Experimental Study on Characteristics of Grinded Graphene Nanofluids with Surfactants
Abstract
:1. Introduction
2. Materials and Methods
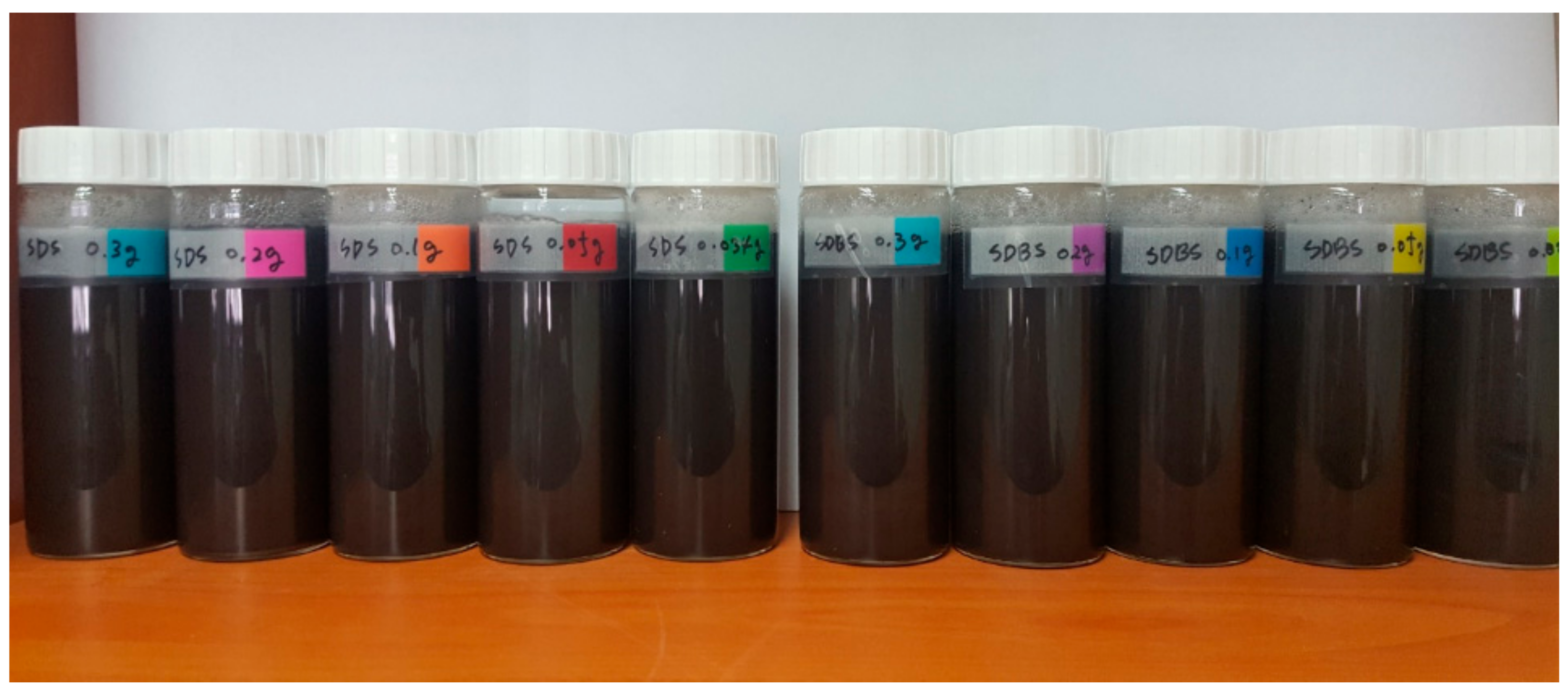
2.1. Nanofluid Preparation
2.2. Measuring Equipments
