Electrochemical Deposition and Nucleation/Growth Mechanism of Ni–Co–Y2O3 Multiple Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Linear Sweep Voltammetry (LSV)
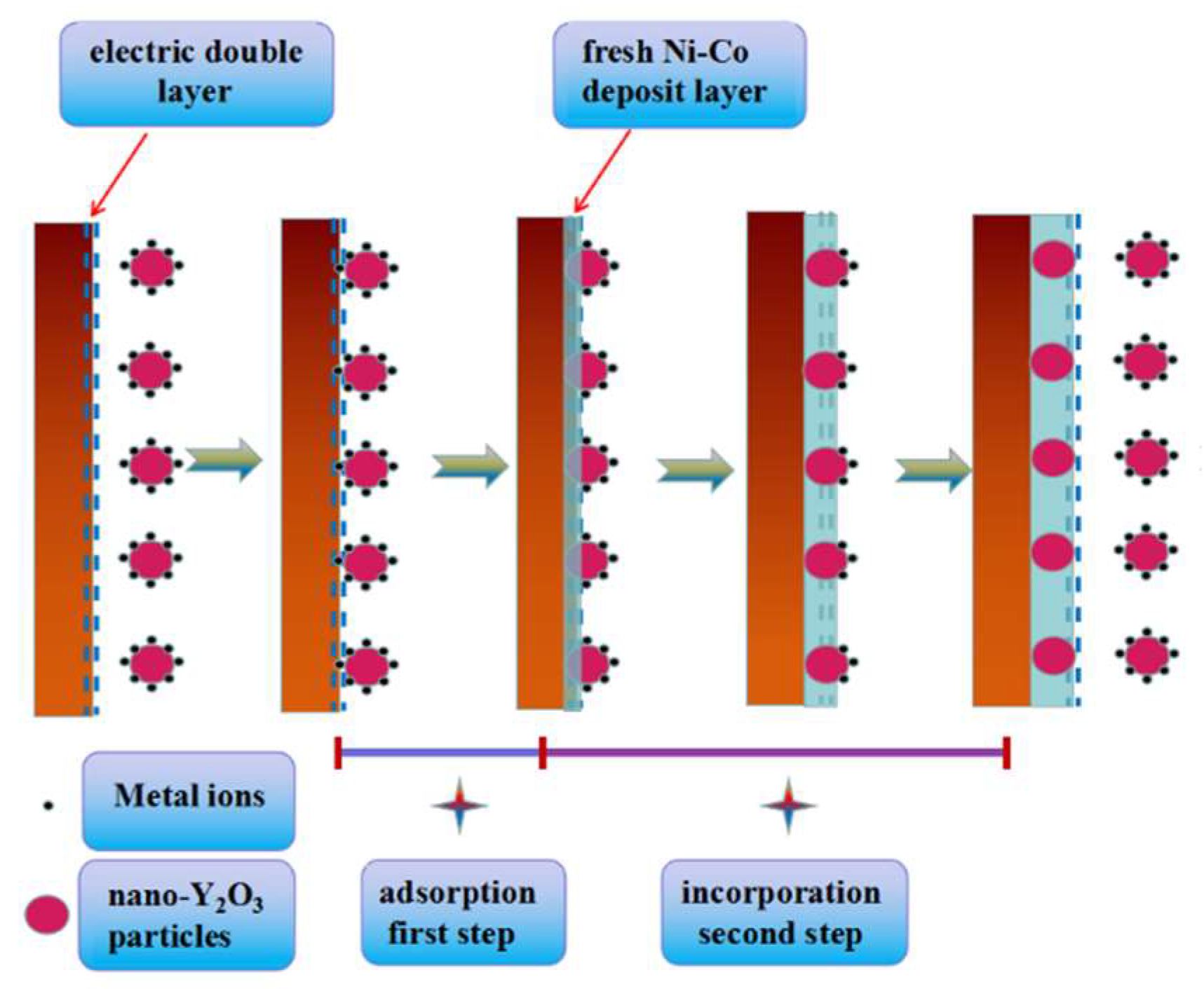
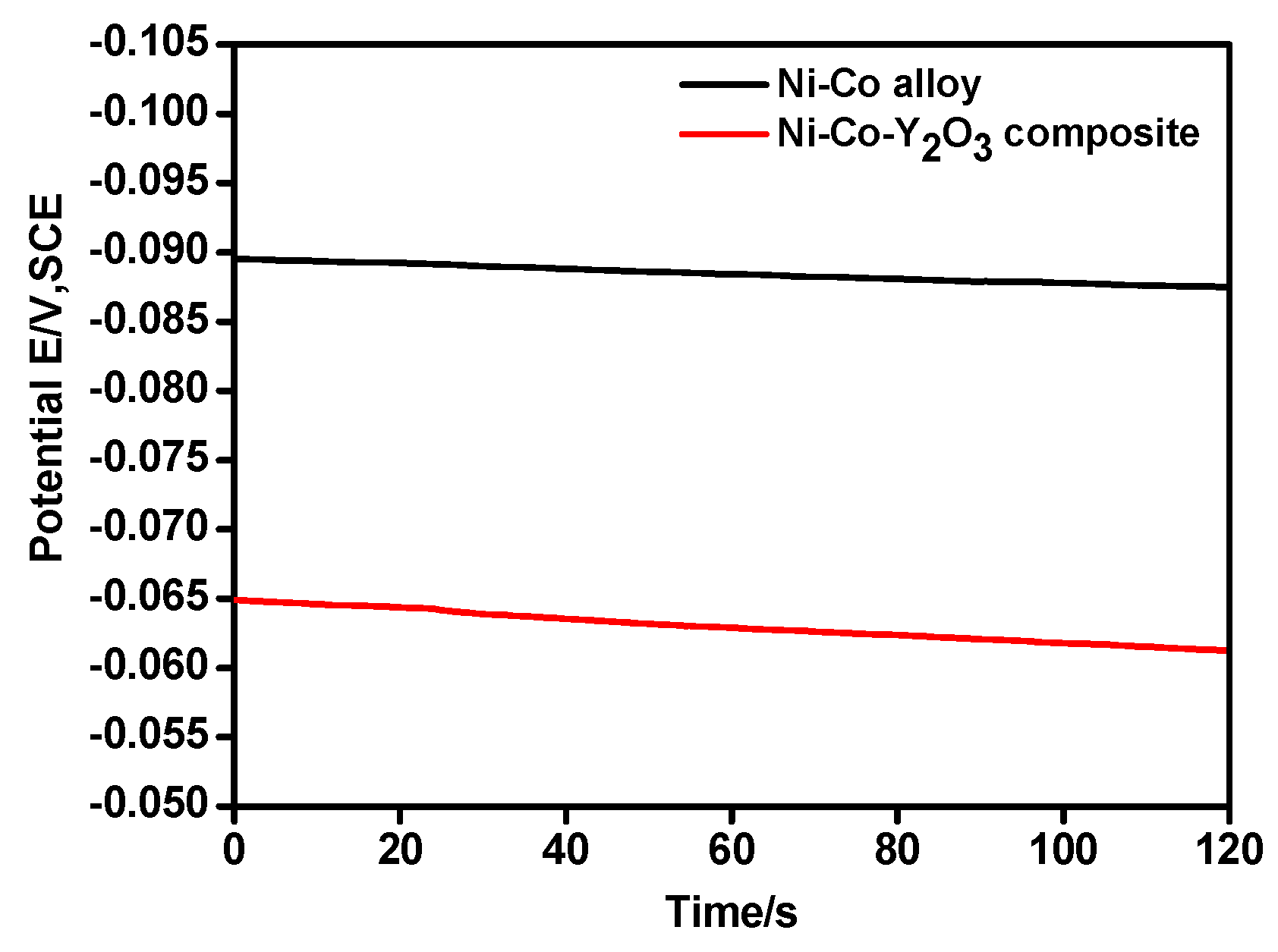
3.2. Chronoamperometry Study
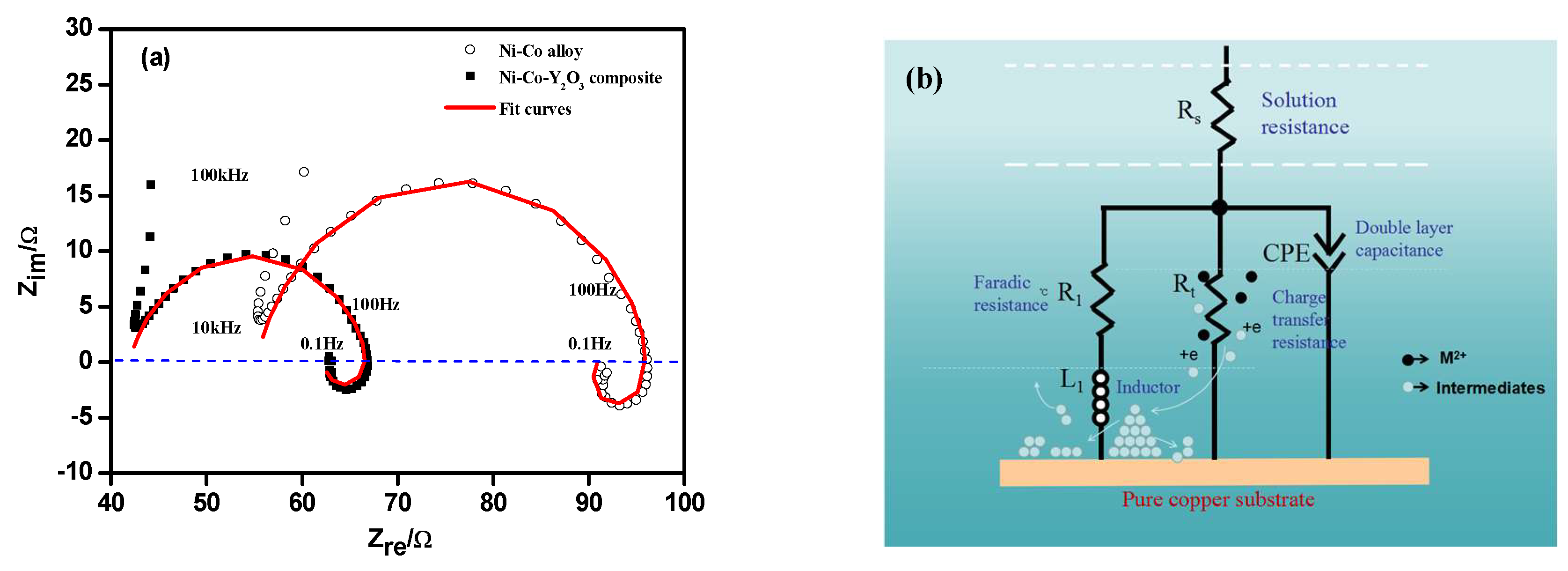
3.3. Electrochemical Impedance Spectroscopy (EIS) Studies
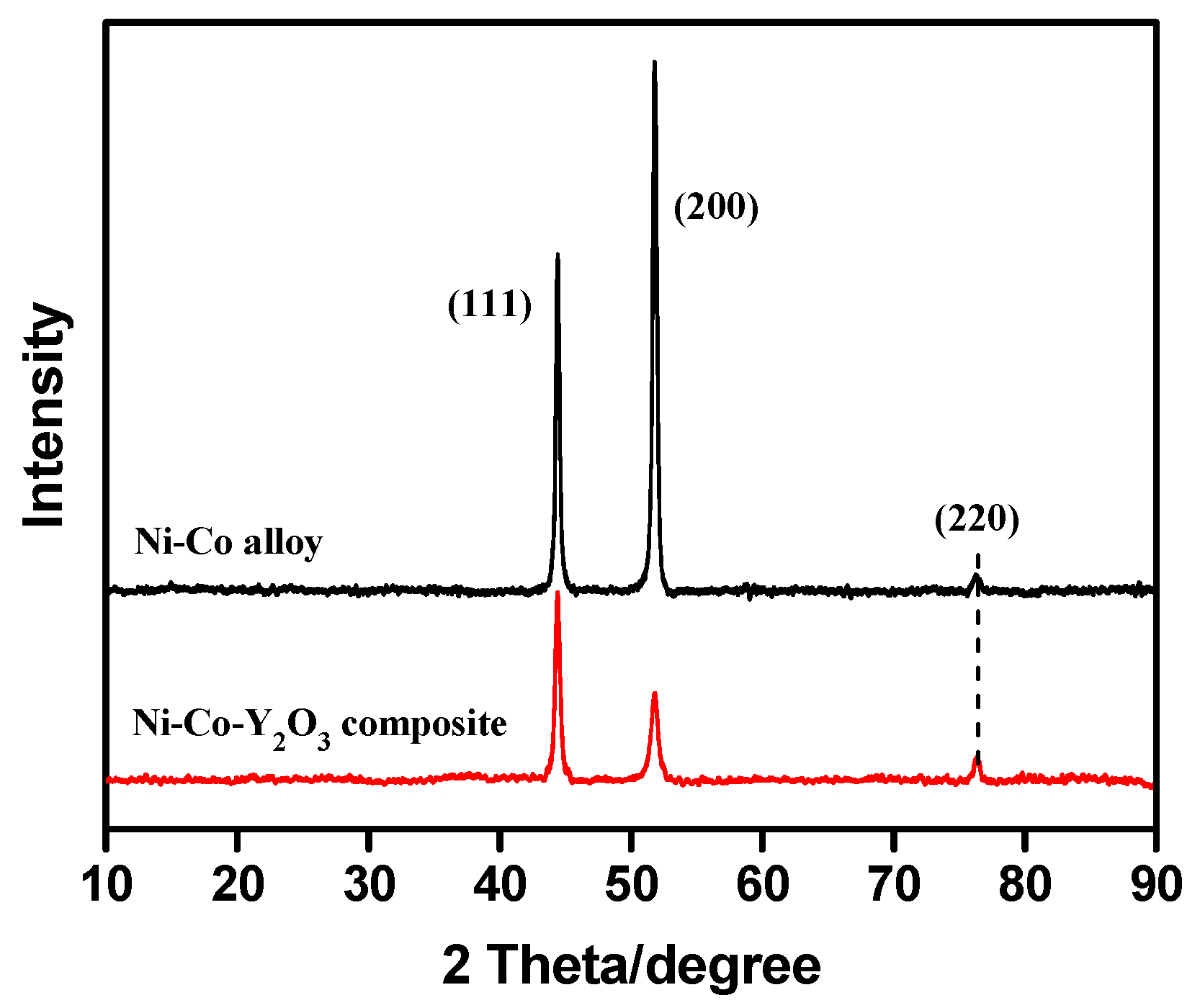
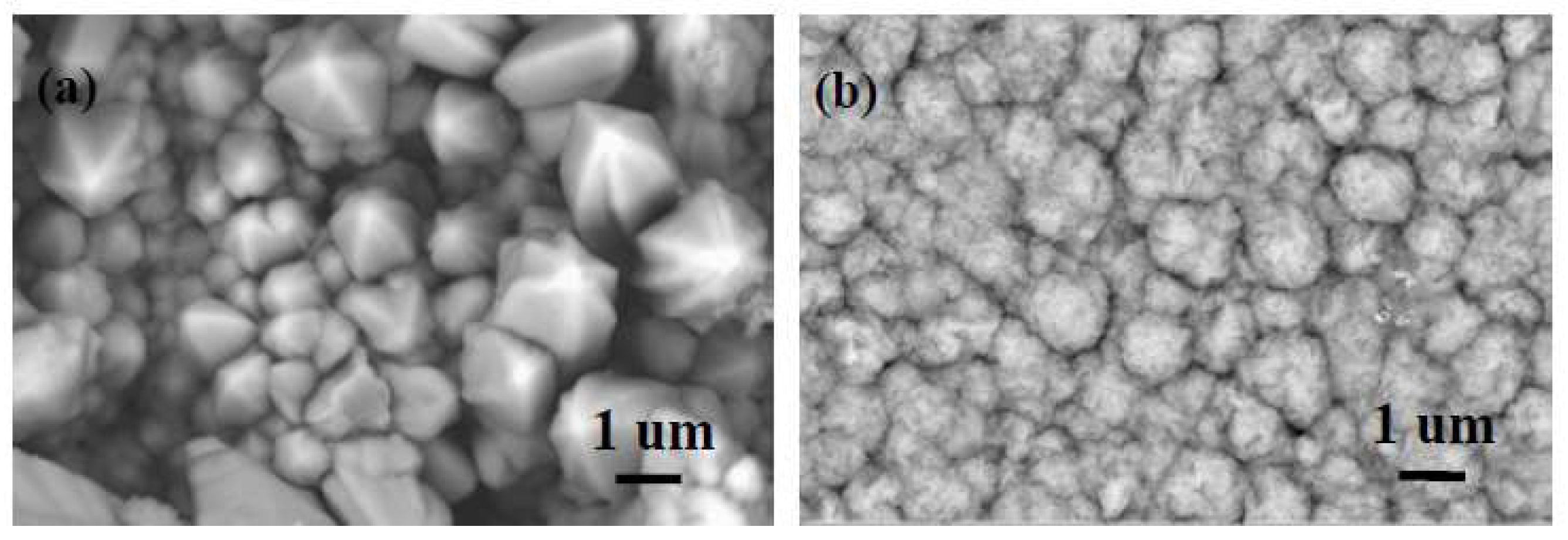
3.4. Microstructure of Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feng, Q.Y.; Li, T.J.; Jin, J.Z. Research on the Mechanism of Composite Electroplating and Its Latest Progress. Rare Met. Mater. Eng. 2007, 36, 559–564. [Google Scholar]
- Walsh, F.C.; Leon, C.P.D. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: An established and diversifying technology. Trans. IMF 2014, 92, 83–98. [Google Scholar] [CrossRef]
- Zhou, H.F.; Du, N.; Zhao, Q. Status of composite electrodeposition techniques. Electeoplating Finish. 2005, 24, 41–46. [Google Scholar]
- Yousefpour, M.; Rahimi, A. Characterization and selection of optimal parameters to achieve the best tribological performance of the electrodeposited Cr nanocomposite coating. Mater. Des. 2014, 54, 382–389. [Google Scholar] [CrossRef]
