A Study on Dissimilar Friction Stir Welded between the Al–Li–Cu and the Al–Zn–Mg–Cu Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
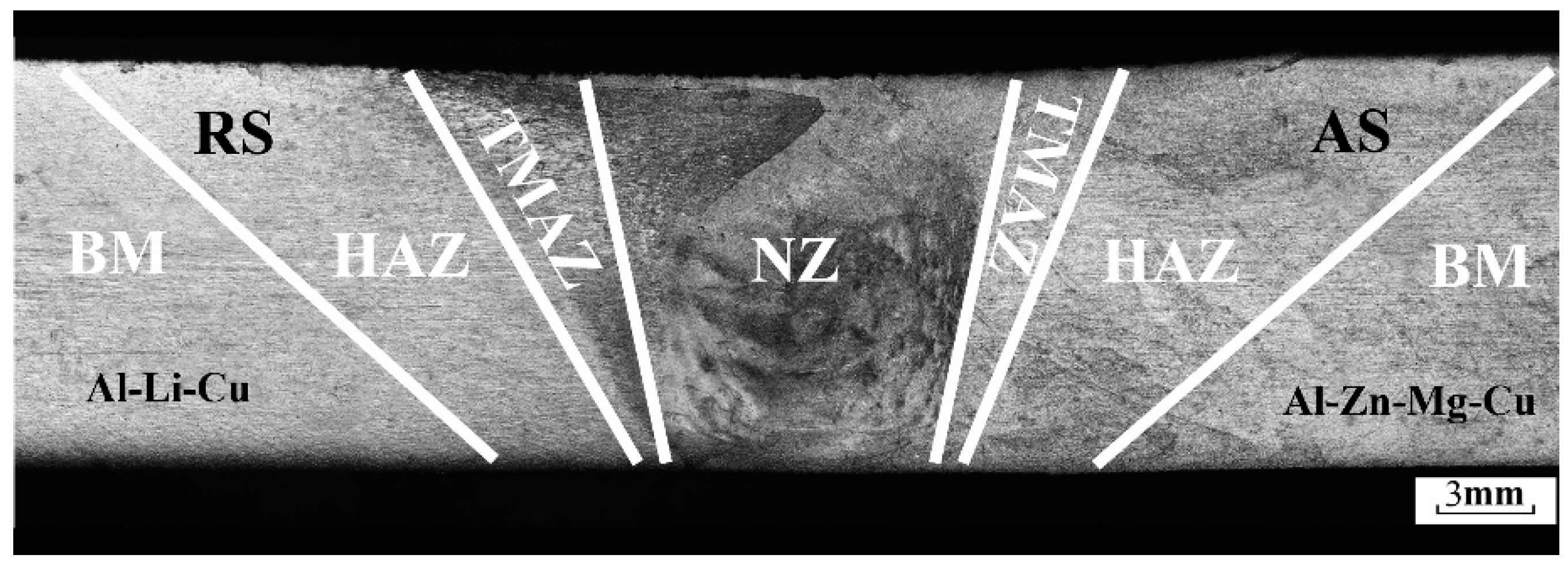
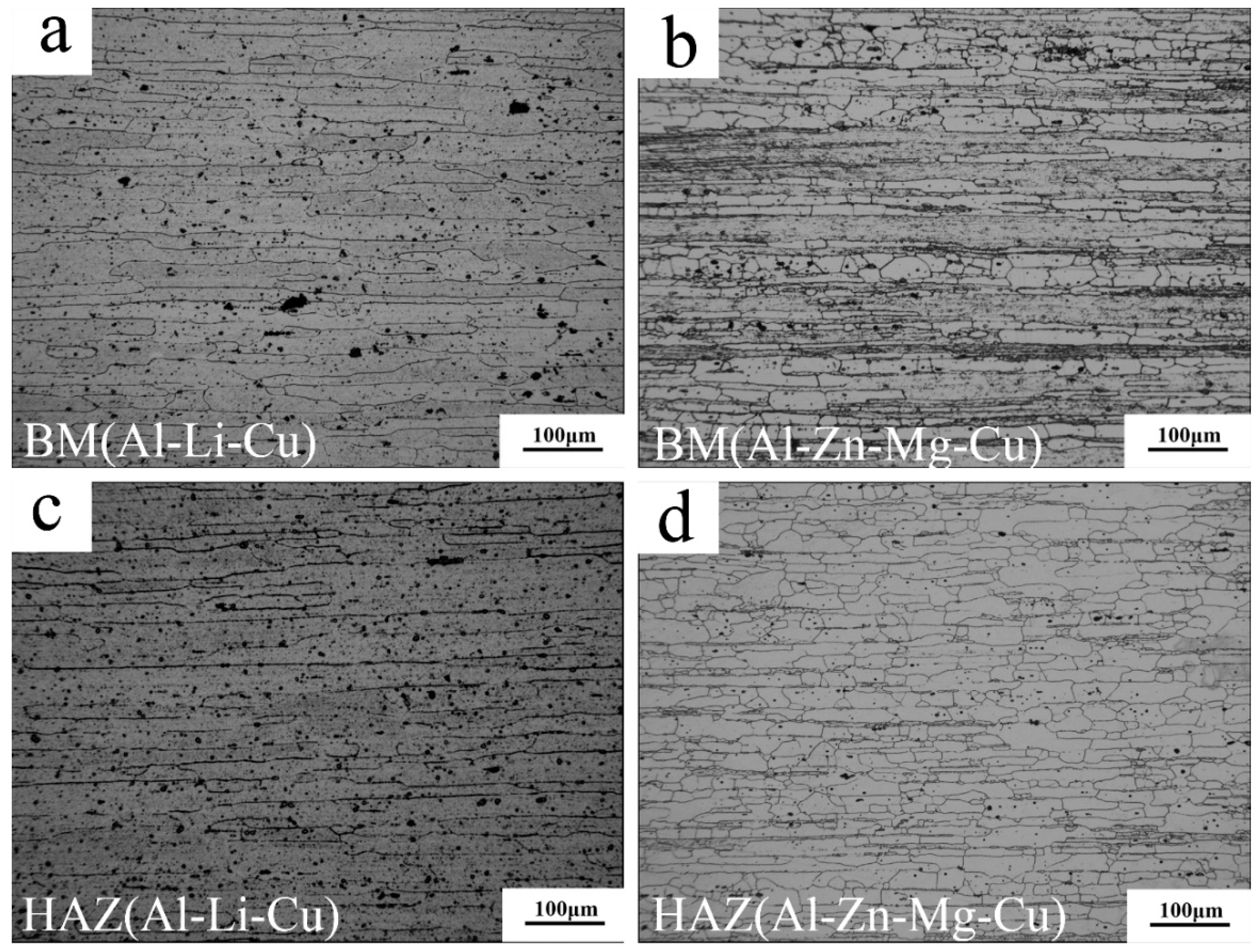
3.1. Microstructure
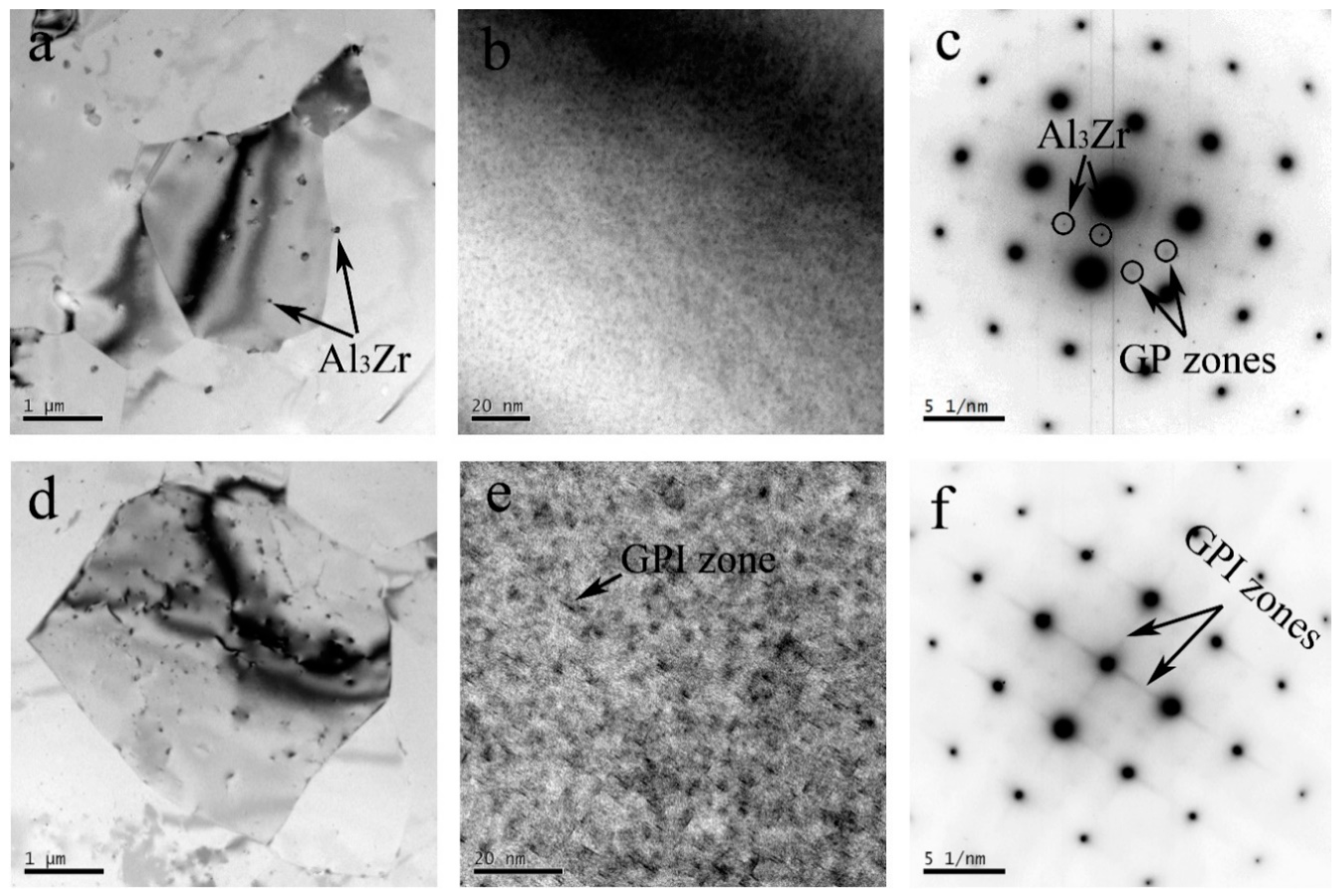
3.2. TEM Observation
3.3. Microhardness of the Weld Zone
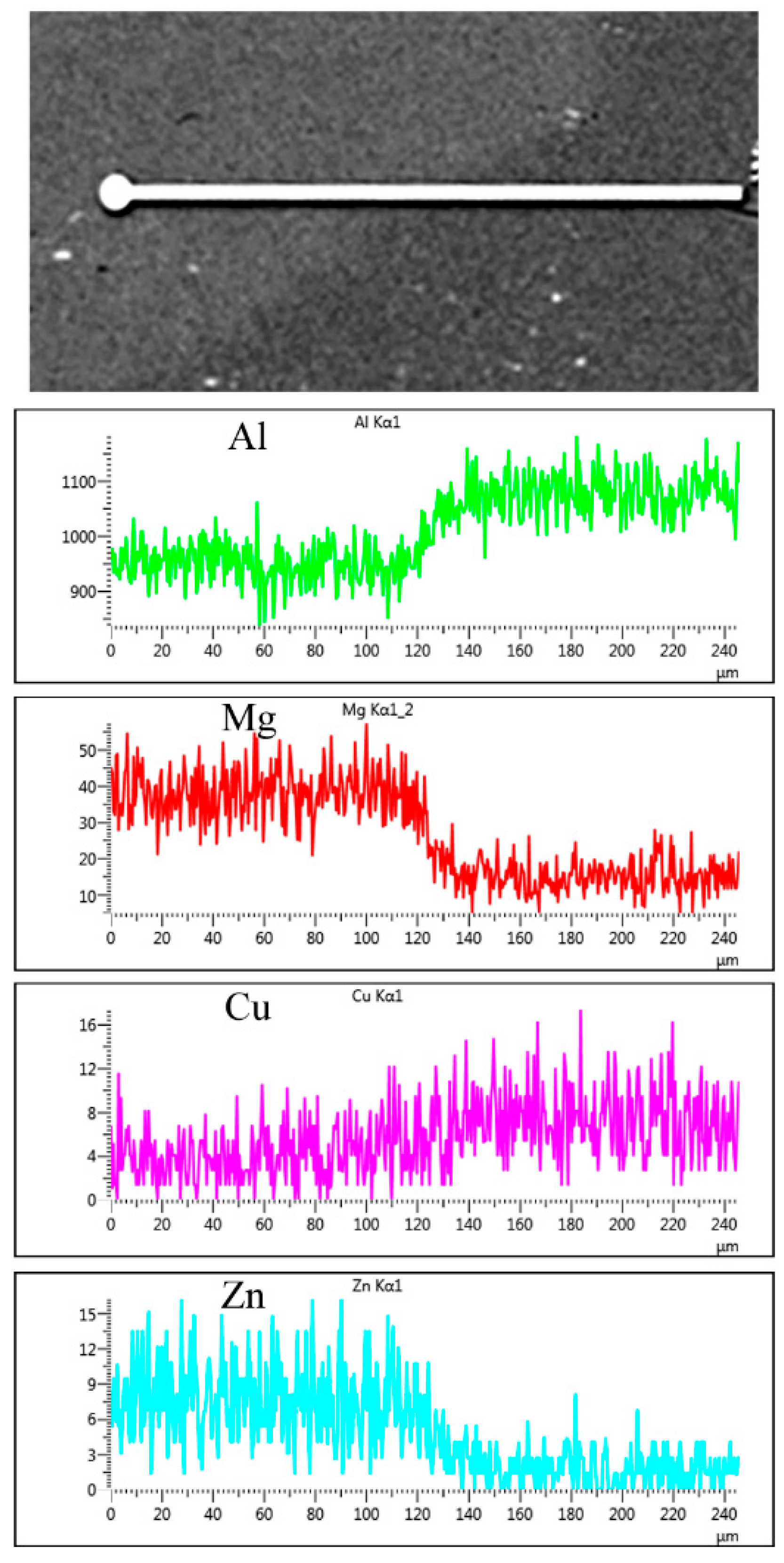
