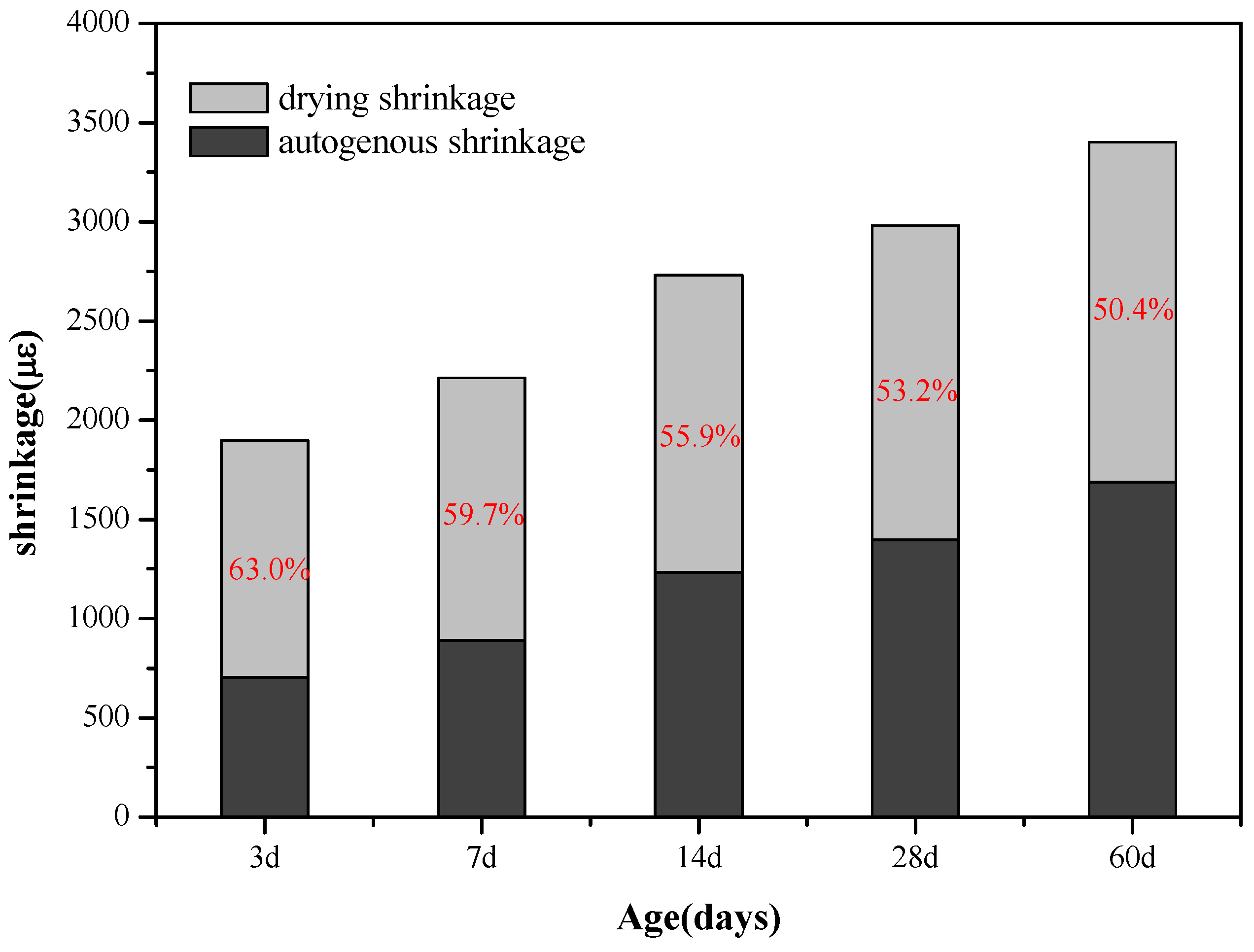3.2. Effect of Synthetic C-A-S-H on the Pore Structure of AAS Mortars
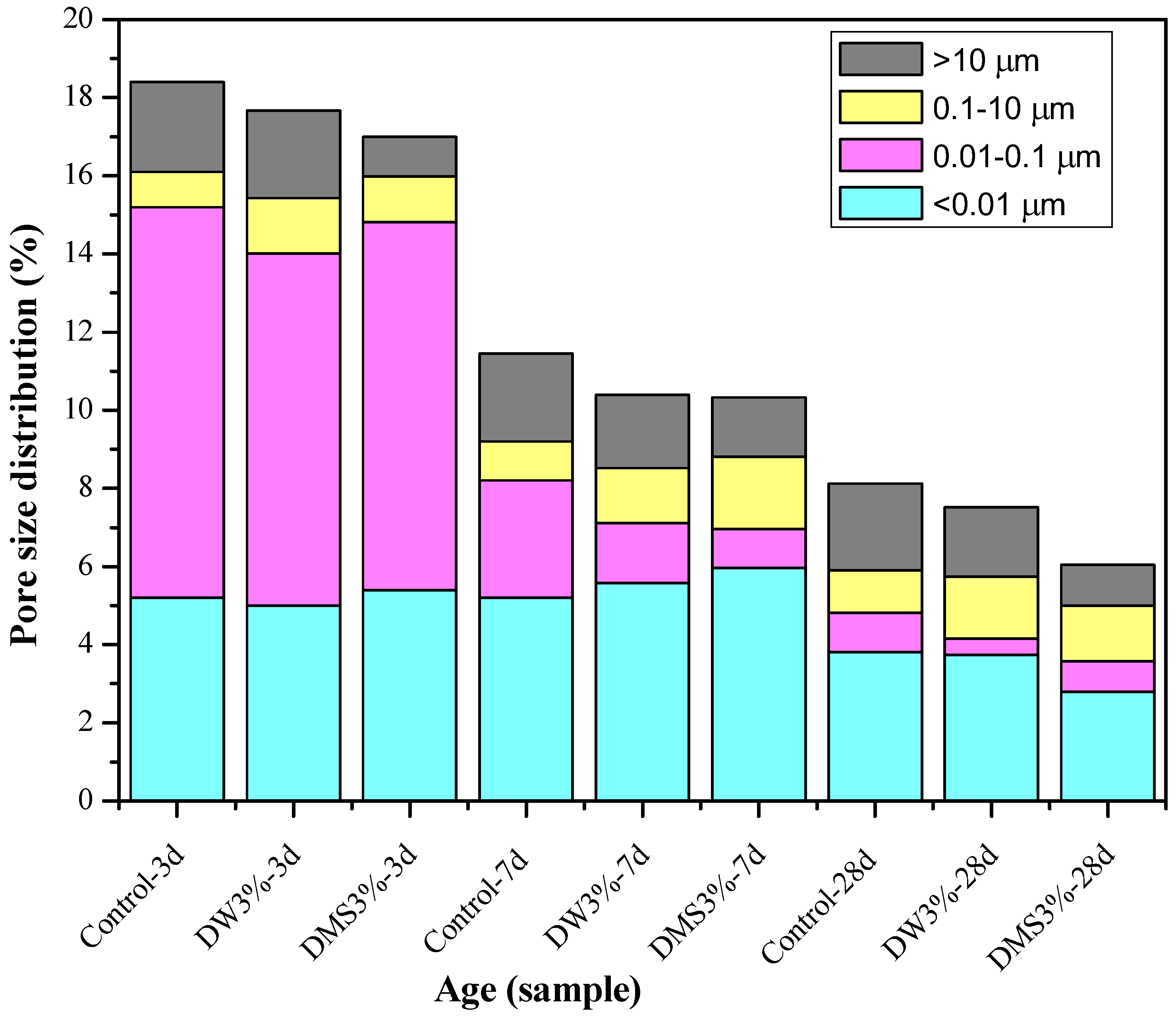
Table 5 shows the total porosity, mesopore volume (<0.05 µm)/total pore volume (%), and average pore diameter of the control samples and the samples in different mixing methods with the addition of 3 wt % synthetic C-A-S-H at 3 days, 7 days, and 28 days.