- Srivastava, M.; Selvi, V.E.; Grips, V.K.W.; Rajam, K.S. Corrosion resistance and microstructure of electrodeposited nickel–cobalt alloy coatings. Surf. Coat. Technol. 2006, 201, 3051–3060. [Google Scholar] [CrossRef]
- Dolati, A.; Sababi, M.; Nouri, E.; Ghorbani, M. A study on the kinetic of the electrodeposited Co–Ni alloy thin films in sulfate solution. Mater. Chem. Phys. 2007, 102, 118–124. [Google Scholar] [CrossRef]
- Marikkannu, K.R.; Kalaignan, G.P.; Vasudevan, T. The role of additives in the electrodeposition of nickel–cobalt alloy from acetate electrolyte. J. Alloys Compd. 2007, 438, 332–336. [Google Scholar] [CrossRef]
- Sr, M.S.; Grips, V.K.W.; Jain, A.; Rajam, K.S. Influence of SiC particle size on the structure and tribological properties of Ni–Co composites. Surf. Coat. Technol. 2007, 202, 310–318. [Google Scholar]
- Srivastava, M.; William Grips, V.K.; Rajam, K.S. Electrochemical deposition and tribological behaviour of Ni and Ni–Co metal matrix composites with SiC nano-particles. Appl. Surf. Sci. 2007, 253, 3814–3824. [Google Scholar] [CrossRef]
- Wu, G.; Li, N.; Wang, D.L.; Zhoub, D.R. Effect of α-Al2O3 particles on the electrochemical codeposition of Co–Ni alloys from sulfamate electrolytes. Mater. Chem. Phys. 2004, 87, 411–419. [Google Scholar] [CrossRef]