3.4. Diffusion at Periphery of the Kissing Line
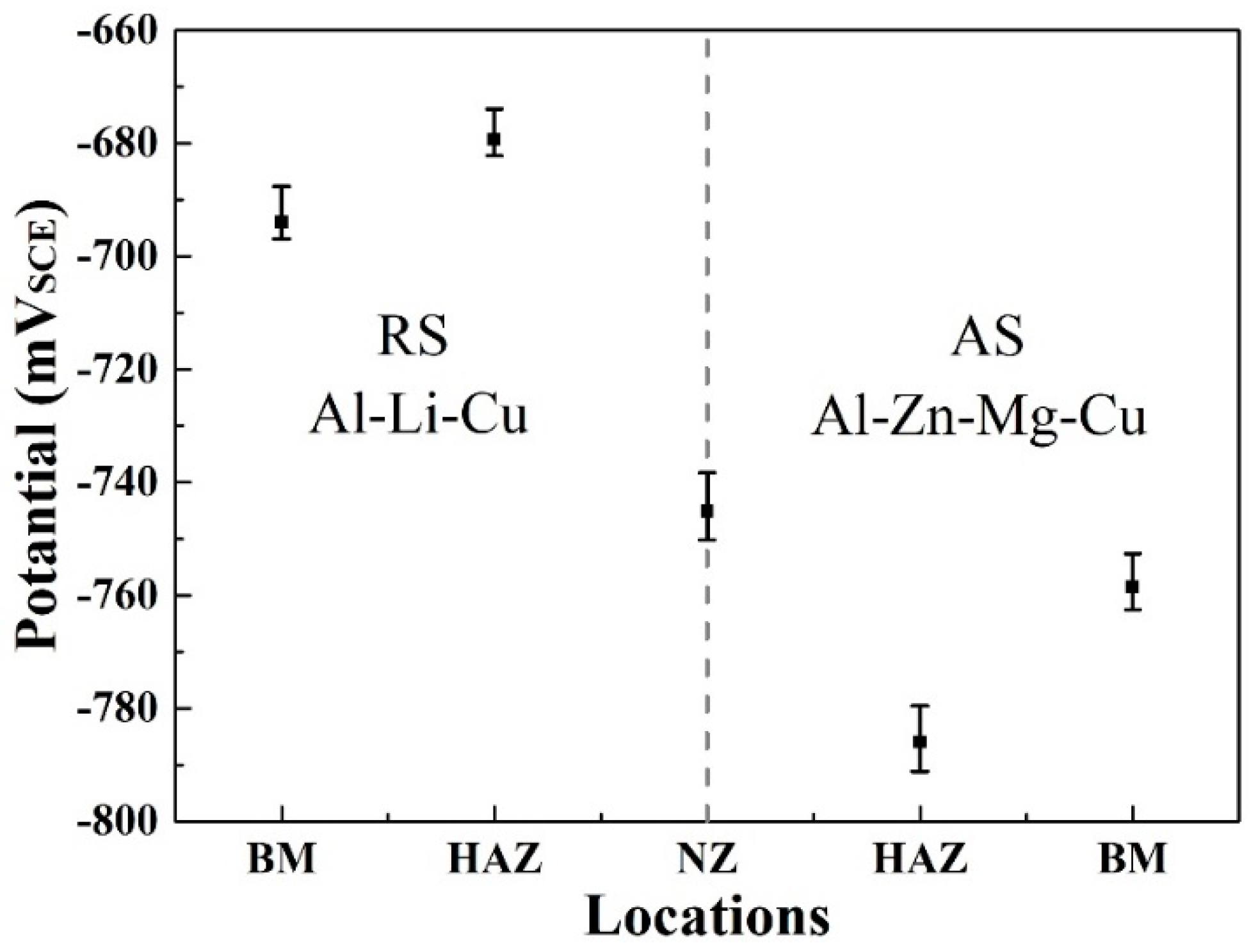
3.5. Corrosion Behavior
4. Discussion
5. Conclusions
- (1)
- During the friction stir welding process, the original η′ phase precipitates in the HAZ of the Al–Zn–Mg–Cu alloy subjected to the thermal transient, coarsening, and dissolving process, occurred simultaneously. The GP zones appeared during the subsequent nature ageing. In the HAZ of the Al–Li–Cu alloy, the θ′ phase dissolved seriously, but most of the T1 precipitates only coarsened, and the density of the T1 precipitates slight decreased, because the thermal stability of the T1 precipitates is much more stable than the θ′ phase precipitates. Because of the nature ageing after welding, the NZ of the Al–Zn–Mg–Cu alloy only contains GP zones, and the NZ of the Al–Li–Cu alloy only contains GP Izones.
- (2)
- During the friction stir welding process, the thermal that came from the NZ led to a different width of the HAZ for two types of alloys. The HAZ of the Al–Zn–Mg–Cu alloy is 7 mm wider than that of the Al–Li–Cu alloy. It is directly observed by the microhardness profile. Thus, the thermal stable temperature of the η′ phase precipitates in Al–Zn–Mg–Cu alloys is approximate to 180 °C, but that of T1 precipitates in Al–Li–Cu alloys is close to 260 °C.
- (3)
- There is only a diffusion of the magnesium element at the periphery of the kissing line during the welding process. However, the content of copper and zinc have no change because of the low diffusion coefficient in the aluminum matrix.
