Application of Low-Cost Me-N-C (Me = Fe or Co) Electrocatalysts Derived from EDTA in Direct Methanol Fuel Cells (DMFCs)
Abstract
:1. Introduction
2. Materials and Methods
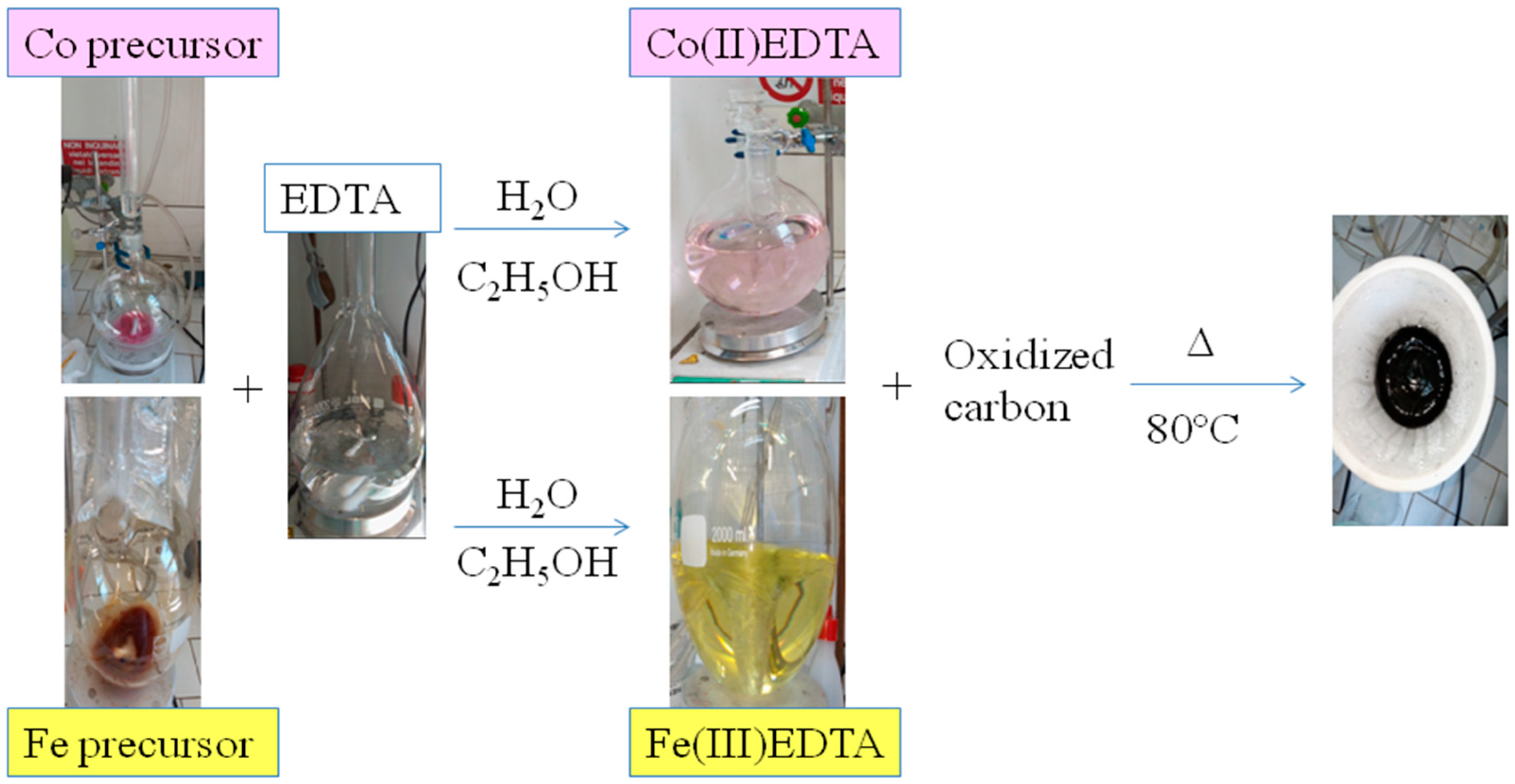
2.1. Preparation of Co-N-C- and Fe-N-C-Based Electrocatalysts
2.2. Physicochemical Characterization
2.3. Electrochemical Measurements
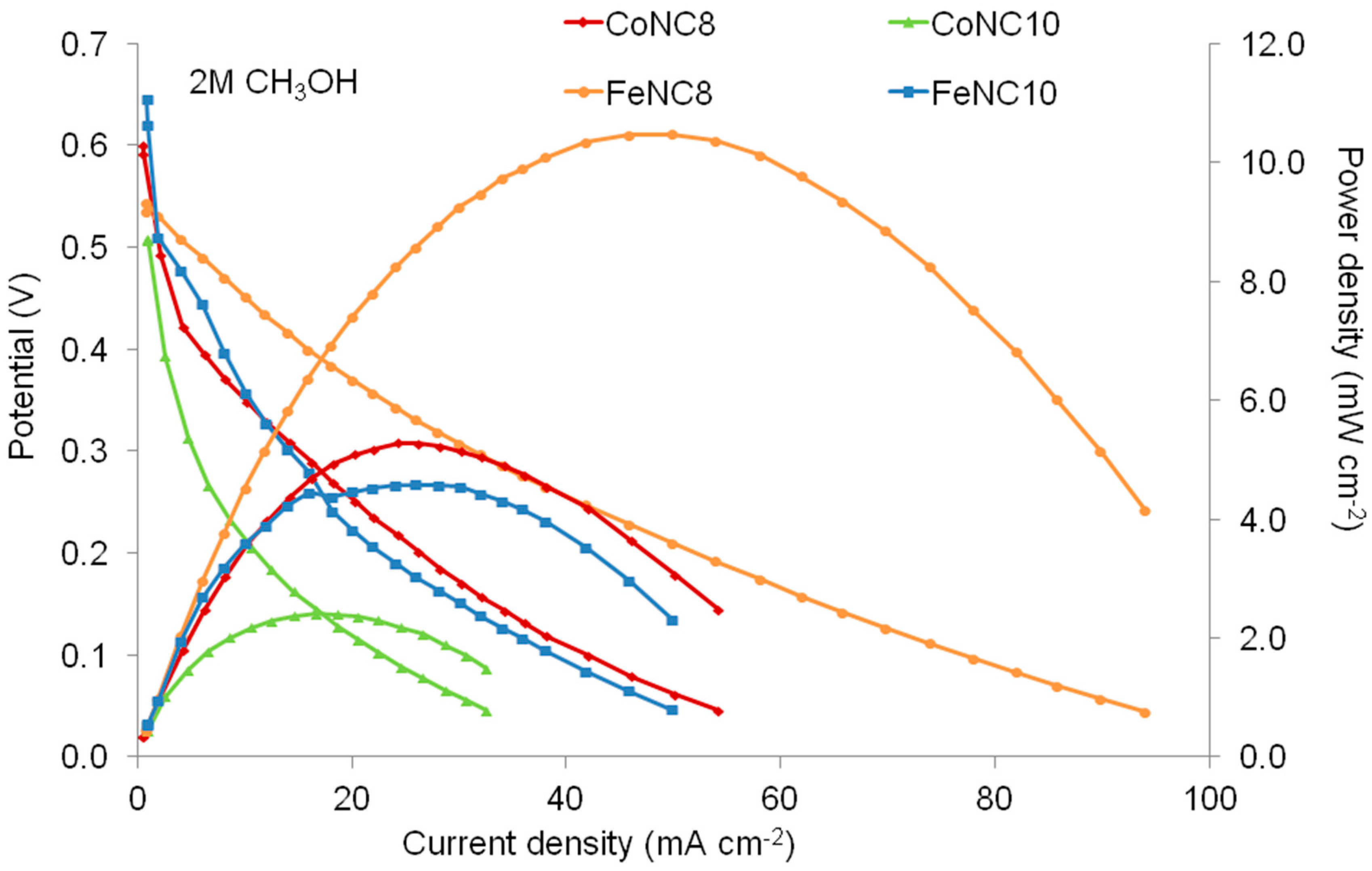
3. Results and Discussion
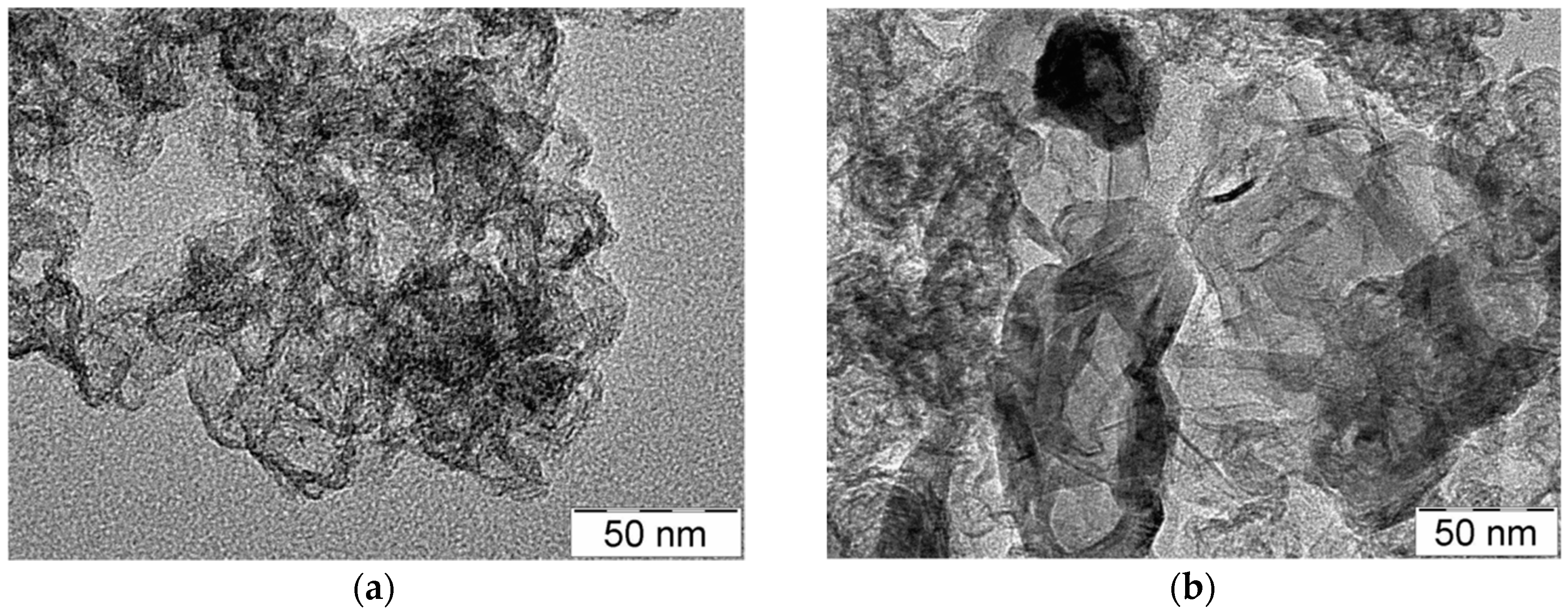
3.1. TEM Analysis
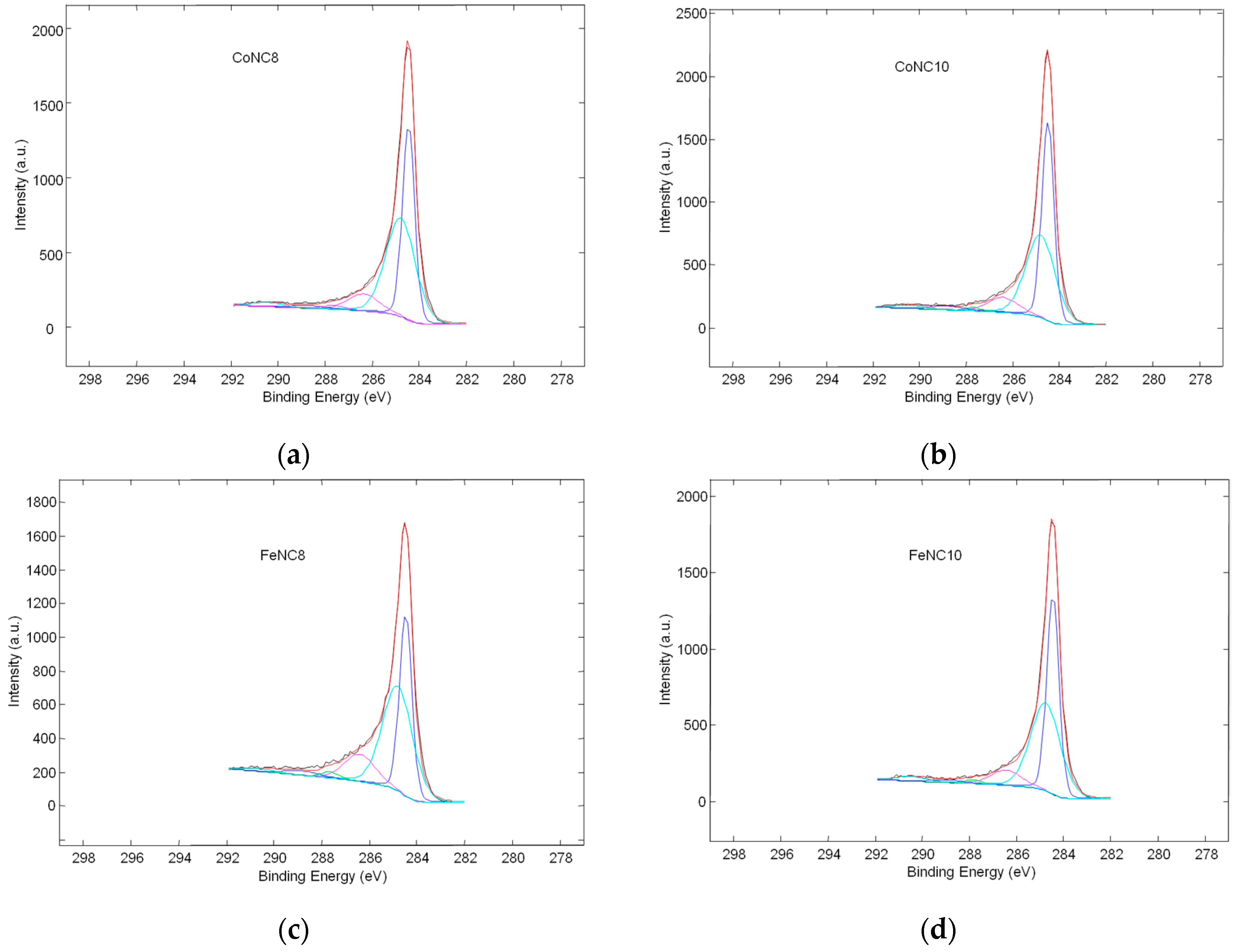
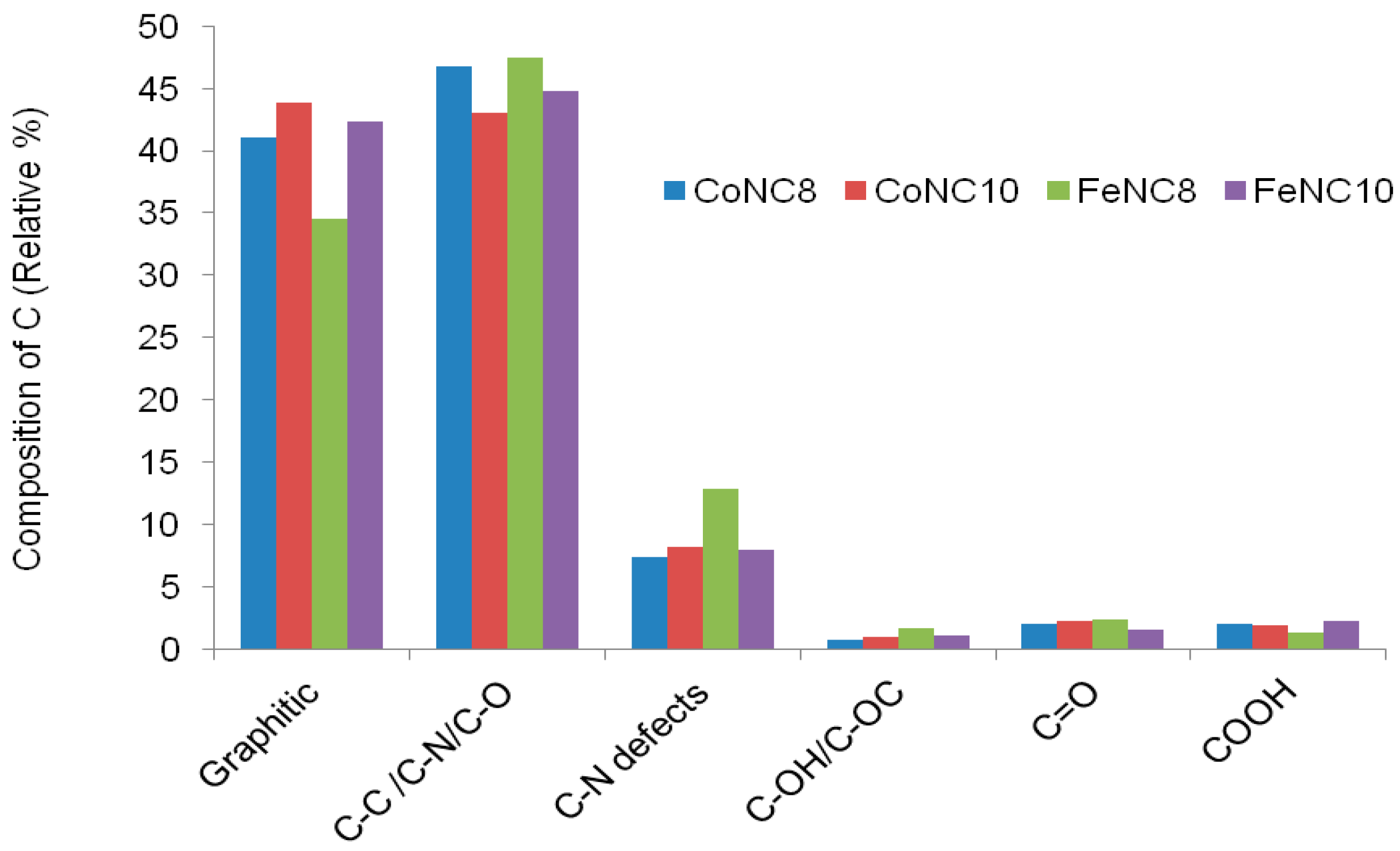
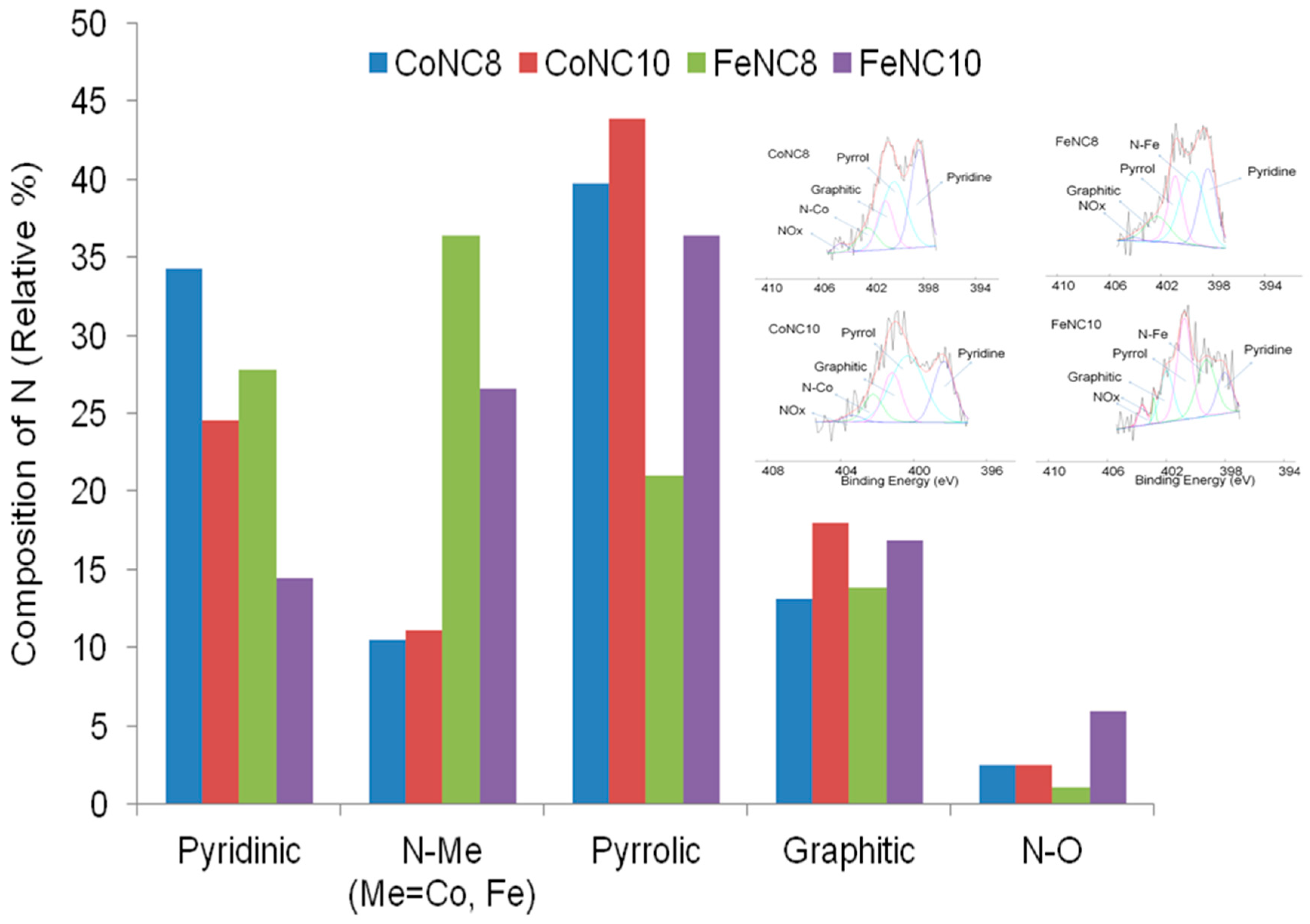
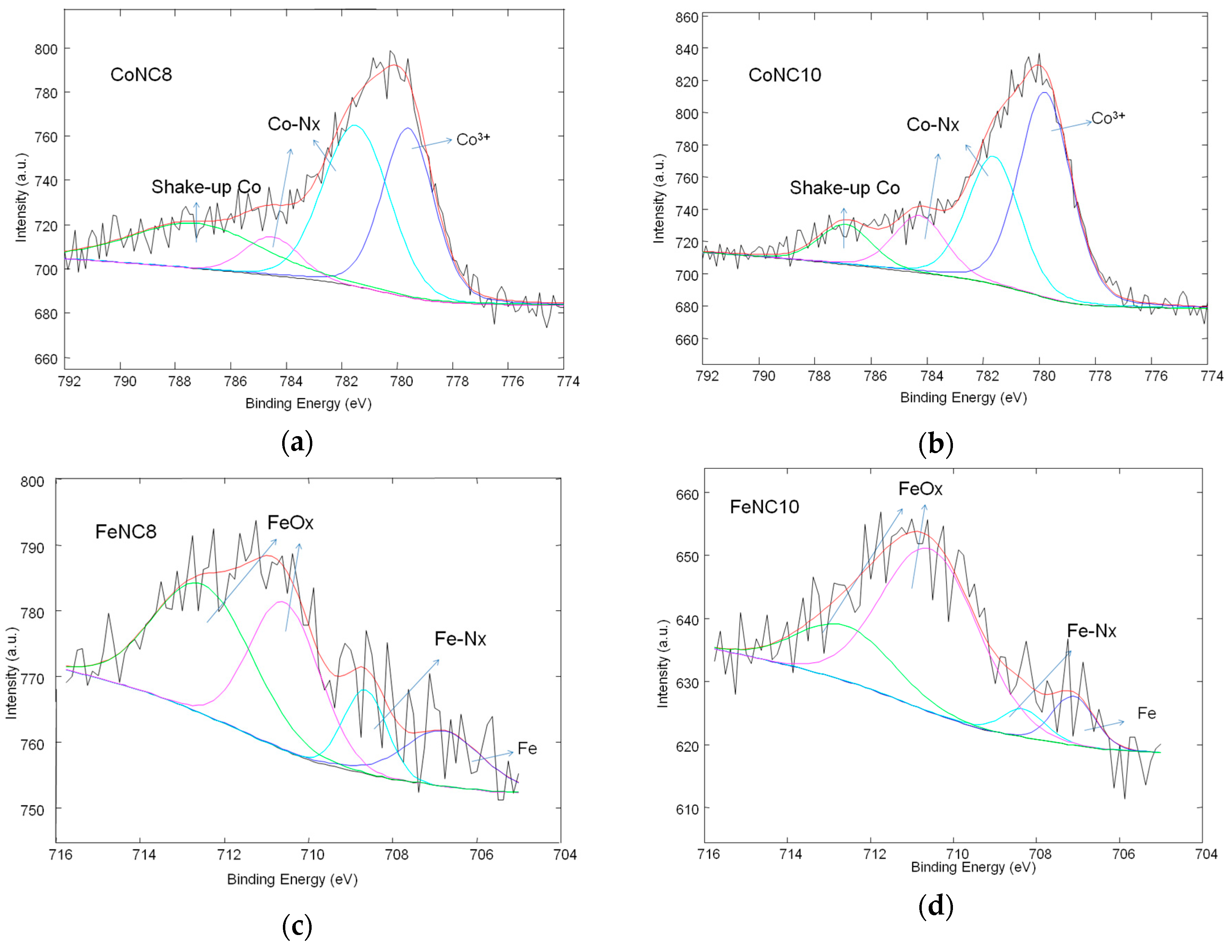
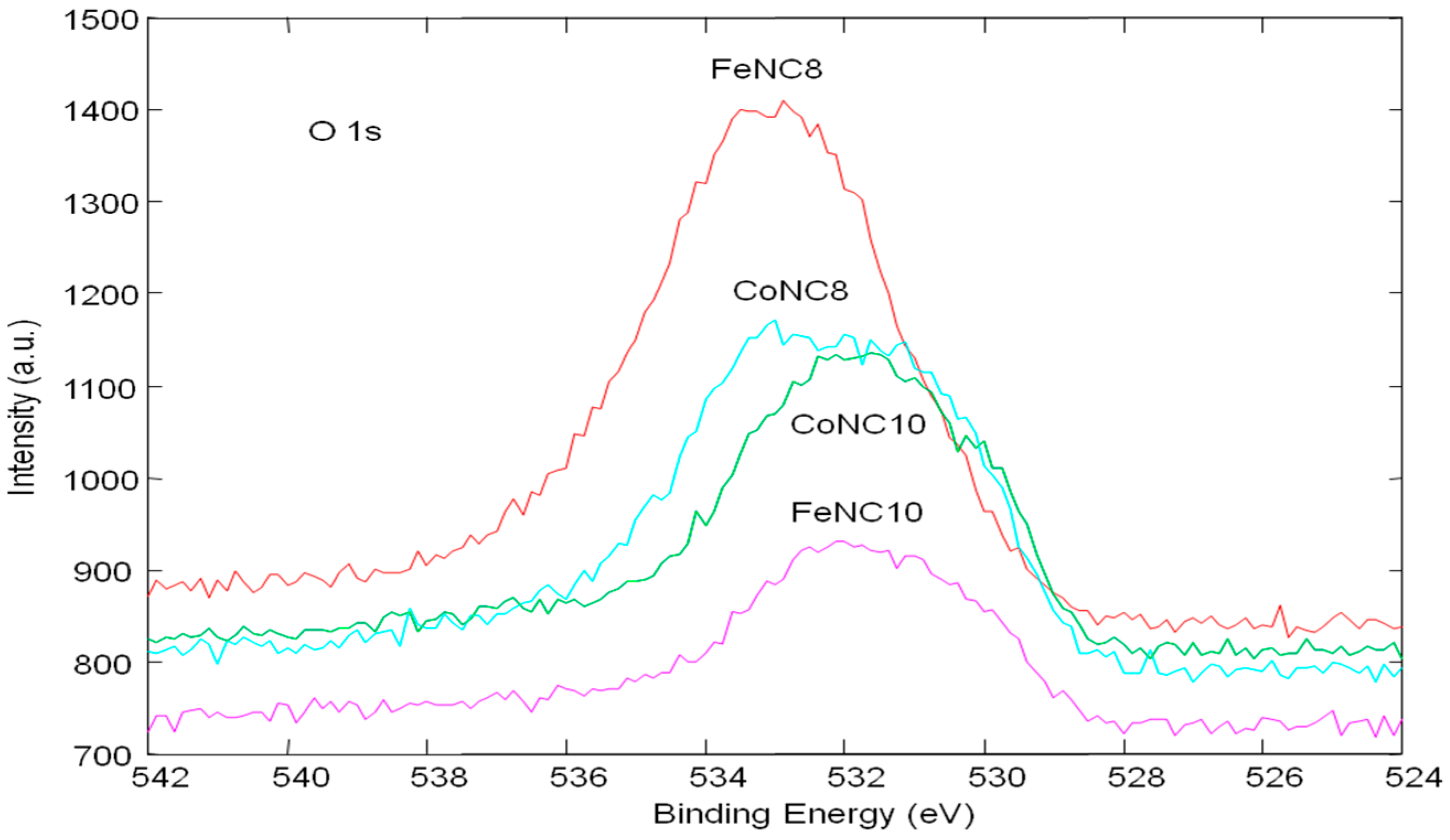
3.2. X-ray Photoelectron Spectroscopy (XPS) Investigation
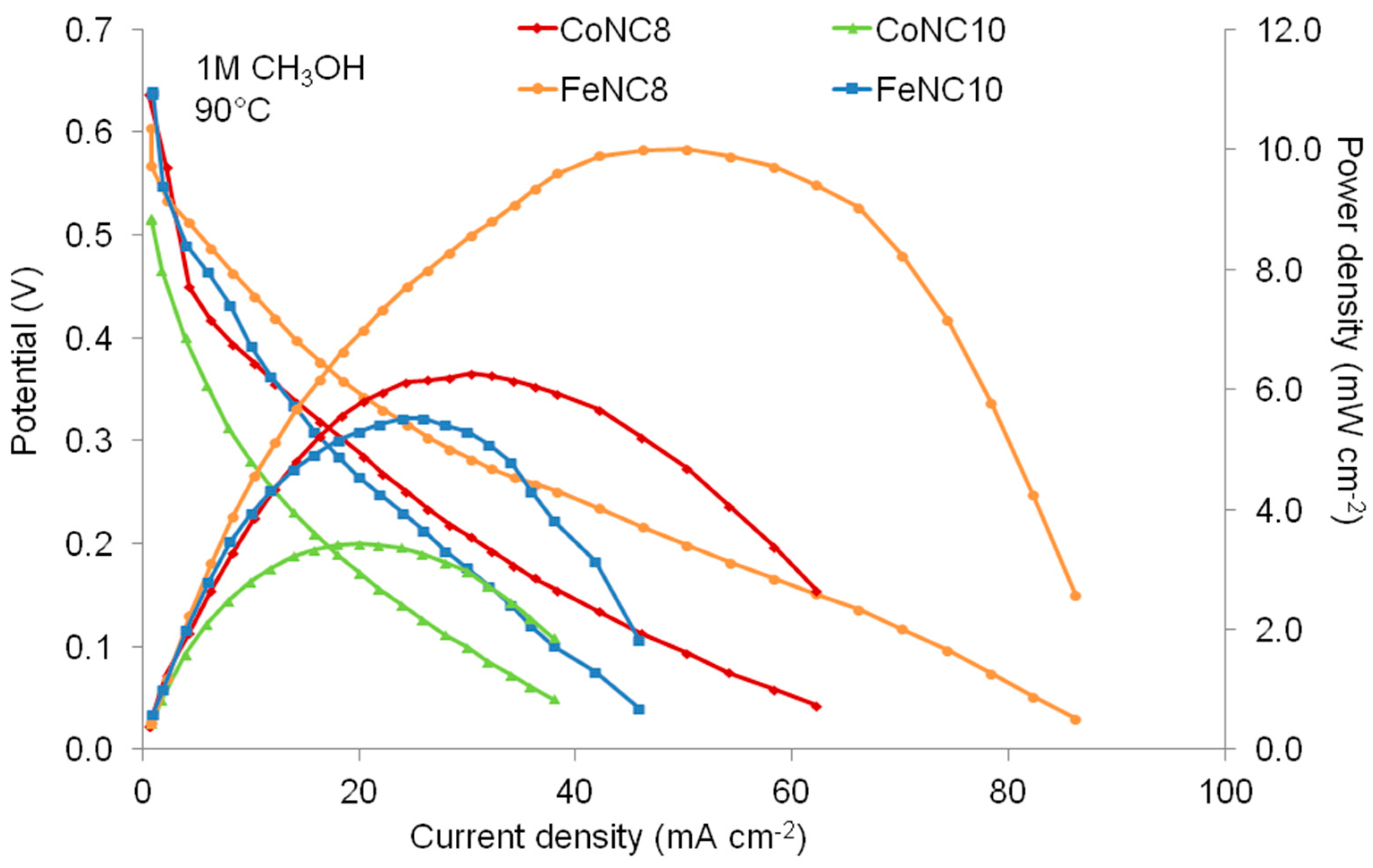
3.3. Direct Methanol Fuel Cell (DMFC) Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wasmus, S.; Küver, A. Methanol oxidation and direct methanol fuel cells: A selective review. J. Electroanal. Chem. 1999, 461, 14–31. [Google Scholar] [CrossRef]
- Serov, A.; Kwak, C. Review of non-platinum anode catalysts for DMFC and PEMFC application. Appl. Catal. B Environ. 2009, 90, 313–320. [Google Scholar] [CrossRef]
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel Cells: Principles, Types, Fuels, and Applications. ChemPhysChem 2000, 1, 162–193. [Google Scholar] [CrossRef]
- Wee, J.H. A feasibility study on direct methanol fuel cells for laptop computers based on a cost comparison with lithium-ion batteries. J. Power Sources 2007, 173, 424–436. [Google Scholar] [CrossRef]
- Sebastián, D.; Stassi, A.; Siracusano, S.; Lo Vecchio, C.; Aricò, A.S.; Baglio, V. Influence of metal oxide additives on the activity and stability of PtRu/C for methanol electro-oxidation. J. Electrochem. Soc. 2015, 162, F713–F717. [Google Scholar] [CrossRef]
