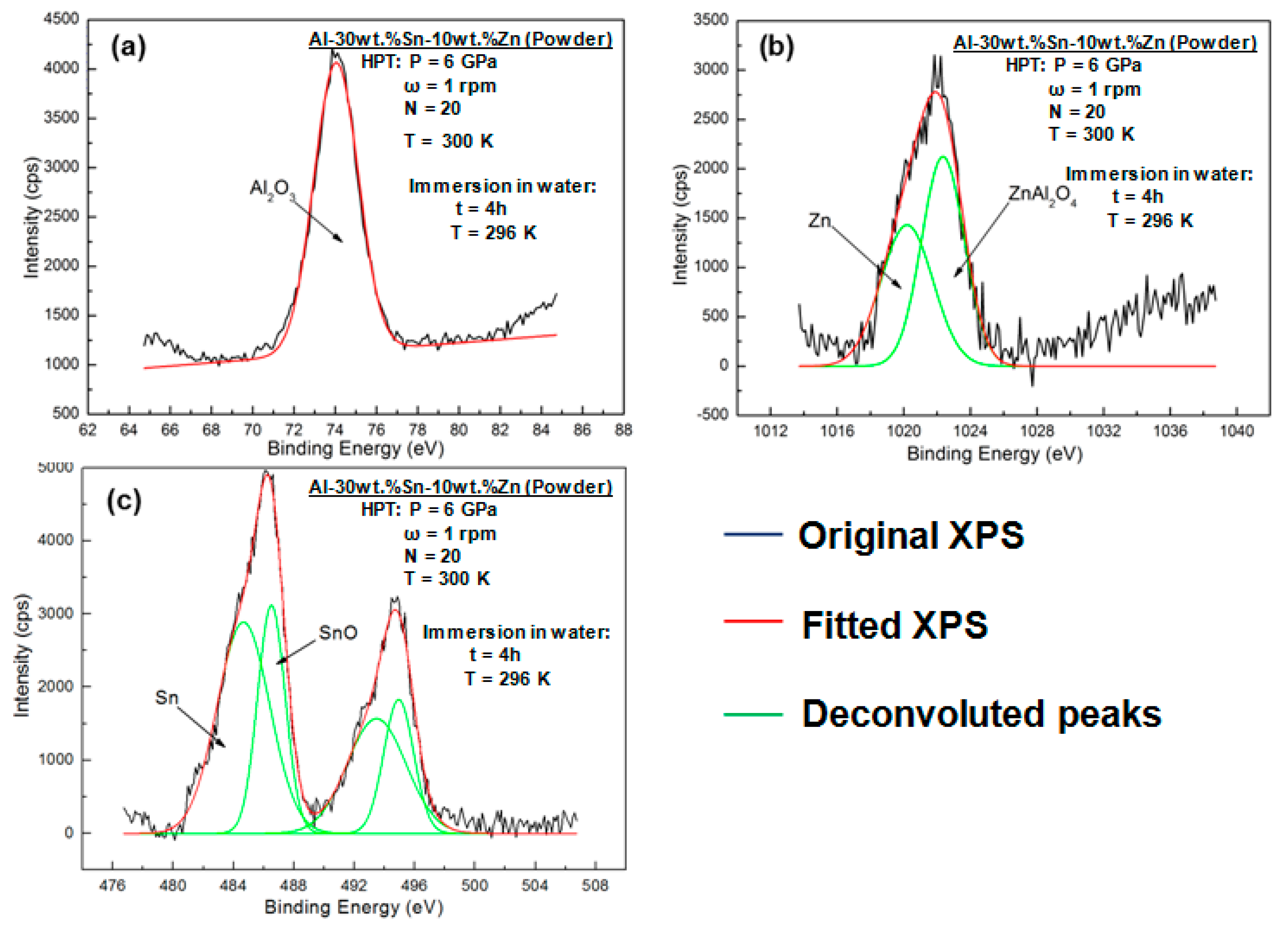
| Element | Purity (wt %) | Size (μm) |
|---|---|---|
| Al | 99.99 | 75 |
| Sn | 99.99 | 38 |
| Zn | 99.99 | 75 |
| Fe | 99.999 | 5 |
| Ni | 99.99 | 150 |
| Cu | 99.9 | 75 |
| Mg | 99.9 | 180 |
| Si | 99.999 | 75 |
| O | Zn | Al | Sn | |
|---|---|---|---|---|
| 1 | 31.14 | 0 | 50.99 | 17.87 |
| 2 | 34.65 | 0 | 50.75 | 14.60 |
| 3 | 10.78 | 15.86 | 42.17 | 31.18 |
| 4 | 10.66 | 34.67 | 31.30 | 23.37 |
| 5 | 22.23 | 17.80 | 39.67 | 20.69 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Edalati, K.; Arita, M.; Horita, Z. Hydrolytic Hydrogen Production on Al–Sn–Zn Alloys Processed by High-Pressure Torsion. Materials 2018, 11, 1209. https://doi.org/10.3390/ma11071209
Zhang F, Edalati K, Arita M, Horita Z. Hydrolytic Hydrogen Production on Al–Sn–Zn Alloys Processed by High-Pressure Torsion. Materials. 2018; 11(7):1209. https://doi.org/10.3390/ma11071209
Chicago/Turabian StyleZhang, Fan, Kaveh Edalati, Makoto Arita, and Zenji Horita. 2018. "Hydrolytic Hydrogen Production on Al–Sn–Zn Alloys Processed by High-Pressure Torsion" Materials 11, no. 7: 1209. https://doi.org/10.3390/ma11071209
APA StyleZhang, F., Edalati, K., Arita, M., & Horita, Z. (2018). Hydrolytic Hydrogen Production on Al–Sn–Zn Alloys Processed by High-Pressure Torsion. Materials, 11(7), 1209. https://doi.org/10.3390/ma11071209