2.3. Measurements Procedure of the Thermal Conductivity
3. Results and Discussion
4. Conclusions
- (1)
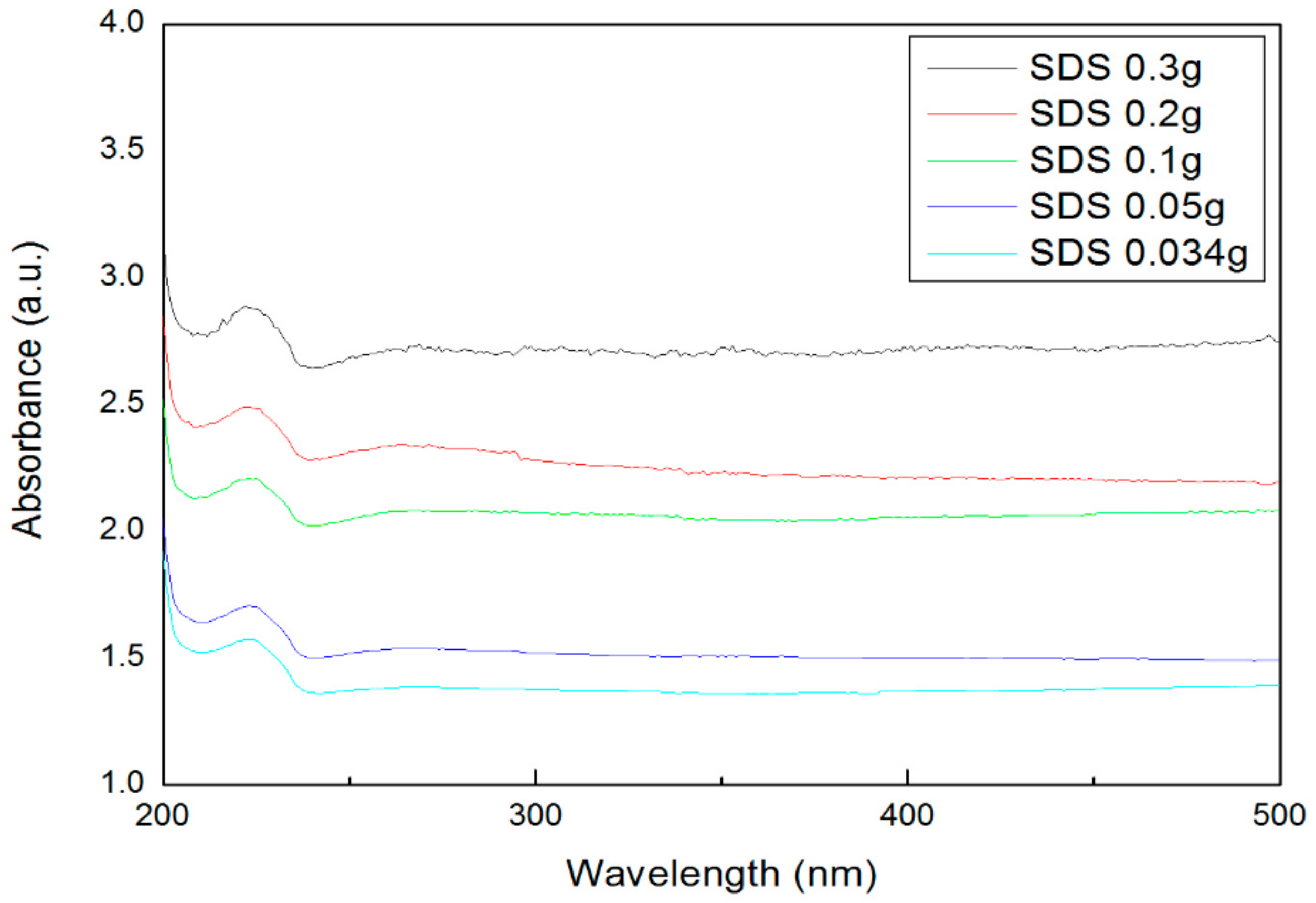
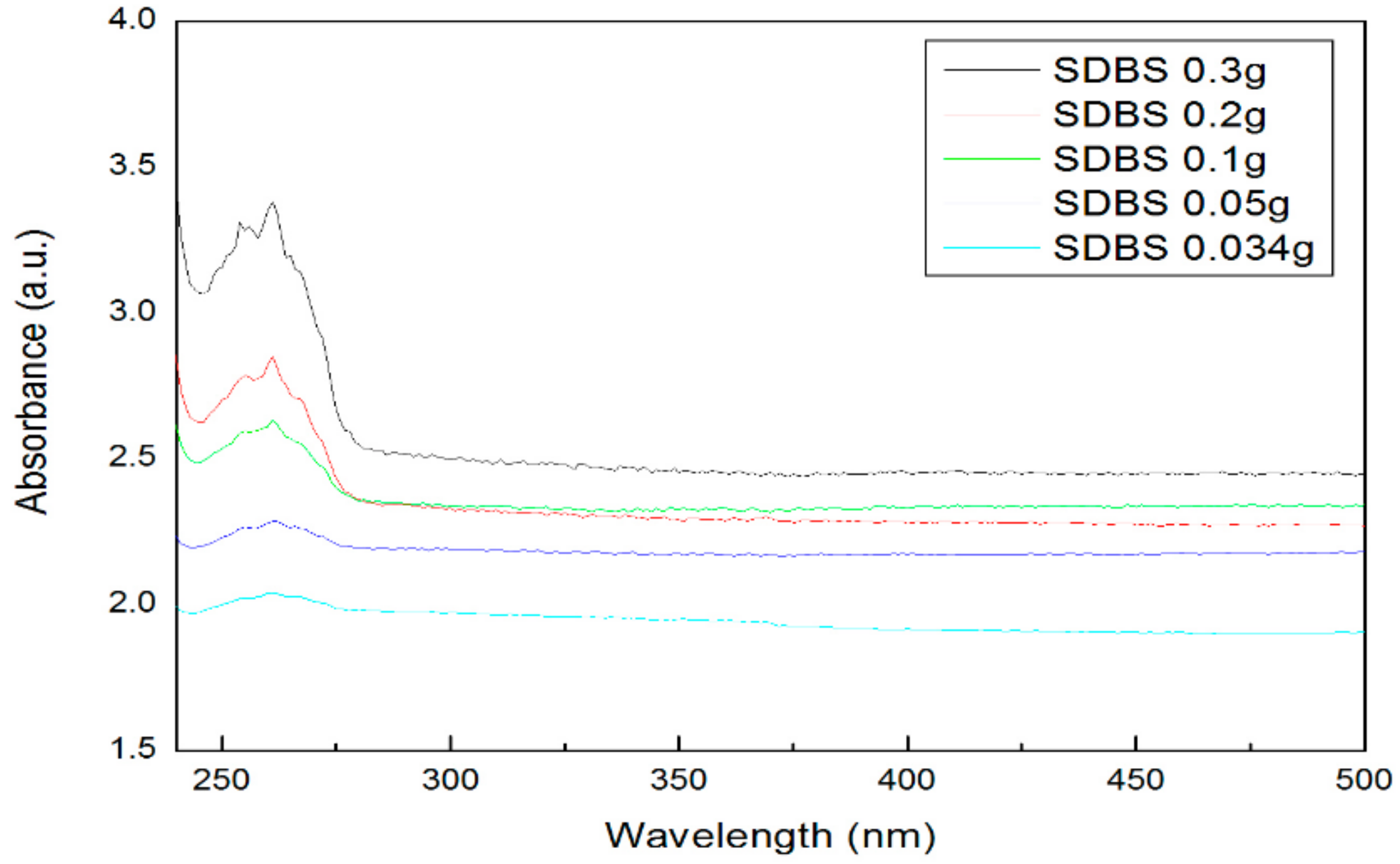
- The absorbance was measured to confirm the dispersibility. The highest absorbance was obtained when 0.3 g of SDS and SDBS were used, and the lowest absorbance was obtained when 0.034 g of SDS and SDBS were used. As a result, as the surfactant ratio increases, the nanofluid containing SDS and SDBS produced higher absorbance values.
