Degradation of Glaukonite Sandstone as a Result of Alkali-Silica Reactions in Cement Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Alkali Reactivity Testing
2.3. Physical Properties of Sandstone
2.4. Microscopic Observations
3. Results
3.1. Identification of Reactive Phases
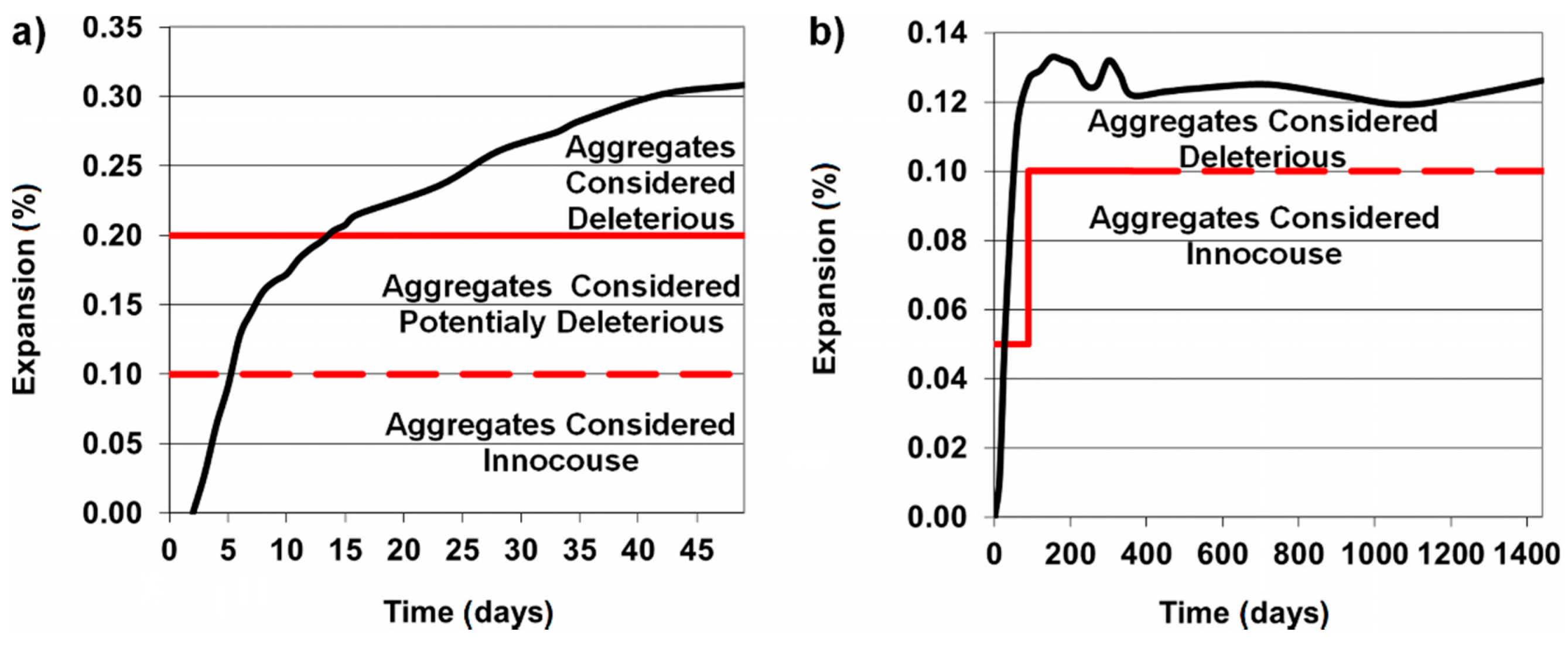
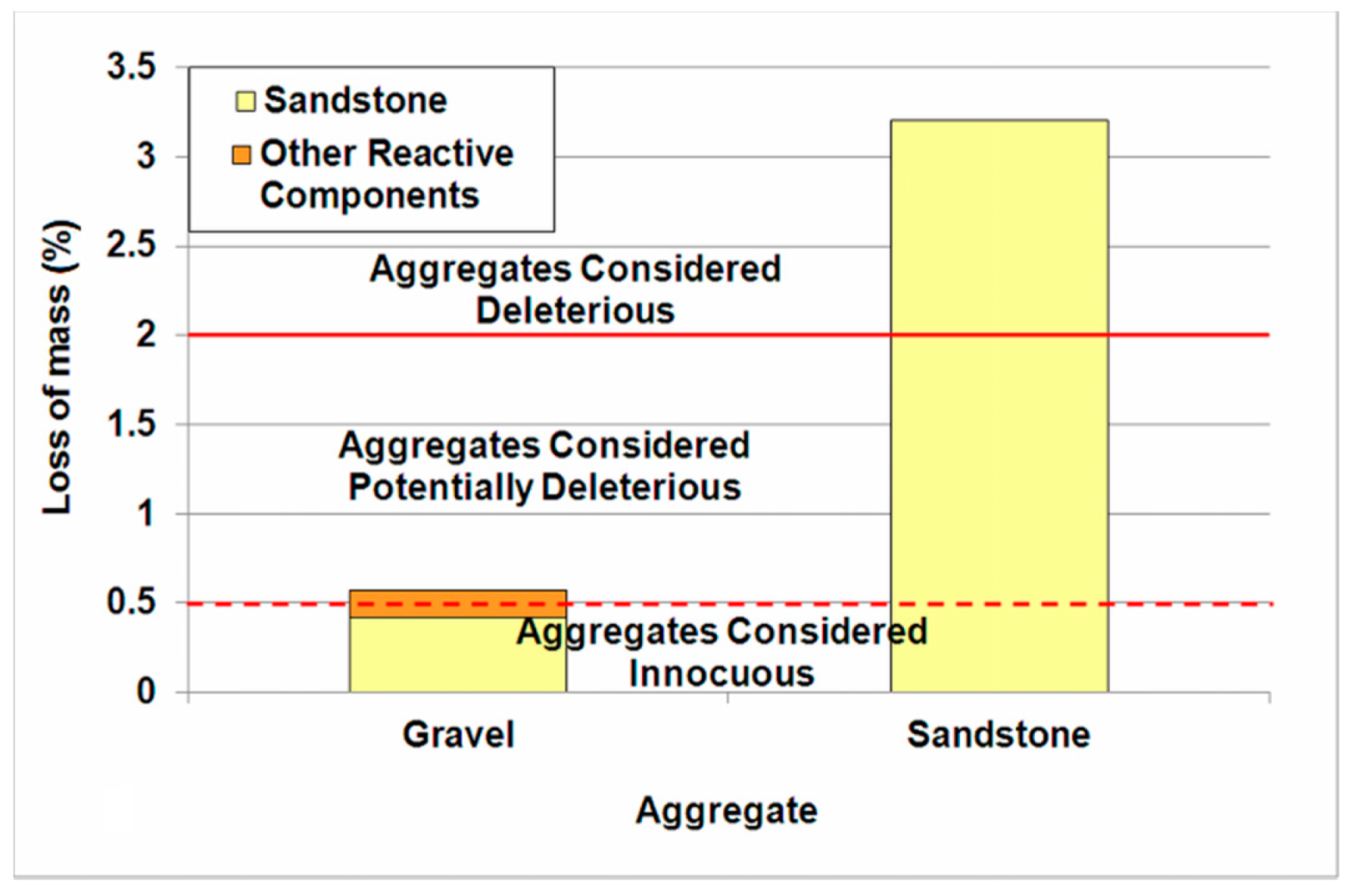
3.2. Gravel and Sandstone Reactivity
3.3. Physical Properties
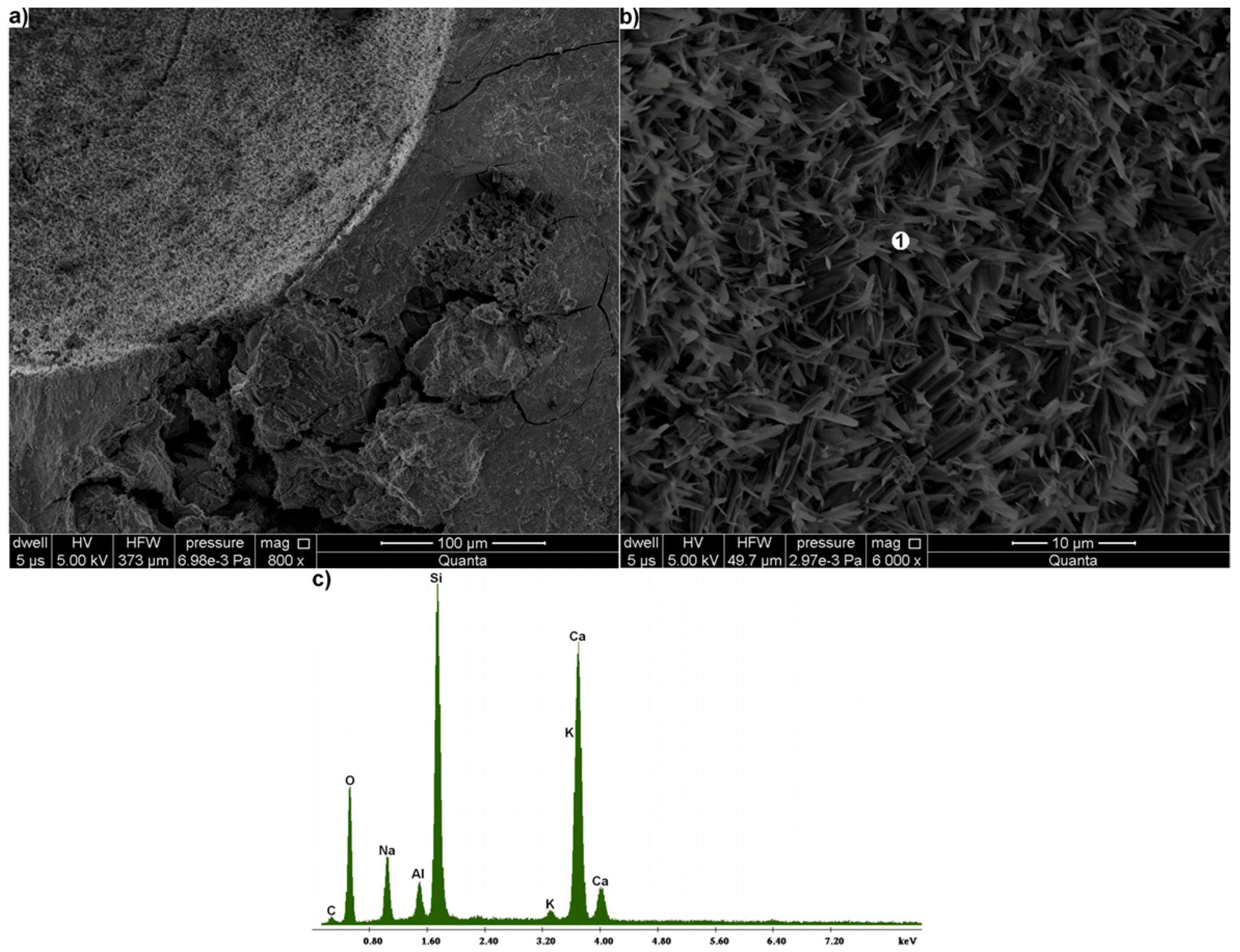
3.4. Sandstone Degradation in Mortar
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Pignatelli, R.; Comi, C.; Monteiro, P.J.M. A coupled mechanical and chemical damage model for concrete affected by alkali-silica reaction. Cem. Concr. Res. 2013, 53, 196–210. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Fournier, B.; Folliard, K.J. Report No. FHWA-HIF-13-019: Alkali-Aggregate Reactivity (AAR) Facts Book; Office of Pavement Technology Federal Highway Administration: Washington, DC, USA, 2013. [Google Scholar]
- Katayama, T. The so-called alkali-carbonate reaction (ACR)—Its mineralogical and geochemical detail, with special reference to ASR. Cem. Concr. Res. 2010, 40, 643–675. [Google Scholar] [CrossRef]