- Sebastián, D.; Nieto-Monge, M.J.; Pérez-Rodrìguez, S.; Pastor, E.; Làzaro, M.J. Nitrogen Doped Ordered Mesoporous Carbon as Support of PtRu Nanoparticles for Methanol Electro-Oxidation. Energies 2018, 11, 831. [Google Scholar] [CrossRef]
- Lu, S.; Li, H.; Sun, J.; Zhuang, Z. Promoting the methanol oxidation catalytic activity by introducing surface nickel on platinum nanoparticles. Nano Res. 2018, 11, 2058–2068. [Google Scholar] [CrossRef]
- Baglio, V.; Arico, A.S.; Stassi, A.; D’Urso, C.; Di Blasi, A.; Castro Luna, A.M.; Antonucci, V. Investigation of Pt-Fe catalysts for oxygen reduction in low temperature direct methanol fuel cells. J. Power Sources 2006, 159, 900–904. [Google Scholar] [CrossRef]
- Yang, D.; Yan, Z.; Li, B.; Higgins, D.C.; Wang, J.; Lv, H.; Chen, Z.; Zhang, C. Highly active and durable Pt–Co nanowire networks catalyst for the oxygen reduction reaction in PEMFCs. Int. J. Hydrogen Energy 2016, 41, 18592–18601. [Google Scholar] [CrossRef]
- Salgado, J.R.C.; Antolini, E.; Gonzalez, E.R. Carbon supported Pt-Co alloys as methanol-resistant oxygen-reduction electrocatalysts for direct methanol fuel cells. Appl. Catal. B Environ. 2005, 57, 283–290. [Google Scholar] [CrossRef]
- Alipour Moghadam Esfahani, R.; Rivera Gavidia, L.M.; García, G.; Pastor, E.; Specchia, S. Highly active platinum supported on Mo-doped titanium nanotubes suboxide (Pt/TNTS-Mo) electrocatalyst for oxygen reduction reaction in PEMFC. Renew. Energy 2018, 120, 209–219. [Google Scholar] [CrossRef]
- Antolini, E. Formation of carbon-supported PtM alloys for low temperature fuel cells: A review. Mater. Chem. Phys. 2003, 78, 563–573. [Google Scholar] [CrossRef]