- (2)
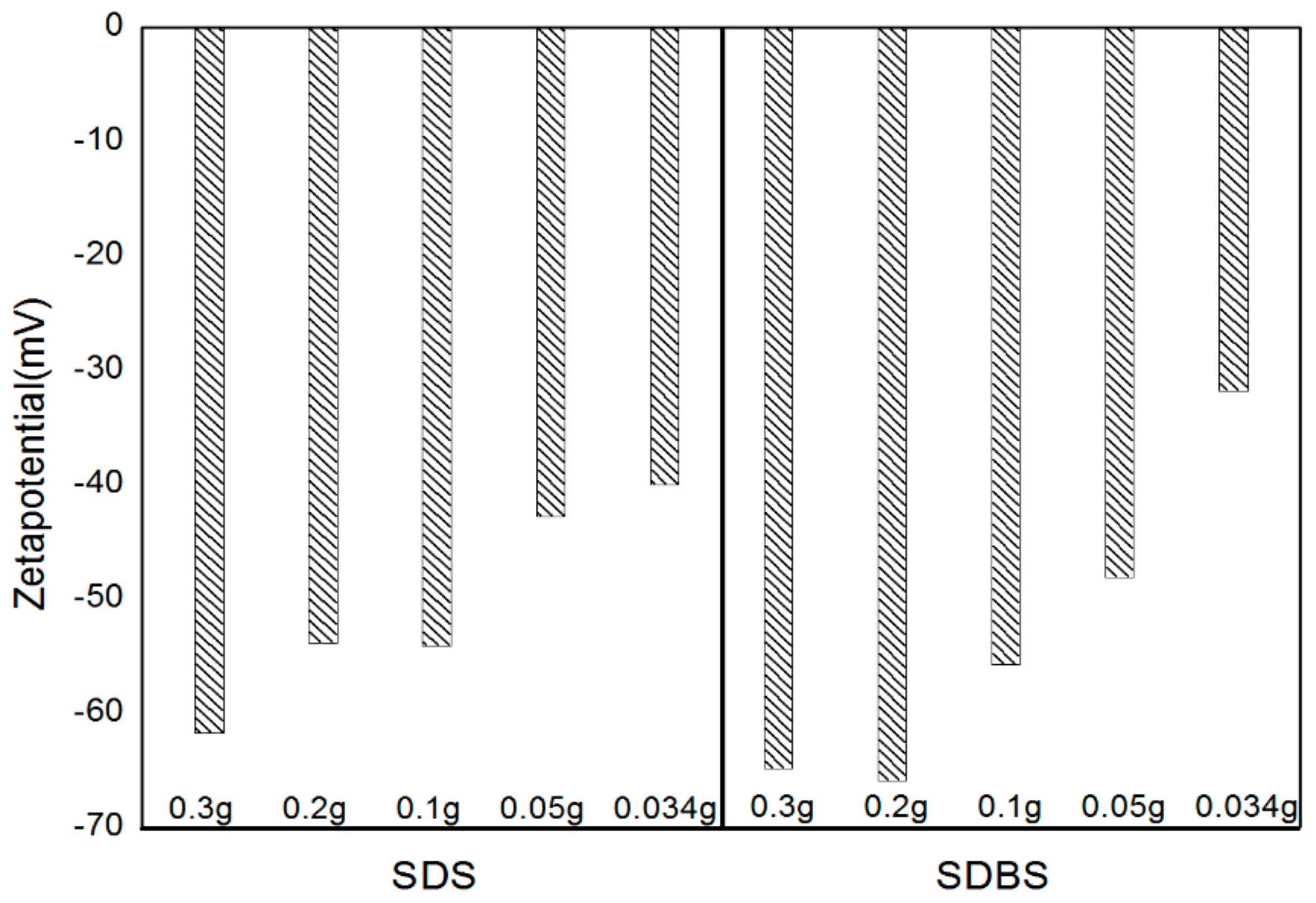
- The zeta potential was measured to figure out the dispersion stability. Absolute values of 30 mV or more were obtained under all of the conditions, and it could be judged that the value of the dispersion stability was sufficiently high. In this experiment, SDBS showed that the zeta potential value was higher than SDS, and had a higher dispersion stability as the amount of surfactant was increased.