- Grattan-Bellew, P.E.; Mitchell, L.D.; Margesin, J.; Min, D. Is alkali-carbonate reaction just a variant of silica reaction ACR = ASR? Cem. Concr. Res. 2010, 40, 556–562. [Google Scholar] [CrossRef]
- Qian, G.; Deng, M.; Lan, X.; Xu, Z.; Tang, M. Alkali carbonate reaction expansion of dolomitic limestone aggregate with porphyrotopic texture. Eng. Geol. 2002, 63, 17–19. [Google Scholar] [CrossRef]
- Jensen, V. Reclassification of Alkali Aggregate Reaction. In Proceedings of the 14th International Conference on Alkali-Aggregate Reaction in Concrete, Austin, TX, USA, 20–25 May 2012. [Google Scholar]
- Islam, M.S. Prediction of ultimate expansion of ASTM C 1260 for various alkali solutions using the proposed decay model. Constr. Build. Mater. 2015, 77, 317–326. [Google Scholar] [CrossRef]
- Owsiak, Z. Dependence between the composition of pore solution and the expansion of mortar containing reactive aggregate. Ceram.-Silik. 2005, 49, 109–114. [Google Scholar]
- Broekmans, M.A.T.M. Structural properties of quartz and their potential role for ASR. Mater. Charact. 2004, 53, 129–140. [Google Scholar] [CrossRef]
- Fernandes, I.; Ribeiro, M.A.; Broekmans, M.A.T.M.; Sims, I. RILEM Recommended Test Method: Detection of Aggregates Regarding Potential Reactivity to Alkalis; Springer Verlag: Berlin/Heidenberg, Germany, 2016; ISBN 978-94-017-7251-8. [Google Scholar]