- (4)
- The HAZ of the Al–Zn–Mg–Cu alloy has the most negative open circuit potential compared with the other welding zones, and is responsible for the severely localized corrosion. Additionally, all of the zones of the Al–Li–Cu alloy show a good corrosion resistance and have a more positive potential compared with the Al–Zn–Mg–Cu ally.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Polmear, I.J.; Chester, R.J. Abnormal age hardening in an Al-Cu-Mg alloy containing silver and lithium. Scr. Mater. 1989, 23, 1213–1217. [Google Scholar] [CrossRef]
- Tsivoulas, D.; Robson, J.D.; Sigli, C.; Prangnella, P.B. Interactions between zirconium and manganese dispersoid-forming elements on their combined addition in Al–Cu–Li alloys. Acta Mater. 2012, 60, 5245–5259. [Google Scholar] [CrossRef]
- Huang, B.P.; Zheng, Z.Q. Effects of Li Content on Precipitation in Al-Cu-(Li)-Mg-Ag-Zr Alloys. Scr. Mater. 1998, 38, 357–362. [Google Scholar] [CrossRef]
- Woo, K.D.; Kim, S.W. The mechanical properties and precipitation behavior of an Al-Cu-Li-(In,Be) alloy. J. Mater. Sci. 2002, 37, 411–416. [Google Scholar] [CrossRef]
- Khachaturyan, A.G.; Lindsey, T.F.; Morris, J.W. Theoretical investigation of the precipitation of δ’ in Al-Li. Metall. Mater. Trans. A. 1988, 19, 249–258. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Moran, J.P.; Stoner, G.E. Localized corrosion behavior of alloy2090-the role of microstructure heterogeneity. Corrosion 1990, 46, 610–617. [Google Scholar] [CrossRef]
- Niskanen, P.; Sanders, T.H.; Rinker, J.G.; Marek, M. Corrosion of aluminum alloys containing lithium. Corros. Sci. 1982, 22, 283–304. [Google Scholar] [CrossRef]
- Proton, V.; Alexis, J.; Andrieu, E.; Delfosse, J.; Deschamps, A.; Geuser, F.D.; Lafont, M.C.; Blanc, C. The influence of artificial ageing on the corrosion behavior of a 2050 aluminium–copper–lithium alloy. Corros. Sci. 2014, 80, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Starke, E.A., Jr.; Staley, J.T. Application of modern aluminum alloys to aircraft. Aerosp. Sci. Technol. 1996, 32, 131–172. [Google Scholar] [CrossRef]
- Fang, X.; Du, Y.; Song, M.; Li, K.; Jiang, C. Effects of Cu content on the precipitation process of Al–Zn–Mg alloys. J. Mater. Sci. 2012, 47, 8174–8187. [Google Scholar] [CrossRef]
- Bendo, A.; Matsuda, K.; Lee, S.; Nishimura, K.; Nunomura, N.; Toda, H.; Yamaguchi, M.; Tsuru, T.; Hirayama, K.; Shimizu, K.; et al. Atomic scale HAADF-STEM study of η′, and η1, phases in peak-aged Al–Zn–Mg alloys. J. Mater. Sci. 2018, 53, 4598–4611. [Google Scholar] [CrossRef]
- Thomas, W.M.; Nicholas, E.D.; Needham, J.C.; Murch, M.G.; Temple-smith, P.; Dawes, C.J. Friction Welding. U.S. Patent 54603171995, 24 October 1995. [Google Scholar]
- Mishra, R.S.; Ma, Z.Y. Friction stir welding and processing. Mater. Sci. Eng. R. 2005, 50, 1–78. [Google Scholar] [CrossRef]
- Shukla, A.K.; Baeslack, W.A., III. Study of microstructural evolution in friction-stir welded thin-sheet Al–Cu–Li alloy using transmission-electron microscopy. Scr. Mater. 2007, 56, 513–516. [Google Scholar] [CrossRef]
- Su, J.Q.; Nelson, T.W.; Mishra, R. Microstructural investigation of friction stir welded 7050-T651 aluminium. Acta Mater. 2003, 51, 713–729. [Google Scholar] [CrossRef]
- Ahmed, M.M.Z.; Ataya, S.; Seleman, E.S.; Ammar, H.R.; Ahmed, E. Friction stir welding of similar and dissimilar AA7075 and AA5083. J. Mater. Process. Technol. 2017, 242, 77–91. [Google Scholar] [CrossRef]
- Jamshidi Aval, H.; Serajzadeh, S.; Kokabi, A.H.; Loureiro, A. Effect of tool geometry on mechanical and microstructural behaviours in dissimilar friction stir welding of AA5086–AA6061. Sci. Technol. Weld. Join. 2013, 16, 597–604. [Google Scholar] [CrossRef]