- Kumar, K.; Chandramohan, R.; Kalyanaraman, D. Effect of heat treatment on cobalt and nickel electroplated surfaces with Cr2O3, dispersions. Appl. Surf. Sci. 2004, 227, 383–386. [Google Scholar] [CrossRef]
- Shi, L.; Sun, C.F.; Gao, P.; Zhoua, F.; Liu, W.M. Electrodeposition and characterization of Ni–Co–carbon nanotubes composite coatings. Surf. Coat. Technol. 2006, 200, 4870–4875. [Google Scholar] [CrossRef]
- Mouzon, J.; Maitre, A.; Frisk, L.; Lehto, N.; Odén, M. Fabrication of transparent yttria by HIP and the glass-encapsulation method. J. Eur. Ceram. Soc. 2009, 29, 311–316. [Google Scholar] [CrossRef]
- Xue, Y.J.; Shen, C.; Li, J.S.; Li, H.; Si, D.H. Corrosion Resistance of Ni-Y2O3 Composite Coating Prepared by Electrodeposition under Ultrasonic Condition. Adv. Mater. Res. 2010, 97–101, 1235–1238. [Google Scholar] [CrossRef]
- Tian, L.; Xu, J. Electrodeposition and characterization of Ni–Y2O3, composite. Appl. Surf. Sci. 2011, 257, 7615–7620. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.F. Mechanistic aspects of electrodeposition of Ni–Co–SiC composite nano-coating on carbon steel. Electrochim. Acta 2013, 109, 638–644. [Google Scholar] [CrossRef]