Figure 9 shows the total porosity and pore size distribution of AAS mortars. From the data in
Table 5, it can be inferred that the addition of an appropriate amount of C-A-S-H gel can reduce the total porosity while reducing the percentage of mesopore volume. The change can also be observed in the average pore size of the slag mortar activated by sodium water glass, as the addition of C-A-S-H gel reduced the average pore size of mortar. From
Table 5 and
Figure 9, it can be seen that the addition of the synthetic C-A-S-H gel via both mixing methods resulted in different degrees of porosity reduction in the AAS mortar at all three ages. Combining the C-A-S-H gel particle size distribution diagram in
Figure 2, it can be seen that because the particle size of the C-A-S-H gel is one order of magnitude smaller than that of the slag particles, one the one hand, it is possible to fill some large pores to increase the internal packing density of the mortar; on the other hand, Zhou et al. [
32] believed that the hydration process of AAS is consistent with the OPC system, so the dilution and nucleation effect can be used to explain that the addition of C-A-S-H gel that promotes early hydration of the ore fines, thereby reducing the porosity of the mortar porosity and refining the pore size in the early stage.
As far as the results from MIP and 3.1.1 shrinkage are concerned, from the perspectives of the mortar composition and structure, the shrinkage deformation is mainly caused by the contraction of the porous cemented phase (C-A-S-H gel phase), while the crystalline substances formed by aggregates and hydration generally do not shrink to deform. In all ages, the addition of C-A-S-H gel reduced the percentage of voids of 0.01–0.1 µm and greater than 10 µm and increased the proportion of pores at 0.1–10 µm size range. From
Table 5, the percentage of mesopore volume (<0.05 µm) in the total pore volume was reduced. This is probably because the drying shrinkage of AAS mortar is induced by the volume increase of mesopore, and larger mesopore volume can lead to a higher capillary stress in the water meniscus formed in the capillary pores of the mortar, resulting in a higher level of drying shrinkage [
3,
28,
33]. Collins et al. [
8] and Tarek Aly et al. [