- Aval, H.J.; Serajzadeh, S.; Kokabi, A.H. Evolution of microstructures and mechanical properties in similar and dissimilar friction stir welding of AA5086 and AA6061. Mater. Sci. Eng. A 2011, 528, 8071–8083. [Google Scholar] [CrossRef]
- Aval, H.J.; Serajzadeh, S.; Sakharova, N.A.; Kokabi, A.H.; Loureiro, A. A study on microstructures and residual stress distributions in dissimilar friction-stir welding of AA5086–AA6061. J. Mater. Sci. 2012, 47, 5428–5437. [Google Scholar] [CrossRef]
- Aval, H.J.; Serajzadeh, S.; Kokabi, A.H. Thermo-mechanical and microstructural issues in dissimilar friction stir welding of AA5086–AA6061. J. Mater. Sci. 2011, 46, 3258–3268. [Google Scholar] [CrossRef]
- Khodir, S.A.; Shibayanagi, T. Friction stir welding of dissimilar AA2024 and AA7075 aluminum alloys. Mater. Sci. Eng. B 2008, 148, 82–87. [Google Scholar] [CrossRef]
- Rodriguez, R.I.; Jordon, J.B.; Allison, P.G.; Rushing, T.; Garcia, L. Microstructure and mechanical properties of dissimilar friction stir welding of 6061-to-7050 aluminum alloys. Mater. Des. 2015, 83, 60–65. [Google Scholar] [CrossRef]
- Senkov, O.N.; Shagiev, M.R.; Senkova, S.V.; Miracle, D.B. Precipitation of Al(Sc,Zr) particles in an Al–Zn–Mg–Cu–Sc–Zr alloy during conventional solution heat treatment and its effect on tensile properties. Acta Mater. 2008, 56, 3723–3738. [Google Scholar] [CrossRef]
- Deng, Y.L.; Wan, L.; Wu, L.H.; Zhang, Y.Y.; Zhang, X.M. Microstructural evolution of Al–Zn–Mg–Cu alloy during homogenization. J. Mater. Sci. 2011, 46, 875–881. [Google Scholar] [CrossRef]
- Chokshi, A.H.; Rosen, A.; Karch, J.; Gleiter, H. On the validity of the hall-petch relationship in nanocrystalline materials. Scr. Metall. 1989, 23, 1679–1683. [Google Scholar] [CrossRef]
- Zhu, A.W.; Starke, E.A., Jr. Strengthening effect of unshearable particles of finite size: A computer experimental study. Acta Mater. 1999, 47, 3263–3269. [Google Scholar] [CrossRef]
- Jariyaboon, M.; Davenport, A.J.; Ambat, R.; Connolly, B.J.; Williams, S.W.; Price, D.A. The effect of welding parameters on the corrosion behaviour of friction stir welded AA2024–T351. Corros. Sci. 2007, 49, 877–909. [Google Scholar] [CrossRef]
- Malard, B.; Geuser, F.D.; Deschamps, A. Microstructure distribution in an AA2050 T34 friction stir weld and its evolution during post-welding heat treatment. Acta Mater. 2015, 101, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Adler, P.N.; Deiasi, R. Calorimetric studies of 7000 series aluminum alloys: II. Comparison of 7075, 7050 and RX720 alloys. Metall. Trans. A 1977, 8, 1185–1190. [Google Scholar] [CrossRef]
- Deng, Y.; Ye, R.; Xu, G.; Yang, J.; Pan, Q.; Peng, B.; Cao, X.; Duan, Y.; Wang, Y.; Lu, L.; et al. Corrosion behaviour and mechanism of new aerospace Al–Zn–Mg alloy friction stir welded joints and the effects of secondary Al3ScxZr1−x, nanoparticles. Corros. Sci. 2015, 90, 359–374. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Moran, J.P.; Stoner, G.E. Electrochemical behavior of the T1(Al2CuLi) intermetallic compound and its role in localized corrosion of Al-2% Li-3% Cu alloys. Corrosion 2012, 50, 120–130. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Deng, Y.; Fan, S.; Ji, H.; Zhang, X. A Study on Dissimilar Friction Stir Welded between the Al–Li–Cu and the Al–Zn–Mg–Cu Alloys. Materials 2018, 11, 1132. https://doi.org/10.3390/ma11071132
Wu P, Deng Y, Fan S, Ji H, Zhang X. A Study on Dissimilar Friction Stir Welded between the Al–Li–Cu and the Al–Zn–Mg–Cu Alloys. Materials. 2018; 11(7):1132. https://doi.org/10.3390/ma11071132
Chicago/Turabian StyleWu, Pengfei, Yunlai Deng, Shitong Fan, Hua Ji, and Xinming Zhang. 2018. "A Study on Dissimilar Friction Stir Welded between the Al–Li–Cu and the Al–Zn–Mg–Cu Alloys" Materials 11, no. 7: 1132. https://doi.org/10.3390/ma11071132