- Aricò, A.S.; Sebastian, D.; Schuster, M.; Bauer, B.; D’Urso, C.; Lufrano, F.; Baglio, V. Selectivity of direct methanol fuel cell membranes. Membranes 2015, 5, 793–809. [Google Scholar] [CrossRef] [PubMed]
- Ercelik, M.; Ozden, A.; Devrim, Y.; Colpan, C.O. Investigation of Nafion based composite membranes on the performance of DMFCs. Int. J. Hydrogen Energy 2017, 42, 2658–2668. [Google Scholar] [CrossRef]
- Borghei, M.; Kanninen, P.; Lundahl, M.; Susi, T.; Sainio, J.; Anoshkin, I.; Nasibulin, A.; Kallio, T.; Tammeveski, K.; Kauppinen, E.; et al. High oxygen reduction activity of few-walled carbon nanotubes with low nitrogen content. Appl. Catal. B Environ. 2014, 158–159, 233–241. [Google Scholar] [CrossRef]
- Yu, Z.; Piao, J.; Liang, Z. Synthesis of 2D nitrogen-doped mesoporous carbon catalyst for oxygen reduction reaction. Materials 2017, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.; Long, G.F.; Liu, M.Y.; Du, L.; Liang, Z.X.; Tsiakaras, P. Nitrogen-doped ordered mesoporous carbon: Synthesis and active sites for electrocatalysis of oxygen reduction reaction. Appl. Catal. B Environ. 2015, 165, 566–571. [Google Scholar] [CrossRef]
- Perazzolo, V.; Durante, C.; Pilot, R.; Paduano, A.; Zheng, J.; Rizzi, G.A.; Martucci, A.; Granozzi, G.; Gennaro, A. Nitrogen and sulfur doped mesoporous carbon as metal-free electrocatalysts for the in situ production of hydrogen peroxide. Carbon 2015, 95, 949–963. [Google Scholar] [CrossRef]
- Tan, A.D.; Wang, Y.F.; Fu, Z.Y.; Tsiakaras, P.; Liang, Z.X. Highly effective oxygen reduction reaction electrocatalysis: Nitrogen-doped hierarchically mesoporous carbon derived from interpenetrated nonporous metal-organic frameworks. Appl. Catal. B Environ. 2017, 218, 260–266. [Google Scholar] [CrossRef]