29] also believed that the most important influence on the dehydration and shrinkage deformation in the mortar is the pore structure. The capillary stress caused by the evaporation and migration of water under relative humidity is one of the main causes of the drying shrinkage deformation, therefore, compared to AAS mortars without C-A-S-H gels, AAS mortars added with C-A-S-H gels had a reduced surface tension of pore water, which helps to eliminate pore water [
15], reducing the capillary stress that may lead to greater drying shrinkage.
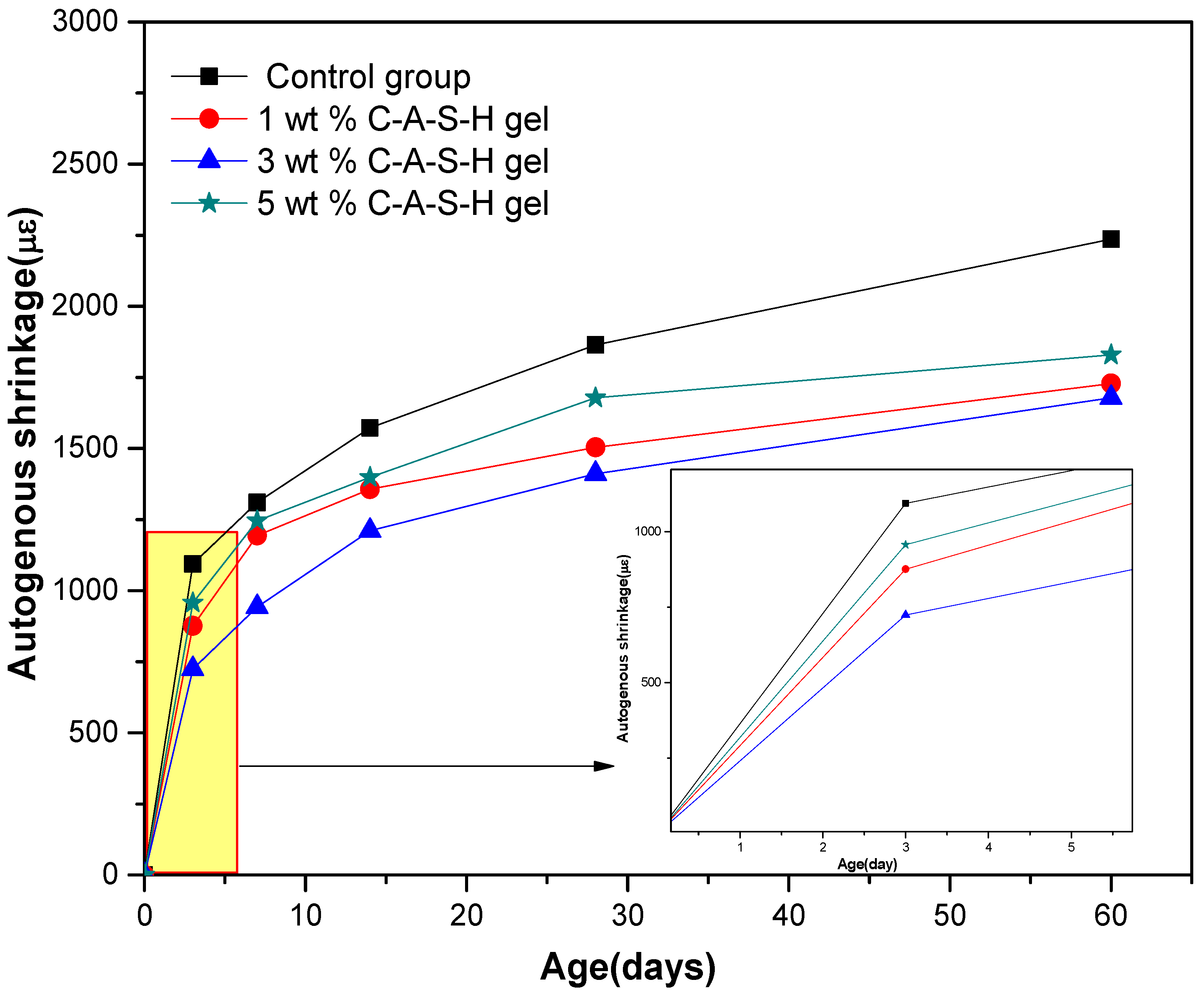
According to research reports [
28], the most direct cause of autogenous shrinkage in AAS is the self-desiccation effect. The chemical shrinkage of the AAS after initial curing time leads to the increase of water-free pores in the slag, therefore, self-desiccation will occur in the AAS mortar without external water supply. At the same time, the water in the pores gradually decreases as the hydration reaction continues; the decrease of the relative humidity in the pore system leads to a water-air meniscus, and the resulting capillary stress in the porous structure that exceeds the tension resistance limit of AAS mortar will lead to drying shrinkage [
33]. As the mesopore volume (<0.05 µm) of the AAS mortar is associated with the drying shrinkage reduction, and such volume in the mortar added with C-A-S-H gel was much smaller than in the control sample, it can be inferred that the autogenous shrinkage of the AAS mortar is mainly due to the self-desiccation in the hardened state, thus that the extensive drying shrinkage in the sodium water glass-activated slag mortar can be explained by its high proportion of mesopores (<0.05 µm); the water meniscus formed in the capillary pores of the mortar leads to considerable stress on the pore walls, which then turns into significant drying shrinkage, whereas the addition of C-A-S-H gels reduces the proportion of mesopore volume (<0.05 µm), thereby reducing the capillary stress generated in the closed system and accordingly reduces the drying shrinkage.
3.3. Effect of Synthetic C-A-S-H on the Microstructure of AAS Mortars
SEM analysis and EDS analysis were used to investigate the effect of C-A-S-H gel addition on the microstructure of AAS mortar.
