Environmentally-Friendly Extraction of Cellulose Nanofibers from Steam-Explosion Pretreated Sugar Beet Pulp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Cellulose Nanofibers (CNFs)
2.2.1. Steam-Explosion Pretreatment
2.2.2. Isolation of CNFs
2.3. Characterization
2.3.1. Fourier Transform–Infrared (FT-IR) Analysis
2.3.2. Thermal Properties Analysis
2.3.3. X-ray Diffraction (XRD) Analysis
2.3.4. Morphology Analysis
3. Results and Discussion
3.1. FT-IR Analysis
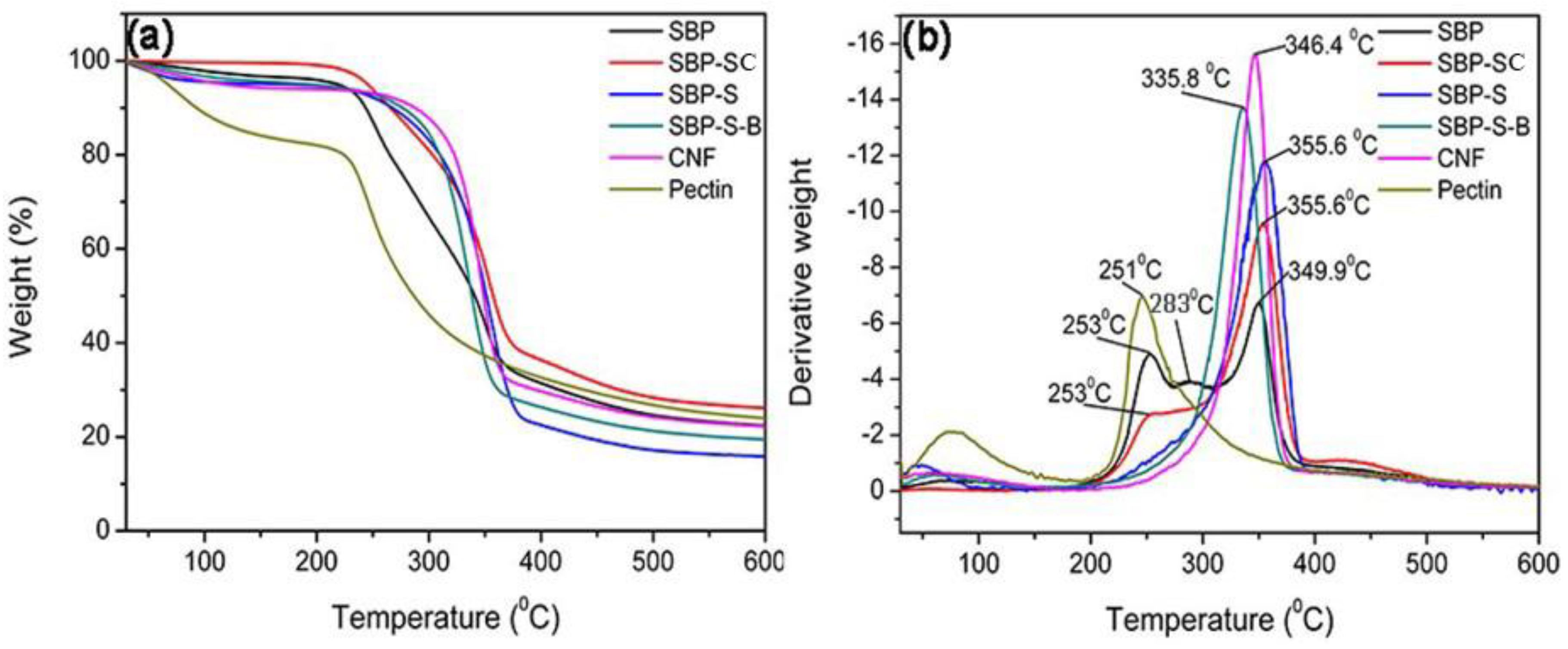
3.2. Thermal Properties
3.3. XRD Analysis
3.4. Morphological Analysis of the CNFs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dinand, E.; Chanzy, H.; Vignon, R.M. Suspensions of cellulose microfibrils from sugar beet pulp. Food Hydrocoll. 1999, 13, 275–283. [Google Scholar] [CrossRef]
- Weibel, M.K. Parenchymal Cell Cellulose and Related Materials. U.S. Patent No. 4,831,127, 16 May 1989. [Google Scholar]
- Lv, C.; Wang, Y.; Wang, L.J.; Li, D.; Adhikari, B. Optimization of production yield and functional properties of pectin extracted from sugar beet pulp. Carbohyd. Polym. 2013, 95, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Fu, X.; Luo, Z.G. Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem. 2015, 168, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Delgado-Aguilar, M.; Tovar, I.G.; Tarrés, Q.; Alcalá, M.; Pèlach, M.À.; Mutjé, P. Approaching a low-cost production of cellulose nanofibers for papermaking application. Bioresources 2015, 10, 5345–5355. [Google Scholar] [CrossRef]
- Oishi, Y.; Nakaya, M.; Matsui, E.; Hotta, A. Structural and mechanical properties of cellulose composites made of isolated cellulose nanofibers and poly (vinyl alcohol). Compos. Part A Appl. S. Manuf. 2015, 73, 72–79. [Google Scholar] [CrossRef]