- Osmieri, L.; Monteverde Videla, A.H.A.; Ocón, P.; Specchia, S. Kinetics of Oxygen Electroreduction on Me–N–C (Me = Fe, Co, Cu) Catalysts in Acidic Medium: Insights on the Effect of the Transition Metal. J. Phys. Chem. C 2017, 121, 17796–17817. [Google Scholar] [CrossRef]
- Osmieri, L.; Escudero-Cid, R.; Monteverde Videla, A.H.A.; Ocón, P.; Specchia, S. Application of a non-noble Fe–N–C catalyst for oxygen reduction reaction in an alkaline direct ethanol fuel cell. Renew. Energy 2018, 115, 226–237. [Google Scholar] [CrossRef]
- Sebastián, D.; Serov, A.; Matanovic, I.; Artyushkova, K.; Atanassov, P.; Aricò, A.S. Nano Energy Insights on the extraordinary tolerance to alcohols of Fe–N–C cathode catalysts in highly performing direct alcohol fuel cells. Nano Energy 2017, 34, 195–204. [Google Scholar] [CrossRef]
- Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Monteverde Videla, A.H.A.; Sebastián, D.; Vasile, N.S.; Osmieri, L.; Aricò, A.S.; Baglio, V.; Specchia, S. Performance analysis of Fe–N–C catalyst for DMFC cathodes: Effect of water saturation in the cathodic catalyst layer. Int. J. Hydrogen Energy 2016, 41, 22605–22618. [Google Scholar] [CrossRef]
- Ota, K.; Ohgi, Y.; Nam, K.D.; Matsuzawa, K.; Mitsushima, S.; Ishihara, A. Development of group 4 and 5 metal oxide-based cathodes for polymer electrolyte fuel cell. J. Power Sources 2011, 196, 5256–5263. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, Q.; Yang, X.; Tavares, A.C.; Sun, S. RRDE experiments on noble-metal and noble-metal-free catalysts: Impact of loading on the activity and selectivity of oxygen reduction reaction in alkaline solution. Appl. Catal. B Environ. 2017, 206, 115–126. [Google Scholar] [CrossRef]