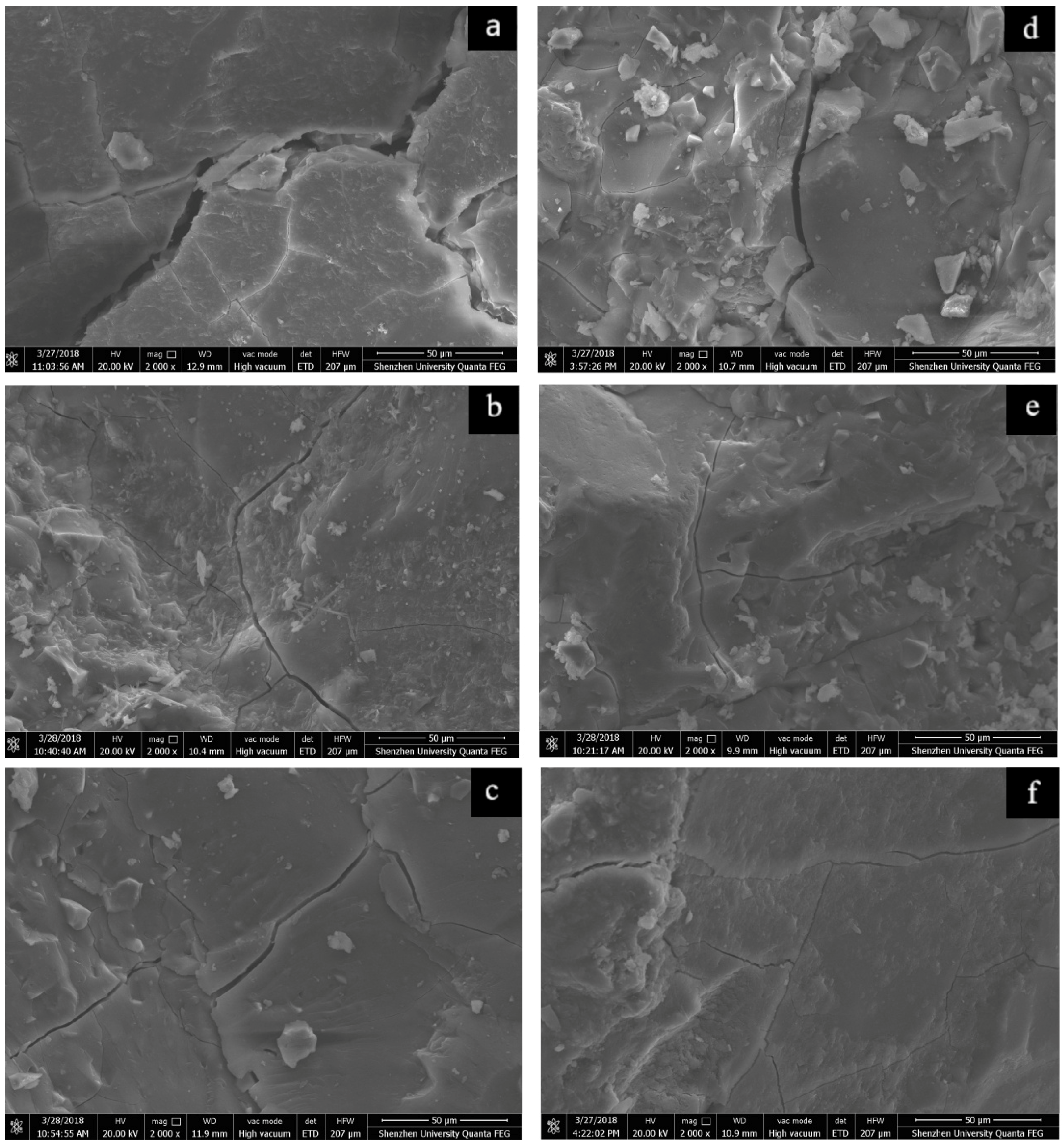
Figure 10a–c show the 50 µm microstructure images of the control sample and the AAS mortars added with 3% C-A-S-H gel via two mixing methods after 3 days of autogenous shrinkage.