- Kelly, J.A.; Shukaliak, A.M.; Cheung, C.C.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Responsive photonic hydrogels based on nanocrystalline cellulose. Angew. Chem. Int. Ed. 2013, 52, 8912–8916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Lu, C.; Deng, Y. Aerogels from crosslinked cellulose nano/micro-fibrils and their fast shape recovery property in water. J. Mater. Chem. 2012, 22, 11642–11650. [Google Scholar] [CrossRef]
- Chen, W.; Abe, K.; Uetani, K.; Yu, H.; Liu, Y.; Yano, H. Individual cotton cellulose nanofibers: Pretreatment and fibrillation technique. Cellulose 2014, 21, 1517–1528. [Google Scholar] [CrossRef]
- Andrade-Mahecha, M.M.; Pelissari, F.M.; Tapia-Blácido, D.R.; Menegalli, F.C. Achira as a source of biodegradable materials: Isolation and characterization of nanofibers. Carbohyd. Polym. 2015, 123, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Wei, X.; Wang, F.; Han, D.; Wang, Q.; Kong, L. Homogeneous isolation of nanocelluloses by controlling the shearing force and pressure in microenvironment. Carbohyd. Polym. 2014, 113, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Gao, W.; Chen, L.; Lan, W.; Zhu, J.Y.; Runge, T. A comparison of cellulose nanofibrils produced from Cladophora glomerata algae and bleached eucalyptus pulp. Cellulose 2016, 23, 493–503. [Google Scholar] [CrossRef]
- Vartiainen, J.; Pöhler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Hiekkataipale, P. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- Hoeger, I.C.; Nair, S.S.; Ragauskas, A.J.; Deng, Y.; Rojas, O.J.; Zhu, J.Y. Mechanical deconstruction of lignocelluloses cell walls and their enzymatic saccharification. Cellulose 2013, 20, 807–818. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Huang, Z.; Liu, S. Nanocellulose fibrils isolated from BHKP using ultrasonication and their reinforcing properties in transparent poly (vinyl alcohol) films. J. Polym. Res. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Jiang, Z.; Yu, Z.; Yu, Y. Isolating nanocellulose fibrils from bamboo parenchymal cells with high intensity ultrasonication. Holzforschung 2016, 70, 401–409. [Google Scholar] [CrossRef]
- Janardhnan, S.; Sain, M.M. Isolation of cellulose microfibrils-an enzymatic approach. Bioresources 2006, 1, 176–188. [Google Scholar]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues-Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, L.J.; Li, D.; Cheng, Y.L.; Adhikari, B. Preparation and characterization of cellulose nanofibers from de-pectinated sugar beet pulp. Carbohyd. Polym. 2014, 102, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Agoda-Tandjawa, G.; Durand, S.; Berot, S.; Blassel, C.; Gaillard, C.; Garnier, C.; Doublier, J.L. Rheological characterization of microfibrillated cellulose suspensions after freezing. Carbohyd. Polym. 2010, 80, 677–686. [Google Scholar] [CrossRef]
- Stelte, W.; Sanadi, A.R. Preparation and characterization of cellulose nanofibers from two commercial hardwood and softwood pulps. Ind. Eng. Chem. Res. 2009, 48, 11211–11219. [Google Scholar] [CrossRef]
- Sun, X.F.; Xu, F.; Sun, R.C.; Fowler, P.; Baird, M.S. Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohyd. Res. 2005, 340, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Cherian, B.M.; Leão, A.L.; de Souza, S.F.; Thomas, S.; Pothan, L.A.; Kottaisamy, M. Isolation of nanocellulose from pineapple leaf fibres by steam explosion. Carbohyd. Polym. 2010, 81, 720–725. [Google Scholar] [CrossRef]