- Ramos, V.; Fernandes, I.; Silva, A.S.; Soares, D.; Fournier, B.; Leal, S.; Noronha, F. Assessment of the potential reactivity of granitic rocks—Petrography and expansion test. Cem. Concr. Res. 2016, 86, 63–77. [Google Scholar] [CrossRef]
- Jarmontowicz, A.; Krzywobłocka-Laurów, R. Instrukcja ITB 317: Ocena Potencjalnej Reaktywności Kruszywa Żwirowego w Stosunku do Alkalii na Podstawie Badań Instrumentalnych; Instytut Techniki Budowlanej: Warsaw, Poland, 1993. [Google Scholar]
- Leemann, A.; Lothenbach, B.; Thalmann, C. Influence of superplasticizers on pore solution and on expansion of concrete due to alkali-silica reaction. Constr. Build. Mater. 2011, 25, 344–350. [Google Scholar] [CrossRef]
- Owsiak, Z. Contribution of alkali from aggregate to pore solution of concrete. Cem. Lime Concr. 2001, 6, 149–153. [Google Scholar]
- Owsiak, Z. The alkali-silica reaction in concrete. Pol. Cer. Bull. Ceram. 2002, 72, 5–107. [Google Scholar]
- Holleman, A.F.; Wiberg, N.; Wiberg, E. Lehrbuch der Anorganischen Chemie; Walter Gruyter Verlag: Berlin, Germany; New York, NY, USA, 1985; ISBN 3-11-007511-3. [Google Scholar]