- Simari, C.; Baglio, V.; Lo Vecchio, C.; Aricò, A.S.; Agostino, R.G.; Coppola, L.; Oliviero Rossi, C.; Nicotera, I. Reduced methanol crossover and enhanced proton transport in nanocomposite membranes based on clay−CNTs hybrid materials for direct methanol fuel cells. Ionics 2017, 23, 2113–2123. [Google Scholar] [CrossRef]
- Yilmaz, E.; Can, E. Cross-Linked Poly(aryl ether sulfone) Membranes for Direct Methanol Fuel Cell Applications. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 558–575. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Liu, X.; Dong, J.; Wang, J.; Yang, Z.; Cheng, H. Cross-linked fully aromatic sulfonated polyamide as a highly efficiency polymeric filler in SPEEK membrane for high methanol concentration direct methanol fuel cells. J. Mater. Sci. 2018, 53, 5501–5510. [Google Scholar] [CrossRef]
- Sebastián, D.; Serov, A.; Artyushkova, K.; Gordon, J.; Atanassov, P.; Aricò, A.S.; Baglio, V. High Performance and Cost-Effective Direct Methanol Fuel Cells: Fe-N-C Methanol-Tolerant Oxygen Reduction Reaction Catalysts. ChemSusChem 2016, 9, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Lo Vecchio, C.; Alegre, C.; Sebastiàn, D.; Stassi, A.; Aricò, A.S.; Baglio, V. Investigation of supported Pd-based electrocatalysts for the oxygen reduction reaction: Performance, Durability and Methanol Tolerance. Materials 2015, 8, 7997–8008. [Google Scholar] [CrossRef] [PubMed]
- Asteazaran, M.; Cespedes, G.; Bengió, S.; Moreno, M.S.; Triaca, W.E.; Castro Luna, A.M. Research on methanol-tolerant catalysts for the oxygen reduction reaction. J. Appl. Electrochem. 2015, 45, 1187–1193. [Google Scholar] [CrossRef]