- (3)
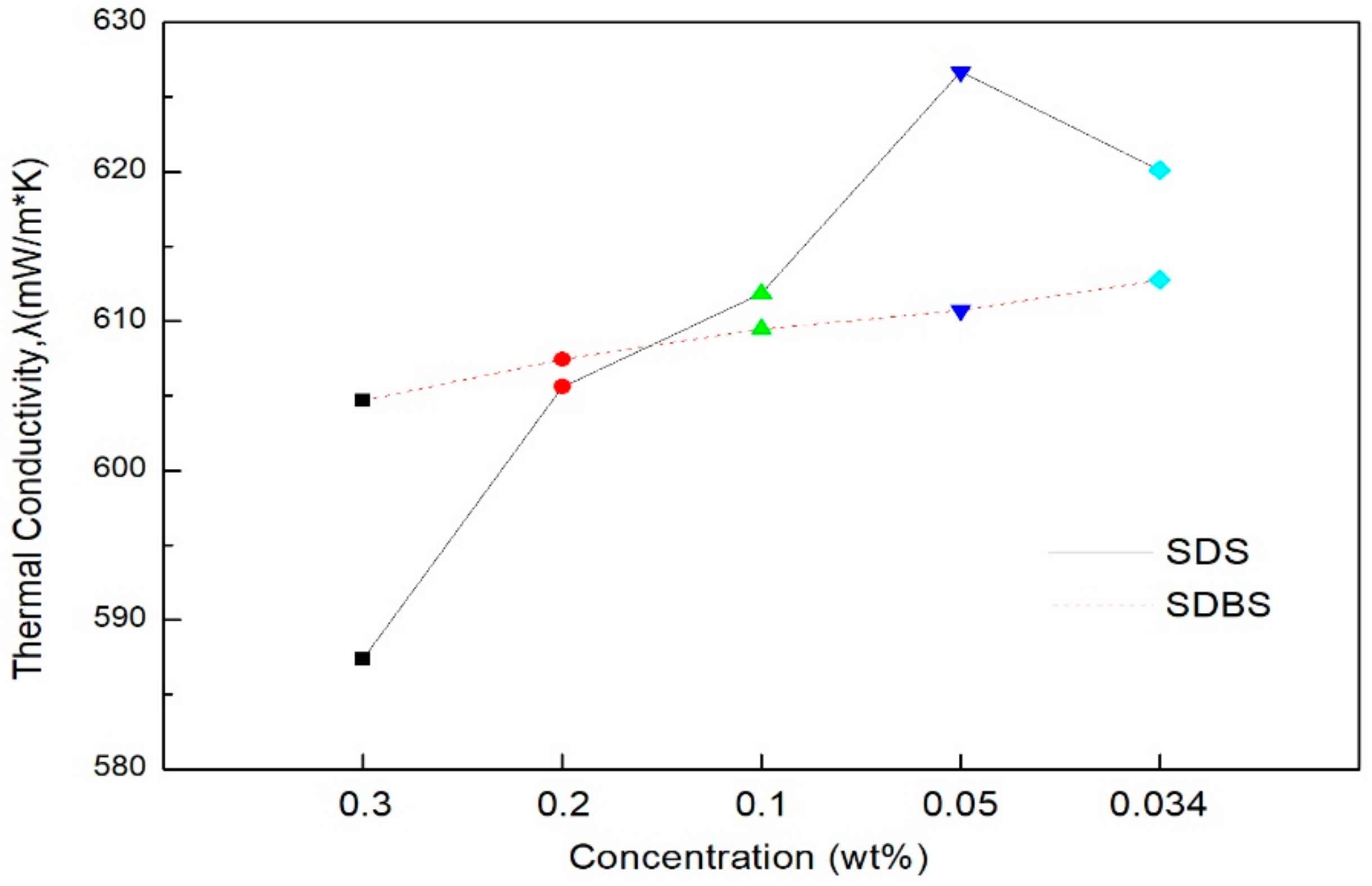
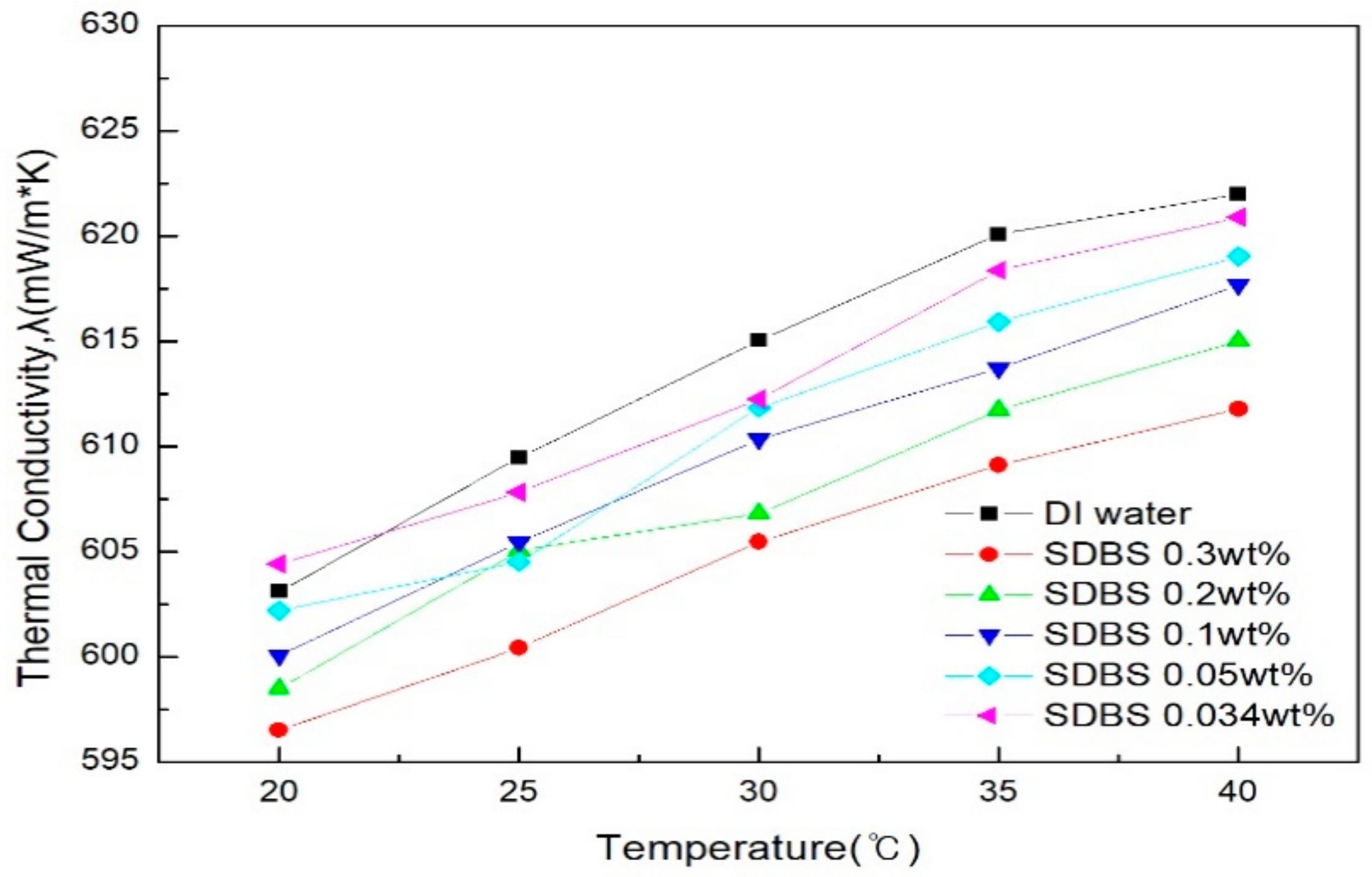
- The thermal conductivity was measured in comparison with distilled water. Graphene nanofluid with SDS added showed a higher thermal conductivity than when SDBS was added. SDS showed a higher thermal conductivity than distilled water at the ratio of graphene to surfactant of 2:1 (0.05 g) and 3:1 (0.034 g) and generally, SDBS showed a lower thermal conductivity than distilled water at all of the conditions. When the mean thermal conductivity of the two surfactants was compared, SDS showed a higher increase in thermal conductivity than the SDBS as the amount of surfactant decreased. When the ratio of graphene to SDS was 2:1 (0.05 g), it showed the highest thermal conductivity. As a result of thermal conductivity measurement, it was confirmed that the thermal conductivity becomes smaller according to the amount of the surfactant.