Figure 10d–f show the 50 µm microstructure images of the control sample and AAS mortars added with 3 wt % C-A-S-H gel via two mixing methods after 7 days of autogenous shrinkage. It can be seen that in the absence of C-A-S-H gel, the microstructure of the samples cured at relative humidity shows strong cracks due to significant shrinkage. The structures of the C-A-S-H gel samples are denser than that of the control samples, and the addition of C-A-S-H gel reduces these crack widths. In the electron microscope image analysis software, first draw a line segment of the same length against the 50 µm ruler of the a, b, c, d, e, f pictures, and input the actual length of 50, unit µm, this line segment is the metric value; Then plot a distance on the cracks at different positions in each picture and measure ten different positions to average value, that is measuring the width of the crack. After measurement, the crack width of the control samples (a) at the 3-day age is about 7.41 µm, while the crack width at the 3 days age of the C-A-S-H gel samples (b, c) is about 1.53 µm and 1.58 µm, respectively; The crack width of the control samples (d) at the 7 day age is about 3.11 µm, while the crack widths of samples (e, f) added with C-A-S-H gel at the 7 days age are about 1.16 µm and 1.06 µm, respectively.
Figure 11 shows the morphology of the control samples and AAS mortars added with 3 wt % synthetic C-A-S-H gel at 3 day and 7 days under a scanning electron microscope with back-scattered electron.
Table 6 shows the elemental ratios of these phases as derived from EDS point analyses. Similar to most alkali activated cementitious materials, the hydration products of AAS are mainly gel-like fine particles [
34,
35], which are microscopic size, disordered structure, variability in composition, and tight binding. From these results in
Figure 11 and
Table 6, the bright, unhydrated slag particles are all surrounded by grey hydration products, containing calcium-alumina-silicate-hydrate (C-A-S-H) and other hydrated phases. The addition of C-A-S-H gel did not significantly change the phase composition of the hydration products of the AAS mortar, but the difference in microstructure between the control samples and the samples added with C-A-S-H gel can be clearly observed from the graph. As shown in
Figure 11a, when the control group curing age was 3 days, many unhydrated slag particles (the gray parts) 5–10 µm in diameter and some pores (the black parts) were observed in the control specimen which did not have a dense matrix structure, and the microscopic appearance demonstrated a looser structure. Compared to the control samples (a), the samples added with C-A-S-H gel (b) had some pores gradually reduce, as indicated in
Figure 11b. When the curing period continues to extend to 7 days, the sample added with 3 wt % C-A-S-H gels had fewer pores and a more compact structure, unhydrated slag particles become smaller, as shown in
Figure 11c,d. In addition, the morphology at day 7 is clearly smoother than at day 3, because a large number of hydration products form a dense and uniform matrix structure, greatly increasing the density.
To further understand the composition of these products,