- Li, Q.; Wang, T.; Havas, D.; Zhang, H.; Xu, P.; Han, J.; Cho, J.; Wu, G. High-Performance Direct Methanol Fuel Cells with Precious-Metal-Free Cathode. Adv. Sci. 2016, 3, 1600140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Vecchio, C.; Aricò, A.S.; Monforte, G.; Baglio, V. EDTA-derived Co–N–C and Fe–N–C electro-catalysts for the oxygen reduction reaction in acid environment. Renew. Energy 2018, 120, 342–349. [Google Scholar] [CrossRef]
- Birss, V.; Sirk, A. Oxygen Reduction Catalyst. Patent Application No. US20030228972, 2003. [Google Scholar]
- Subramanian, N.P.; Kumaraguru, S.P.; Colon-Mercado, H.; Kim, H.; Popov, B.N.; Black, T.; Chen, D.A. Studies on Co-based catalysts supported on modified carbon substrates for PEMFC cathodes. J. Power Sources 2006, 157, 56–63. [Google Scholar] [CrossRef]
- Nallathambi, V.; Lee, J.-W.; Kumaraguru, S.P.; Wu, G.; Popov, B.N. Development of high performance carbon composite catalyst for oxygen reduction reaction in PEM Proton Exchange Membrane fuel cells. J. Power Sources 2008, 183, 34–42. [Google Scholar] [CrossRef]
- Baglio, V.; Stassi, A.; Matera, F.V.; Di Blasi, A.; Antonucci, V.; Aricò, A.S. Optimization of properties and operating parameters of a passive DMFC mini-stack at ambient temperature. J. Power Sources 2008, 180, 797–802. [Google Scholar] [CrossRef]
- Gokhale, R.; Chen, Y.; Serov, A.; Artyushkova, K.; Atanassov, P. Direct synthesis of platinum group metal-free Fe-N-C catalyst for oxygen reduction reaction in alkaline media. Electrochem. Commun. 2016, 72, 140–143. [Google Scholar] [CrossRef] [Green Version]
- Sebastiàn, D.; Serov, A.; Artyushkova, K.; Atanassov, P.; Aricò, A.S.; Baglio, V. Performance, methanol tolerance and stability of Fe-aminobenzimidazole derived catalyst for direct methanol fuel cells. J. Power Sources 2016, 319, 235–246. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics: Chanhassen, MN, USA, 1995. [Google Scholar]