Author Contributions
Funding
Conflicts of Interest
References
- Kwon, Y.H.; Kim, D.; Li, C.G.; Lee, J.K.; Hong, D.S.; Lee, J.G.; Lee, S.H.; Cho, Y.H.; Kim, S.H. Heat transfer and pressure drop characteristics of nanofluids in a plate heat exchanger. J. Nanosci. Nanotechnol. 2011, 11, 5769–5774. [Google Scholar] [CrossRef] [PubMed]
- Afgam, N. New Developments in Heat Exchangers; Gordon and Breach Publishers: Philadelphia, PA, USA, 1996; ISBN 905699512X. [Google Scholar]
- Shirvan, K.M.; Mamourian, M.; Mirzakhanlari, S.; Ellahi, R. Two phase simulation and sensitivity analysis of effective parameters on combined heat transfer and pressure drop in a solar heat exchanger filled with nanofluid by RSM. J. Mol. Liq. 2016, 220, 888–901. [Google Scholar] [CrossRef]
- Chen, H.; Ding, Y.; Tan, C. Rheological behaviour of nanofluids. New J. Phys. 2007, 9, 367. [Google Scholar] [CrossRef]
- Ebrahimnia-Bajestan, E.; Moghadam, M.C.; Niazmand, H.; Daungthongsuk, W.; Wongwises, S. Experimental and numerical investigation of nanofluids heat transfer characteristics for application in solar heat exchangers. Int. J. Heat Mass Transf. 2016, 92, 1041–1052. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Gavrilov, A.N.; McCloskey, J.M.; Tolmachev, Y.V.; Sprunt, S.; Lopatina, L.M.; Selinger, J.V. Thermal conductivity and particle agglomeration in alumina nanofluids: Experiment and theory. Phys. Rev. E 2007, 76, 061203. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.U.S.; Zhang, Z.G.; Yu, W.; Lockwood, F.E.; Grulke, E.A. Anomalous thermal conductivity enhancement in nanotube suspensions. Appl. Phys. Lett. 2001, 79, 2252–2254. [Google Scholar] [CrossRef]
- Das, S.K.; Choi, S.U.; Yu, W.; Pradeep, T. Nanofluids: Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 9780470180693. [Google Scholar] [CrossRef]
- Machrafi, H.; Lebon, G. The role of several heat transfer mechanisms on the enhancement of thermal conductivity in nanofluids. Contin. Mech. Thermodyn. 2016, 28, 1461–1475. [Google Scholar] [CrossRef] [Green Version]
- Anoop, K.B.; Kabelac, S.; Sundararajan, T.; Das, S.K. Rheological and flow characteristics of nanofluids: Influence of electroviscous effects and particle agglomeration. J. Appl. Phys. 2009, 106, 034909. [Google Scholar] [CrossRef]
- Machrafi, H.; Lebon, G.; Iorio, C.S. Effect of volume-fraction dependent agglomeration of nanoparticles on the thermal conductivity of nanocomposites: Applications to epoxy resins, filled by SiO2, AlN and MgO nanoparticles. Compos. Sci. Technol. 2016, 130, 78–87. [Google Scholar] [CrossRef]
- Akhavan-Zanjani, H.; Saffar-Avval, M.; Mansourkiaei, M.; Sharif, F.; Ahadi, M. Experimental investigation of laminar forced convective heat transfer of Graphene–water nanofluid inside a circular tube. Int. J. Therm. Sci. 2016, 2016 100, 316–323. [Google Scholar] [CrossRef]
- Correa, J.D.; Orellana, P.A.; Pacheco, M. Optoelectronic properties of van der Waals hybrid structures: Fullerenes on graphene nanoribbons. Nanomaterials 2017, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wu, H. Graphene-based nanomaterials: Synthesis, properties, and optical and optoelectronic applications. Adv. Funct. Mater. 2013, 23, 1984–1997. [Google Scholar] [CrossRef]
- Bernardi, M.; Lohrman, J.; Kumar, P.V.; Kirkeminde, A.; Ferralis, N.; Grossman, J.C.; Ren, S. Nanocarbon-based photovoltaics. ACS Nano 2012, 6, 8896–8903. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Lee, H.; Youn, W.; Choi, M. Nanofluids containing multiwalled carbon nanotubes and their enhanced thermal conductivities. J. Appl. Phys. 2003, 94, 4967–4971. [Google Scholar] [CrossRef]