- Owsiak, Z.; Czapik, P. Interfacial transition zone of cement paste-reactive aggregate in cement—Zeolite mortars. Bull. Pol. Acad. Sci. Tech. Sci. 2015, 63, 31–34. [Google Scholar] [CrossRef]
- Zapała-Sławeta, J.; Owsiak, Z. Effect of lithium nitrate on the reaction between opal aggregate and sodium and potassium hydroxides in concrete over a long period of time. Bull. Pol. Acad. Sci. Tech. Sci. 2017, 65, 773–778. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Tang, W.; Hu, N.; Wei, J. Inhibitory Effect of Waste Glass Powder on ASR Expansion Induced by Waste Glass Aggregate. Materials 2015, 8, 6849–6862. [Google Scholar] [CrossRef] [PubMed]
- Owsiak, Z. The effect of delayed ettringite formation and alkali-silica reaction on concrete microstructure. Ceram.-Silik. 2010, 54, 151–153. [Google Scholar]
- Hamoudi, A.; Khouchaf, L.; Depecker, C.; Revel, B.; Montagne, L.; Corrdier, P. Microstructural evolution of amorphous silica following alkali-silica reaction. J. Non-Cryst. Solids 2008, 354, 5074–5078. [Google Scholar] [CrossRef]
- Alnaggar, M.; Di Luzio, G.; Cusatis, G. Modeling Time-Dependent Behavior of Concrete Affected by Alkali Silica Reaction in Variable Environmental Conditions. Materials 2017, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Lukovic, M.; De Schutter, G.; Ye, G.; Taerwe, L. Elastic Modulus of the Alkali-Silica Reaction Rim in a Simplified Calcium-Alkali-Silicate System Determined by Nano-Indentation. Materials 2016, 9, 787. [Google Scholar] [CrossRef] [PubMed]