- Abyaneh, M.Y.; Saez, V.; González-García, J.; Mason, T.J. Electrocrystallization of lead dioxide: Analysis of the early stages of nucleation and growth. Electrochim. Acta 2010, 55, 3572–3579. [Google Scholar] [CrossRef]
- Vasilakopoulos, D.; Bouroushian, M.; Spyrellis, N. Electrocrystallisation of zinc from acidic sulphate baths; A nucleation and crystal growth process. Electrochim. Acta 2009, 54, 2509–2514. [Google Scholar] [CrossRef]
- Ghaziof, S.; Kilmartin, P.A.; Gao, W. Electrochemical studies of sol-enhanced Zn–Ni–Al2O3, composite and Zn–Ni alloy coatings. J. Electroanal. Chem. 2015, 755, 63–70. [Google Scholar] [CrossRef]
- Tebbakh, S.; Beniaiche, A.; Fenineche, N.; Azizi, A.; Schmerber, G.; Dinia, A. Electrochemical nucleation behaviours and properties of electrodeposited Co–Ni alloy thin films. Trans. Inst. Met. Finish. 2013, 91, 17–23. [Google Scholar] [CrossRef]
- Zhao, X.S.; Tan, C.Y.; Cheng, W.J.; Zheng, Z.-Q. Nucleation kinetics analysis of Ni-SiC composite film during early electro crystallization processes. Chin. J. Nonferrous Met. 2008, 18, 823–828. [Google Scholar]
- Guglielmi, N. Codeposition of inert particles in a metallic matrix. J. Electrochem. Soc. 1972, 119, 1009. [Google Scholar] [CrossRef]