- Kaushik, A.; Singh, M. Isolation and characterization of cellulose nanofibrils from wheat straw using steam explosion coupled with high shear homogenization. Carbohyd. Res. 2011, 346, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Manhas, N.; Balasubramanian, K.; Prajith, P.; Rule, P.; Nimje, S. PCL/PVA nanoencapsulated reinforcing fillers of steam exploded/autoclaved cellulose nanofibrils for tissue engineering applications. RSC Adv. 2015, 5, 23999–24008. [Google Scholar] [CrossRef]
- Phatak, L.; Chang, K.C.; Brown, G. Isolation and characterization of pectin in sugar-beet pulp. J. Food Sci. 1988, 53, 830–833. [Google Scholar] [CrossRef]
- Segal, L.C.; Creely, J.; Martin, A.E.J.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Zeronian, S.H.; Inglesby, M.K. Bleaching of cellulose by hydrogen peroxide. Cellulose 1995, 2, 265–272. [Google Scholar] [CrossRef]
- Fan, M.; Dai, D.; Huang, B. Fourier transform infrared spectroscopy for natural fibers. In Fourier Transform Mater. Anal; Salih, S., Ed.; InTech: Shanghai, China, 2012; Volume 3, pp. 45–69. ISBN 978-953-51-0594-7. [Google Scholar]
- Kaliyan, N.; Morey, R.V. Factors affecting strength and durability of densified biomass products. Biomass Bioenergy 2009, 33, 337–359. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicelluloses, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—a review. Cell. Chem. Technol. 2010, 44, 353. [Google Scholar]
- Aburto, J.; Moran, M.; Galano, A.; Torres-Garcia, E. Non-isothermal pyrolysis of pectin: A thermochemical and kinetic approach. J. Anal. Appl. Pyrol. 2015, 112, 94–104. [Google Scholar] [CrossRef]
- Chirayil, C.J.; Joy, J.; Mathew, L.; Mozetic, M.; Koetz, J.; Thomas, S. Isolation and characterization of cellulose nanofibrils from Helicteres isora plant. Ind. Crops Prod. 2014, 59, 27–34. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, T.; Feng, Y.; Qu, J.; He, H.; Yu, X. Isolating cellulose nanofibers from steam-explosion pretreated corncobs using mild mechanochemical treatments. Bioresources 2017, 12, 9183–9197. [Google Scholar]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohyd. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, T.; Yang, W.; Ma, P.; He, H.; Yin, X.; Yu, X. Characteristics and environmentally friendly extraction of cellulose nanofibrils from sugarcane bagasse. Ind. Crop. Prod. 2018, 111, 285–291. [Google Scholar] [CrossRef]
- Uetani, K.; Yano, H. Nanofibrillation of wood pulp using a high-speed blender. Biomacromolecules 2010, 12, 348–353. [Google Scholar] [CrossRef] [PubMed]


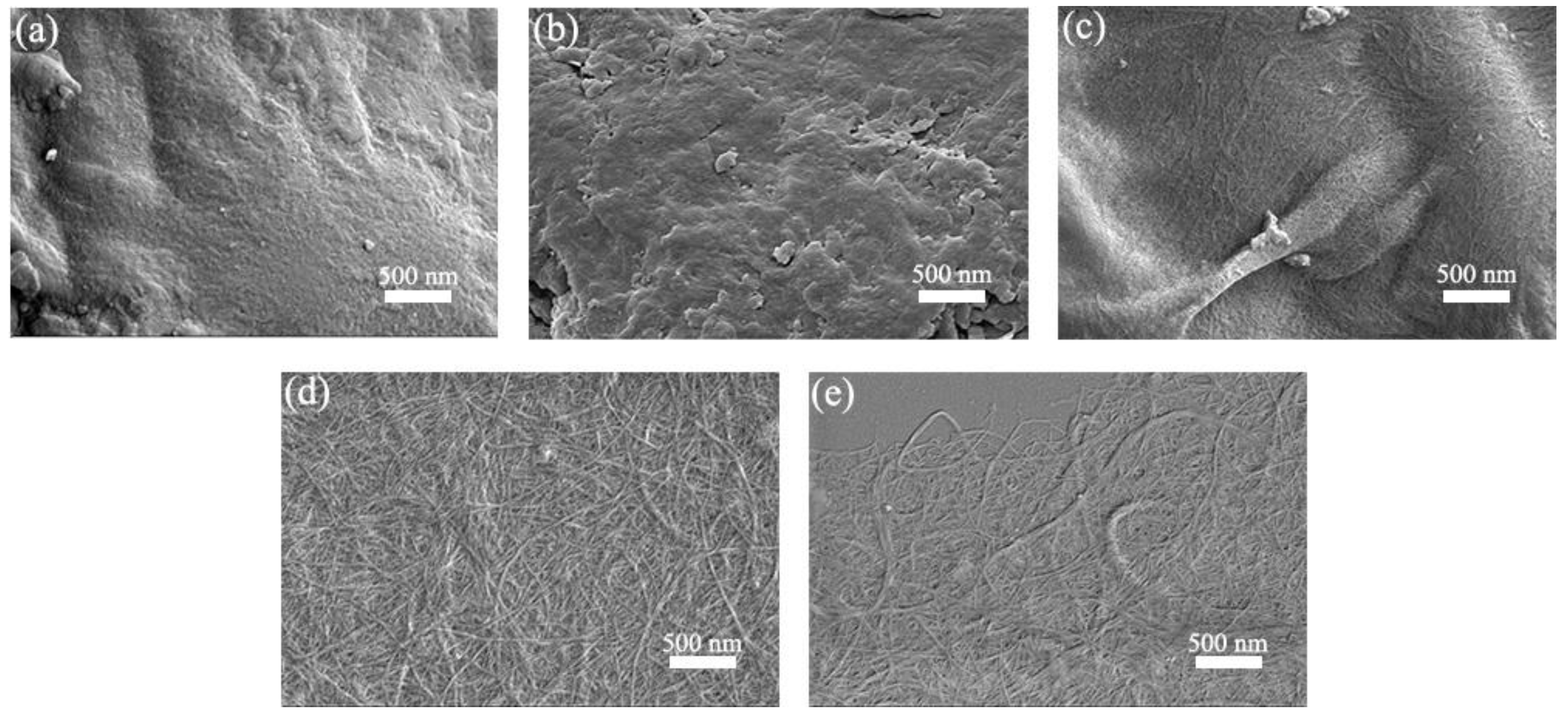

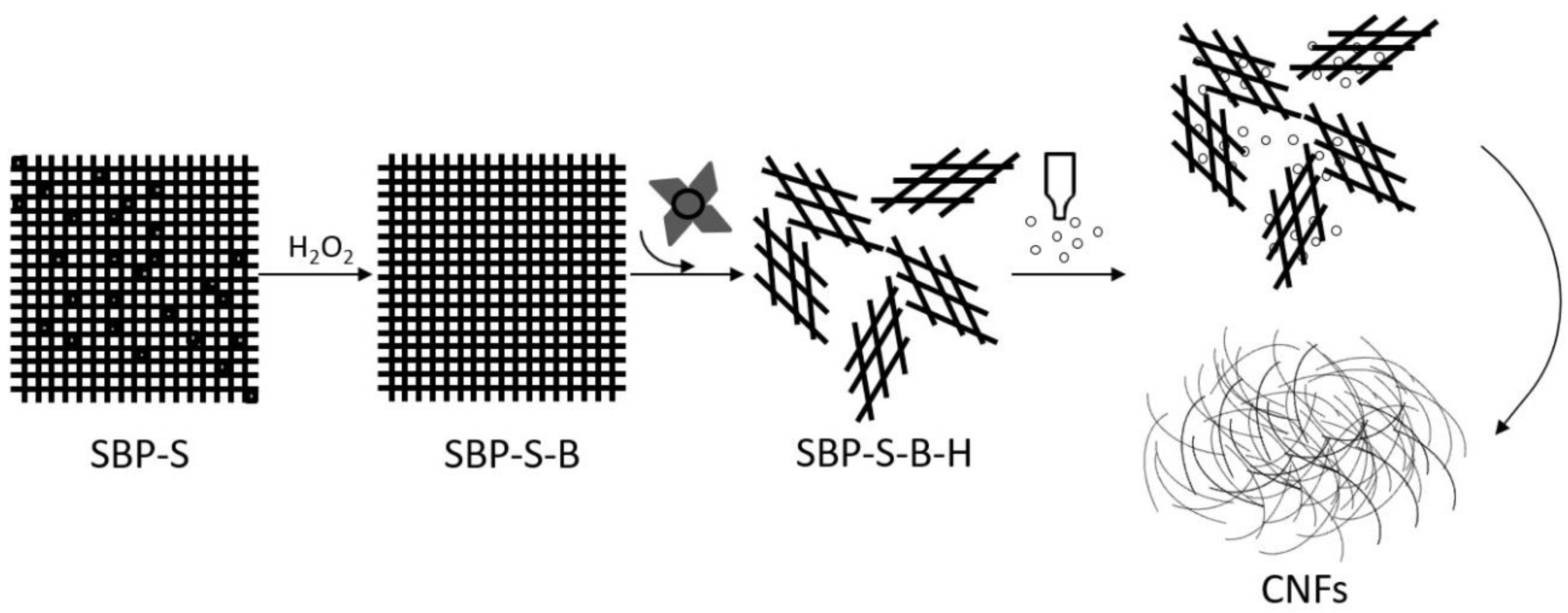
| Sample | Treatment |
|---|---|
| SBP | sugar beet pulp |
| SBP-SC | steam-cooked sbp |
| SBP-S | steam-explosion treated SBP |
| SBP-S-B | bleached SBP-S |
| SBP-S-B-H | high-speed blending treated SBP-S-B |
| CNF | cellulose nanofibers |
| Sample | Maximum Degradation Temperature (°C) | Crystallinity Index (%) |
|---|---|---|
| SBP | 253, 283, 349.9 | 29.31 |
| SBP-SC | 253, 355.6 | 37.12 |
| SBP-S | 355.6 | 47.79 |
| SBP-S-B | 335.8 | 59.01 |
| CNF | 346.4 | 62.30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Feng, Y.; He, H.; Yang, Z. Environmentally-Friendly Extraction of Cellulose Nanofibers from Steam-Explosion Pretreated Sugar Beet Pulp. Materials 2018, 11, 1160. https://doi.org/10.3390/ma11071160
Yang W, Feng Y, He H, Yang Z. Environmentally-Friendly Extraction of Cellulose Nanofibers from Steam-Explosion Pretreated Sugar Beet Pulp. Materials. 2018; 11(7):1160. https://doi.org/10.3390/ma11071160
Chicago/Turabian StyleYang, Wengang, Yanhong Feng, Hezhi He, and Zhitao Yang. 2018. "Environmentally-Friendly Extraction of Cellulose Nanofibers from Steam-Explosion Pretreated Sugar Beet Pulp" Materials 11, no. 7: 1160. https://doi.org/10.3390/ma11071160