- Hin, J.-H.; Struble, L.J.; Kirkpatrick, R.J. Microstructural Changes Due to Alkali-Silica Reaction during Standard Mortar Test. Materials 2015, 8, 8292–8303. [Google Scholar] [CrossRef]
- ASTM C441/C441M. Standard Test Method for Effectiveness of Pozzolans or Ground Blast-Furnace Slag in Preventing Excessive Expansion of Concrete Due to the Alkali-Silica Reaction; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Manecki, A.; Muszyński, M. Przewodnik do Petrografii; AGH: Cracow, Poland, 2008; ISBN 979-83-7464-110-4. [Google Scholar]
- Gao, X.X.; Multon, S.; Cyr, M.; Sellier, A. Alkali-silica reaction (ASR) expansion, Pessimum effect versus scale effect. Cem. Concr. Res. 2013, 44, 25–33. [Google Scholar] [CrossRef]
- Rivard, P.; Ollivier, J.-P.; Ballivy, G. Characterization of the ASR rim. Application to the Potsdam sandstone. Cem. Concr. Res. 2002, 32, 1259–1267. [Google Scholar] [CrossRef]
- Lindgård, J.; Andiç-Çakır, Ö.; Fernandes, I.; Rønning, T.F.; Thomas, M.D.A. Alkali-silica reaction (ASR), Literature review on parameters influencing laboratory performance testing. Cem. Concr. Res. 2012, 42, 223–243. [Google Scholar] [CrossRef]