- Scharifker, B.; Hills, G. Theoretical and experimental studies of multiple nucleation. Electrochim. Acta 1983, 28, 879–889. [Google Scholar] [CrossRef]
- Raeissi, K.; Saatchi, A.; Golozar, M.A. Effect of nucleation mode on the morphology and texture of electrodeposited zinc. J. Appl. Electrochem. 2003, 33, 635–642. [Google Scholar] [CrossRef]
- Epelboin, I.; Joussellin, M.; Wiart, R. Impedance measurements for nickel deposition in sulfate and chloride electrolytes. J. Electroanal. Chem. 1981, 119, 61–71. [Google Scholar] [CrossRef]
- Basavanna, S.; Naik, Y.A. Electrochemical studies of Zn–Ni alloy coatings from acid chloride bath. J. Appl. Electrochem. 2009, 39, 1975–1982. [Google Scholar] [CrossRef]
- Palomar-Pardavé, M.; Scharifker, B.R.; Arce, E.M.; Romero-Romo, M. Nucleation and diffusion-controlled growth of electroactive centers: Reduction of protons during cobalt electrodeposition. Electrochim. Acta 2005, 50, 4736–4745. [Google Scholar] [CrossRef]
- Nowark, P.; Socha, R.P.; Kaisheva, M.; Fransaer, J.; Celis, J.-P.; Stoinov, Z. Electrochemical investigation of the codeposition of SiC and SiO2 particles with nickel. J. Appl. Electrochem. 2004, 21, 429–437. [Google Scholar]