- Alegre, C.; Modica, E.; Di Blasi, A.; Di Blasi, O.; Busacca, C.; Ferraro, M.; Aricò, A.S.; Antonucci, V.; Baglio, V. NiCo-loaded carbon nanofibers obtained by electrospinning: Bifunctional behavior as air electrodes. Renew. Energy 2018, 125, 250–259. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, M.; Dai, B. Cobalt-nitrogen-activated carbon as catalyst in acetylene hydrochlorination. Catal. Commun. 2017, 98, 22–25. [Google Scholar] [CrossRef]
- Negro, E.; Videla, A.H.A.M.; Baglio, V.; Aricò, A.S.; Specchia, S.; Koper, G.J.M. Fe–N supported on graphitic carbon nano-networks grown from cobalt as oxygen reduction catalysts for low-temperature fuel cells. Appl. Catal. B Environ. 2015, 166–167, 75–83. [Google Scholar] [CrossRef]
- Jaouen, F.; Herranz, J.; Lefévre, M.; Dodelet, J.P.; Kramm, U.I.; Herrmann, I.; Bogdanoff, P.; Maruyama, J.; Nagaoka, T.; Garsuch, A.; et al. Cross-laboratory experimental study of non-noble-metal electrocatalysts for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2009, 1, 1623–1639. [Google Scholar] [CrossRef] [PubMed]
- Osmieri, L.; Escudero-Cid, R.; Armandi, M.; Ocón, P.; Monteverde Videla, A.H.A.; Specchia, S. Effects of using two transition metals in the synthesis of non-noble electrocatalysts for oxygen reduction reaction in direct methanol fuel cell. Electrochim. Acta 2018, 266, 220–232. [Google Scholar] [CrossRef]
- Sebastián, D.; Baglio, V.; Aricò, A.S.; Serov, A.; Atanassov, P. Performance analysis of a non-platinum group metal catalyst based on iron-aminoantipyrine for direct methanol fuel cells. Appl. Catal. B Environ. 2016, 182, 297–305. [Google Scholar] [CrossRef]