- López-Buendia, A.M.; Climent, V.; Verdú, P. Lithological influence of aggregate in the alkali-carbonate reaction. Cem. Concr. Res. 2006, 36, 1490–1500. [Google Scholar] [CrossRef]
- Newman, J.; Choo, B.-S. Advanced Concrete Technology Volume II: Concrete Properties; Elsevier: Oxford, UK, 2003. [Google Scholar]
- Owsiak, Z.; Zapała-Sławeta, J.; Czapik, P. Diagnosis of concrete structures distress due to alkali-aggregate reaction. Bull. Pol. Acad. Sci. Tech. Sci. 2015, 63, 23–30. [Google Scholar] [CrossRef]
- Owsiak, Z.; Zapała, J.; Czapik, P. Sources of the gravel aggregate reaction with alkalis in concrete. Cem. Lime Concr. 2012, 17, 149–153. [Google Scholar]
- Piasta, W.; Budzyński, W.; Góra, J. The effect of selected aggregates on the properties of high performance concrete. Cem. Lime Concr. 2015, 20, 171–178. [Google Scholar]
- Jóźwiak-Biedźwiedzka, D.; Gibas, K.; Glinicki, M.A. Petrographic identification of reactive minerals in domestic aggregates and their classification according to RILEM and ASTM recommendation. Road Bridges Drogi i Mosty 2017, 16, 223–239. [Google Scholar] [CrossRef]