- Ding, Y.; Alias, H.; Wen, D.; Williams, R.A. Heat transfer of aqueous suspensions of carbon nanotubes (CNT nanofluids). Int. J. Heat Mass Transf. 2006, 49, 240–250. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, C.; Tang, Y.; Wang, J.; Ren, B.; Yin, Y. Preparation and thermal conductivity of suspensions of graphite nanoparticles. Carbon 2007, 45, 226–228. [Google Scholar] [CrossRef]
- Xie, H.; Yu, W.; Li, Y. Thermal performance enhancement in nanofluids containing diamond nanoparticles. J. Phys. D Appl. Phys. 2009, 42, 095413. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Yarmand, H.; Gharehkhani, S.; Ahmadi, G.; Shirazi, S.F.S.; Baradaran, S.; Montazer, E.; Zubir, M.N.M.; Alehashem, M.S.; Kazi, S. N.; Dahari, M. Graphene nanoplatelets–silver hybrid nanofluids for enhanced heat transfer. Energy Convers. Manag. 2015, 100, 419–428. [Google Scholar] [CrossRef]
- S tankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.B.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.J.; Jeon, J.H.; Noh, J.P.; Jeong, H.M.; Park, J.S.; Huh, S.C. Study on heat transfer characteristics of graphene nanofluids by physical grinding. J. Mech. Sci. Technol. 2017, 37–38. [Google Scholar]
- Kim, G.N.; Kim, J.H.; Kim, B.S.; Jeong, H.M.; Huh, S.C. Study on the thermal conductivity characteristics of graphene prepared by the planetary ball mill. Metals 2016, 6, 234. [Google Scholar] [CrossRef]
- Wang, Y.; Mortimer, M.; Chang, C.H.; Holden, P.A. Alginic acid-aided dispersion of carbon nanotubes, graphene, and boron nitride nanomaterials for microbial toxicity testing. Nanomaterials 2018, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Handy, R.D.; von der Kammer, F.; Lead, J.R.; Hassellov, M.; Owen, R.; Crane, M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 2008, 17, 287–314. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Bentley, J.P. Temperature sensor characteristics and measurement system design. J. Phys. E Sci. Instrum. 1984, 17, 430. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: Cambridge, MA, USA, 2013; ISBN 978-0-12-361961-7. [Google Scholar]
- Kwak, K.; Kim, C. Viscosity and thermal conductivity of copper oxide nanofluid dispersed in ethylene glycol. Korea-Aust. Rheol. J. 2005, 17, 35–40. [Google Scholar]
- Mondragon, R.; Julia, J.E.; Barba, A.; Jarque, J.C. Characterization of silica–water nanofluids dispersed with an ultrasound probe: A study of their physical properties and stability. Powder Technol. 2012, 224, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Al-Anssari, S.; Arif, M.; Wang, S.; Barifcani, A.; Iglauer, S. Stabilising nanofluids in saline environments. J. Colloid Interface Sci. 2017, 508, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Zhu, D.S.; Wang, X.J.; Wang, N.; Gao, J.W.; Li, H. Thermal conductivity enhancement dependent pH and chemical surfactant for Cu-H2O nanofluids. Thermochim. Acta 2008, 469, 98–103. [Google Scholar] [CrossRef]
- Kim, S.; Tserengombo, B.; Choi, S.; Noh, J.; Huh, S.; Choi, B.; Chung, H.; Kim, J.; Jeong, H. Experimental investigation of dispersion characteristics and thermal conductivity of various surfactants on carbon based nanomaterial. Int. Commun. Heat Mass Transf. 2018, 91, 95–102. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, H.; Kim, G.; Jeon, J.; Jeong, H.; Noh, J.; Kim, Y.; Kim, H.; Huh, S. Experimental Study on Characteristics of Grinded Graphene Nanofluids with Surfactants. Materials 2018, 11, 950. https://doi.org/10.3390/ma11060950
Seong H, Kim G, Jeon J, Jeong H, Noh J, Kim Y, Kim H, Huh S. Experimental Study on Characteristics of Grinded Graphene Nanofluids with Surfactants. Materials. 2018; 11(6):950. https://doi.org/10.3390/ma11060950
Chicago/Turabian StyleSeong, HeonJin, GwiNam Kim, JongHoon Jeon, HyoMin Jeong, JungPil Noh, YoungJu Kim, HyunJi Kim, and SunChul Huh. 2018. "Experimental Study on Characteristics of Grinded Graphene Nanofluids with Surfactants" Materials 11, no. 6: 950. https://doi.org/10.3390/ma11060950