Table 6 shows the atomic ratios of the reaction products of the specimens by EDS analyses. Previous studies reported that the hydration products of the alkali-activated slag are usually C(-A)-S-H gels with a low Ca/Si ratio [
36], the Na/Si ratios of other hydrated phases for the specimens were respectively 0.59, 0.47 and 0.46 on average, as listed in
Table 6. This is supported by the presence of Na in other hydrated phases, it can be deduced from these results that a high content of Na was incorporated into the other hydrated phases. Thus, it appears that the Na which dissolved from the sodium water glass activator was more likely to participate in the formation of the C-N-A-S-H gel through the reaction with slag particles, which may be a hybrid type phase of N-A-S-H and C-A-S-H gels [
37,
38], this is supported by the presence of Na in the other hydrated phase found in the EDS analysis, which corresponds to Lee et al.’s research findings [
28]. In addition, as identified from the EDS results, the Ca/Si ratio and Al/Si ratio and chemical composition of the C-A-S-H phase observed by SEM are close to those of the incorporated C-A-S-H gel (
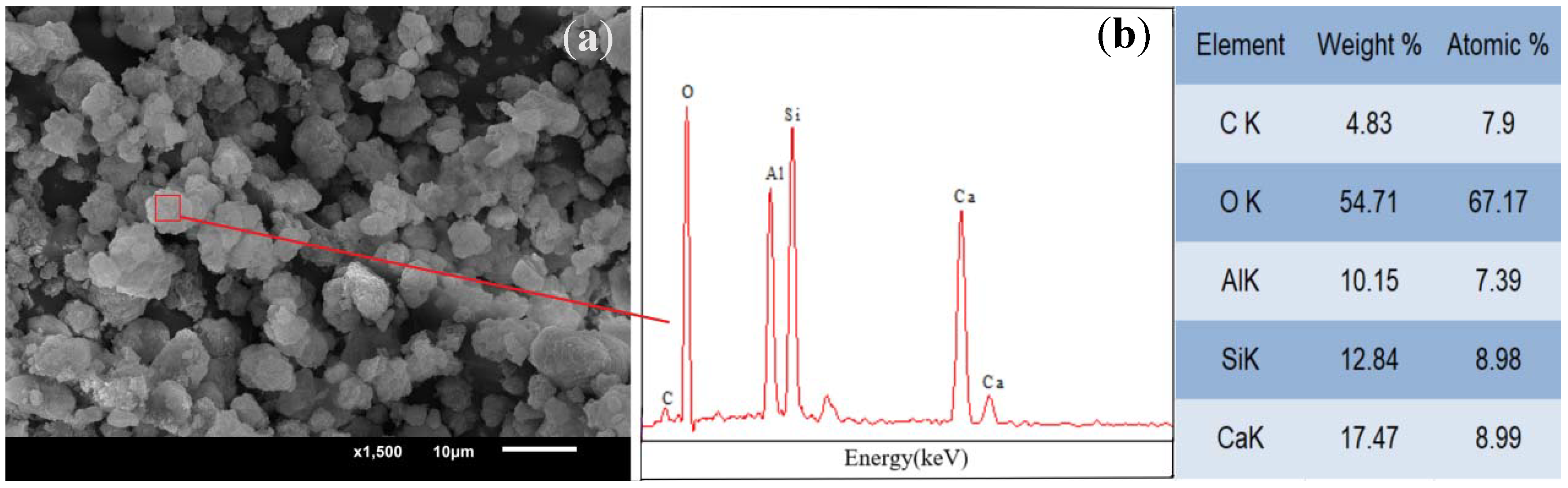
Figure 1). It can be seen that a small amount of fine particulate matter is adsorbed on the surface and is bound together with other hydration products nearby, which corresponds to Lloyd et al.’s observation [
21].
Another mechanism to explain the modification effect of C-S-H seed crystals on Portland cement is that the added C-S-H gel crystals can greatly reduce the nucleation barrier of the hydration products with its dual roles in adsorption and nucleation [
23,
24]. Similarly, in the alkali-activated slag system, since the composition and structure of the synthetic C-A-S-H gel and the gel formed by hydration are similar, it can be seen from
Figure 10 and
Figure 11 that the AAS mortar structure of the control samples is loose and crack width is large, while the AAS mortar structure is gradually compact and the crack width is reduced after the addition of C-A-S-H gel. One possible reason for this is that the surface of the incorporated C-A-S-H gel contains a large number of broken bonds and structural defects, and a higher surface free energy grants C-A-S-H with more capabilities to adsorb ions and molecular. The dual role of adsorption and nucleation changed the hydration process of AAS, so the hydration products obtained a close and uniform structure. Besides, unlike capillary stress, changes in surface free energy can cause overall differential stress in direction, size, and position; capillary stress is more likely to push tighter conical pressures on adjacent solid particles, while surface free energy induces densification of the particles themselves [
5]. On the other hand, the body of hardened mortar of AAS material is mainly dense gels [
39], and gels are prone to dehydrate, which will cause micro-cracks in the interior of the hardened mortar; the inclusion of gels in the form of particles or strips into the hardened mortar may exert a certain traction control effect on the development of cracks, thereby reducing the crack width.