- Hu, W.; Tan, C.; Cui, H.; Liu, Y.; Zheng, Z.-Q. Kinetics analysis of Ni–TiO2 composite system during initial stages of electro-crystallization. J. Cent. South Univ. Technol. 2010, 17, 460–466. [Google Scholar] [CrossRef]
- Benea, L. Electrodeposition and tribocorrosion behaviour of ZrO2–Ni composite coatings. J. Appl. Electrochem. 2009, 39, 1671–1681. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]







| Potential/V (vs. SCE) | Ni–Co Alloy | Ni–Co–Y2O3 | ||
|---|---|---|---|---|
| Im (A·cm−2) | tm (s) | Im (A·cm−2) | tm (s) | |
| −1.05 V | −0.00122 | 76.1 | −0.00160 | 49.2 |
| −1.10 V | −0.00169 | 31.8 | −0.00209 | 22.5 |
| −1.15 V | −0.00197 | 21.9 | −0.00256 | 12.3 |
| −1.20 V | −0.00258 | 18.6 | −0.00296 | 8.5 |
| Materials | Potential | P1* (μA cm−2) | P2 (s−1) | P3 (s−1) | P4 (μA cm−2) | A (s−1) | N0 × 106 (cm−2) |
|---|---|---|---|---|---|---|---|
| Ni–Co | −1.05 V | −1.37 | 0.14 | 0.75 | −1.52 | 0.75 | 3.92 |
| Ni–Co | −1.20 V | −2.07 | 0.33 | 1.98 | −1.59 | 1.98 | 5.47 |
| Ni–Co–Y2O3 | −1.05 V | −1.55 | 0.26 | 1.28 | −1.54 | 1.28 | 4.61 |
| Ni–Co–Y2O3 | −1.20 V | −2.48 | 0.54 | 2.39 | −1.55 | 2.39 | 6.38 |
| Materials | Potential (V) | Rs/Ω·cm2 | CPE1-P/F·cm2 | Rt/Ω·cm2 | R1/Ω·cm2 | L1/H·cm2 |