- Piela, B.; Olson, T.S.; Atanassov, P.; Zelenay, P. Highly methanol-tolerant non-precious metal cathode catalysts for direct methanol fuel cell. Electrochim. Acta 2010, 55, 7615–7621. [Google Scholar] [CrossRef]
- Wei, Y.; Shengzhou, C.; Weiming, L. Oxygen reduction on non-noble metal electrocatalysts supported on N-doped carbon aerogel composites. Int. J. Hydrogen Energy 2012, 37, 942–945. [Google Scholar] [CrossRef]
- Xiao, M.; Zhu, J.; Feng, L.; Liu, C.; Xing, W. Meso/macroporous nitrogen-doped carbon architectures with iron carbide encapsulated in graphitic layers as an efficient and robust catalyst for the oxygen reduction reaction in both acidic and alkaline solutions. Adv. Mater. 2015, 27, 2521–2527. [Google Scholar] [CrossRef] [PubMed]
- Baranton, S.; Coutanceau, C.; Léger, J.-M.; Roux, C.; Capron, P. Alternative cathodes based on iron phthalocyanine catalysts for mini- or micro-DMFC working at room temperature. Electrochim. Acta 2005, 51, 517–525. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Vecchio, C.; Aricò, A.S.; Baglio, V. Application of Low-Cost Me-N-C (Me = Fe or Co) Electrocatalysts Derived from EDTA in Direct Methanol Fuel Cells (DMFCs). Materials 2018, 11, 1193. https://doi.org/10.3390/ma11071193
Lo Vecchio C, Aricò AS, Baglio V. Application of Low-Cost Me-N-C (Me = Fe or Co) Electrocatalysts Derived from EDTA in Direct Methanol Fuel Cells (DMFCs). Materials. 2018; 11(7):1193. https://doi.org/10.3390/ma11071193
Chicago/Turabian StyleLo Vecchio, Carmelo, Antonino Salvatore Aricò, and Vincenzo Baglio. 2018. "Application of Low-Cost Me-N-C (Me = Fe or Co) Electrocatalysts Derived from EDTA in Direct Methanol Fuel Cells (DMFCs)" Materials 11, no. 7: 1193. https://doi.org/10.3390/ma11071193