For the results of MIP and SEM,
Figure 9 shows that the mixing method of C-A-S-H gel has no significant effect on the pore size distribution of the mortar; from the crack size comparison in
Figure 10b,c,e,f, it can be seen that the mixing method of C-A-S-H gel has no significant effect on the width of cracks. However, as shown in
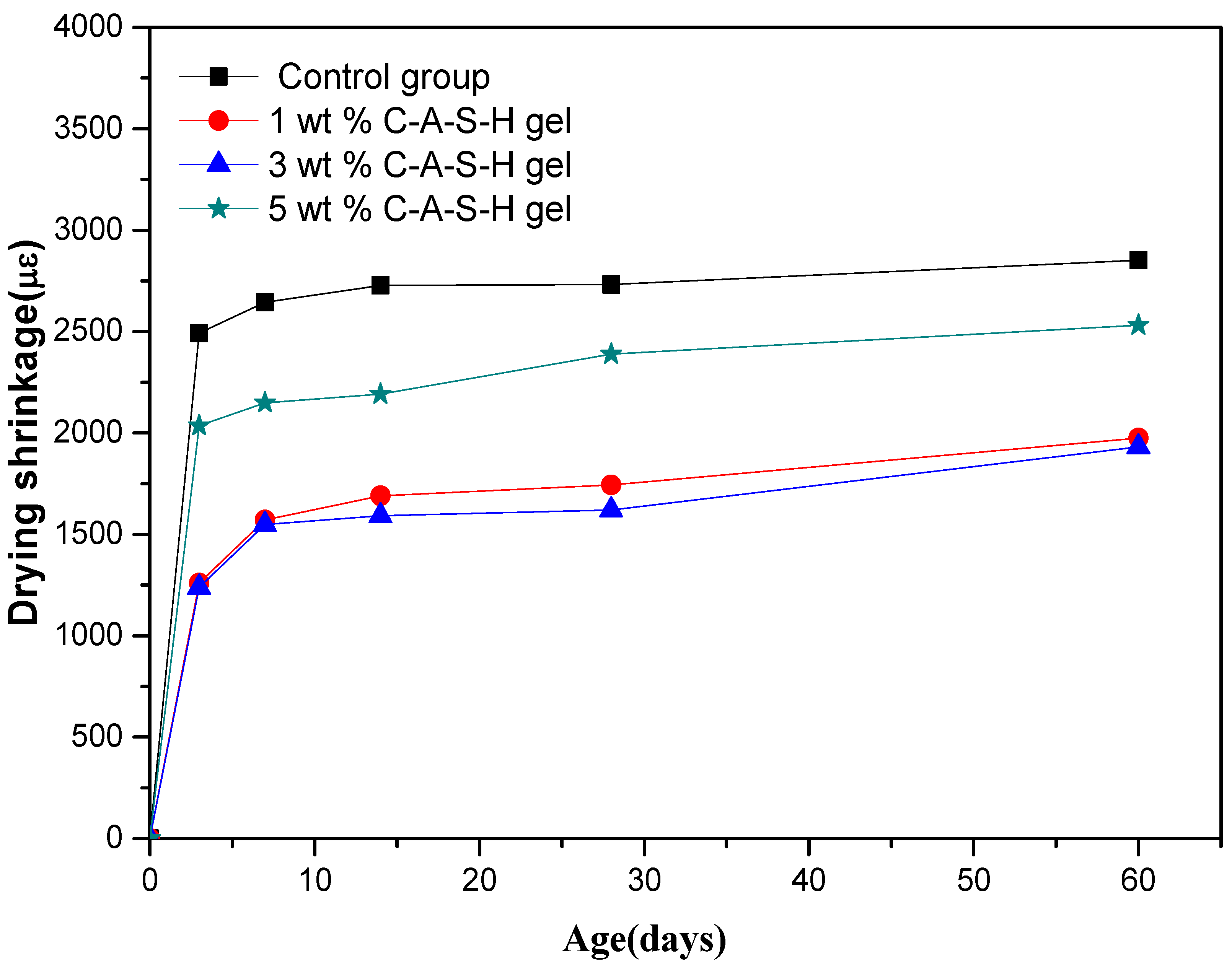
Figure 7 and
Figure 8,
Table 3 and
Table 4, the dispersion method is slightly better than the dry-mixing method. This result can be explained by the report of Land et al. [
40], which stated that the dispersion of nanoscale C-A-S-H gels in water using ultrasonic waves can avoid the flocculation of nanoparticles and thus the dispersed particles are more uniform, resulting in a more effective accelerated hydration compared to that of the dry-mixing method. This may be the main reason for the slight difference in shrinkage reduction between the dispersion method and dry-mixing method.