- ASTM C1260–14. Standard Test Method for Potential Reactivity of Aggregates (Mortar-Bar Method); ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM C227–10. Standard Test Method for Potential Alkali Reactivity of Cement—Aggregate Combinations (Mortar-Bar Method); ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Owsiak, Z.; Czapik, P. The reduction of expansion of mortars produced from reactive aggregate by the clinoptilolite addition. Cem. Lime Concr. 2014, 19, 152–157. [Google Scholar]
- Owsiak, Z.; Czapik, P. Limitation of the effects at AAR in concrete by the addition of zeolite. Cem. Lime Concr. 2013, 18, 310–320. [Google Scholar]
- PN-92/B-06714-46. Mineral Aggregate-Test—Determination of Potential Alkaline Reactivity by the Fast Method; Polish Committee for Standardization: Warsaw, Poland, 1992. [Google Scholar]
- PN-EN 1936:2010. Natural Stone Test Methods—Determination of Real Density and Apparent Density, and of Total and Open Porosity; Polish Committee for Standardization: Warsaw, Poland, 2010. [Google Scholar]
- Leemann, A. Raman microscopy of alkali-silica reaction (ASR) products formed in concrete. Cem. Concr. Res. 2017, 102, 41–47. [Google Scholar] [CrossRef]
- National Ready Mixed Concrete Association. Guide Specifications for Concrete Subject to Alkali-Silica Reactions; NRMCA: Silver Spring, MD, USA, 1993. [Google Scholar]