|---|---|---|---|---|---|---|
| Ni–Co | −1.20 | 55.21 | 5.09 × 10-5 | 41.48 | 221.9 | 15.61 |
| Ni–Co–Y2O3 | −1.20 | 51.92 | 4.98 × 10-5 | 25.29 | 106.8 | 4.72 |
| Materials | Ni | Co | O | Y | Total |
|---|---|---|---|---|---|
| Ni–Co | 67.32 | 32.68 | – | – | 100 |
| Ni–Co–Y2O3 | 59.55 | 20.69 | 14.11 | 5.65 | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Wang, Y.; Liang, Z.; Jin, H. Electrochemical Deposition and Nucleation/Growth Mechanism of Ni–Co–Y2O3 Multiple Coatings. Materials 2018, 11, 1124. https://doi.org/10.3390/ma11071124
Zhou X, Wang Y, Liang Z, Jin H. Electrochemical Deposition and Nucleation/Growth Mechanism of Ni–Co–Y2O3 Multiple Coatings. Materials. 2018; 11(7):1124. https://doi.org/10.3390/ma11071124
Chicago/Turabian StyleZhou, Xinyu, Yiyong Wang, Zhipeng Liang, and Hui Jin. 2018. "Electrochemical Deposition and Nucleation/Growth Mechanism of Ni–Co–Y2O3 Multiple Coatings" Materials 11, no. 7: 1124. https://doi.org/10.3390/ma11071124
APA StyleZhou, X., Wang, Y., Liang, Z., & Jin, H. (2018). Electrochemical Deposition and Nucleation/Growth Mechanism of Ni–Co–Y2O3 Multiple Coatings. Materials, 11(7), 1124. https://doi.org/10.3390/ma11071124