- Ponce, J.M.; Batic, O.R. Different manifestation of the alkali-silica reaction in concrete according to the kinetics of the reactive aggregate. Cem. Concr. Res. 2006, 36, 1148–1156. [Google Scholar] [CrossRef]
- Handke, M. Krystalochemia krzemianów; AGH: Cracow, Poland, 2005. [Google Scholar]
- Monnin, Y.; Dégrugilliers, P.; Bulteel, D.; Garcia-Diaz, E. Petrography study of two siliceous limestones submitted to alkali-silica reaction. Cem. Concr. Res. 2006, 36, 1460–1466. [Google Scholar] [CrossRef]
- Milanesi, C.A.; Marfil, S.A.; Batic, O.R.; Maiza, P.J. The alkali-carbonate reaction and its reaction products an experience with Argentinean dolomite rocks. Cem. Concr. Res. 1996, 26, 1579–1591. [Google Scholar] [CrossRef]
- Kurdowski, W.; Garbacik, A.; Trybalska, B. Application of accelerated test ASTM C1260 to aggregate containing calcium carbonate. Cem. Lime Concr. 2005, 10, 339–348. [Google Scholar]


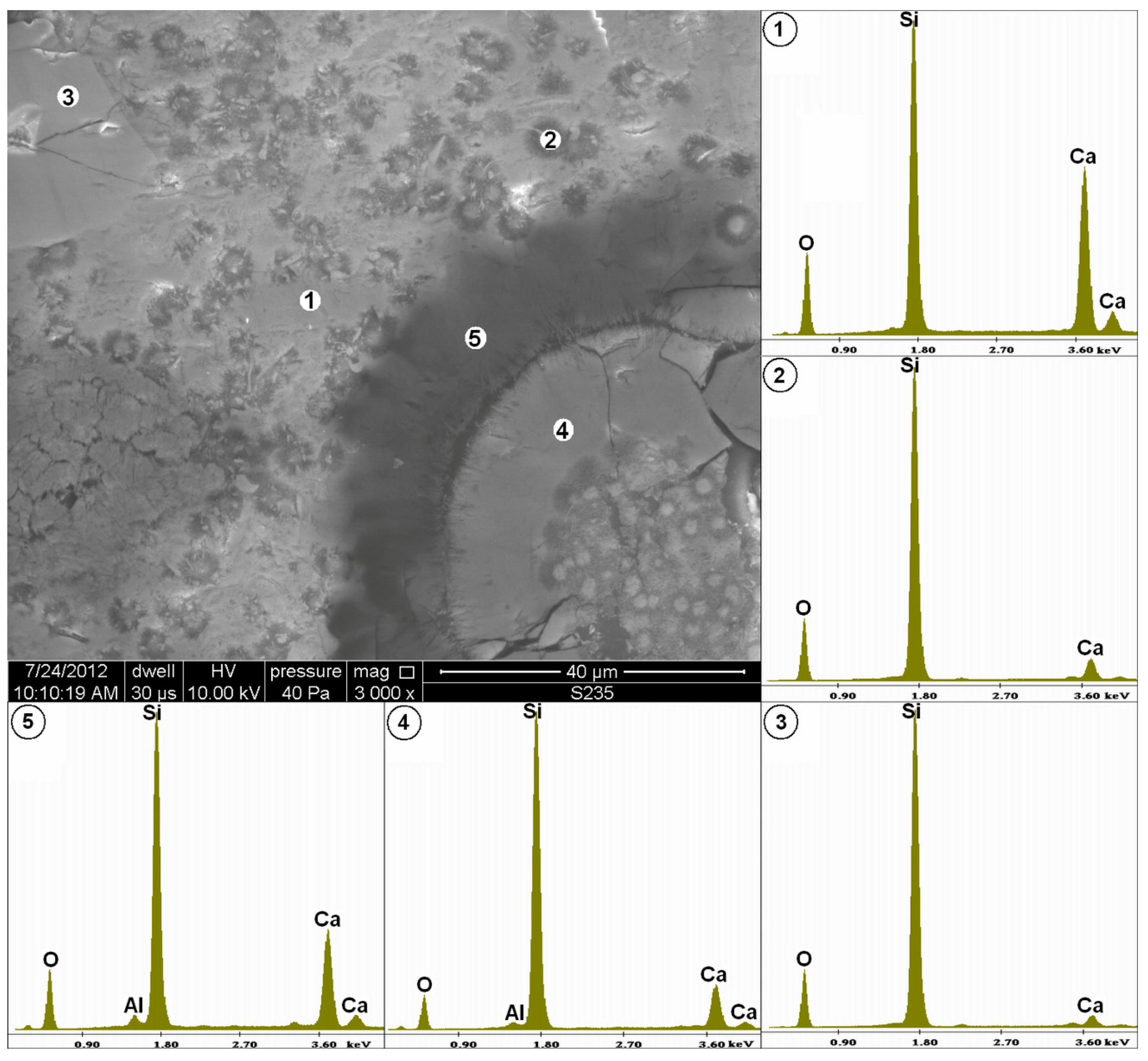

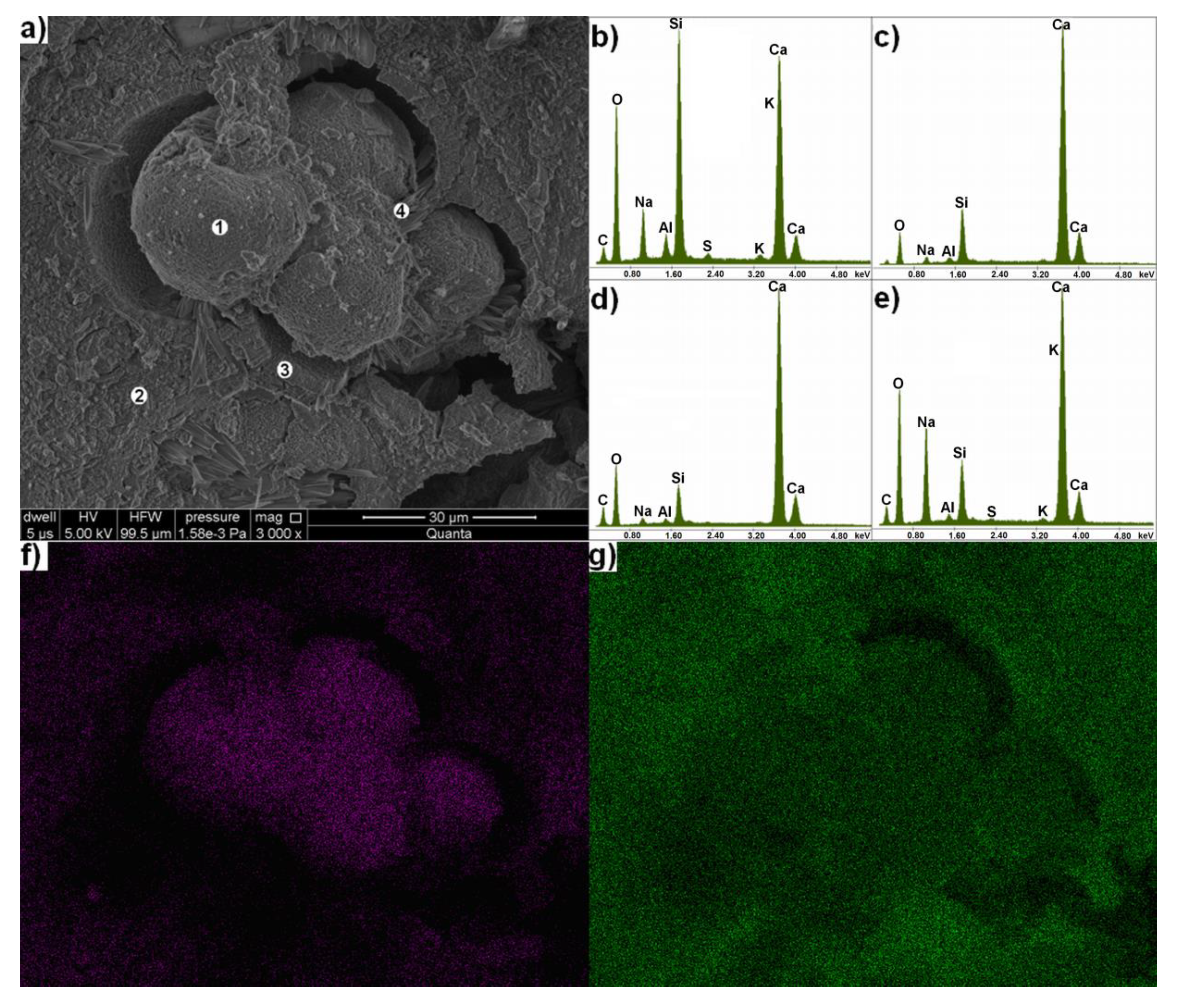
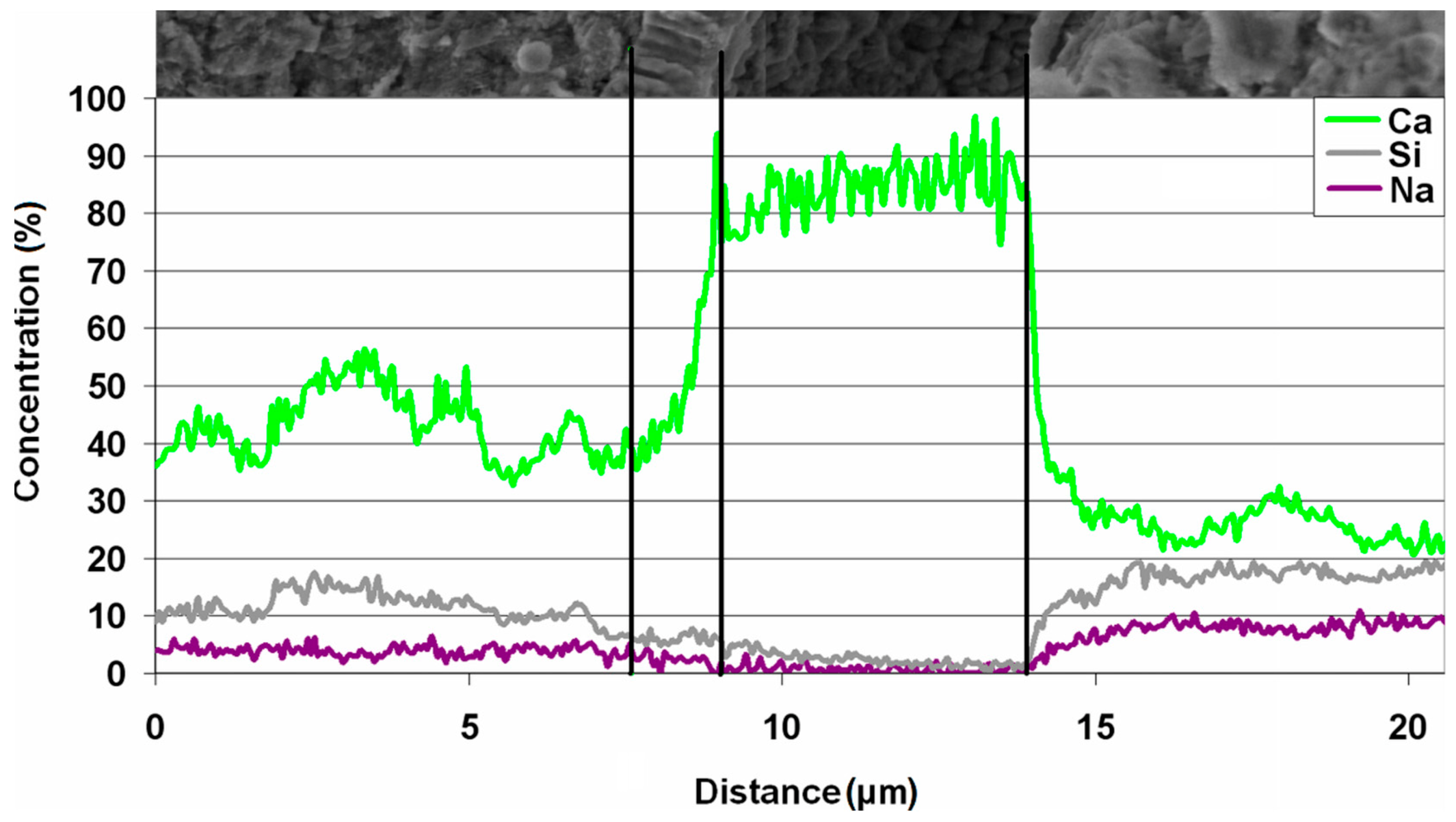
| Quartz-Glaukonite Sandstone | Organo-Dendritic, Sparite-Micrite Limestone | Metamorphous Quartz-Pyroxene Shale with Opal | Feldspar-Biotite Granite | Unreactive Component (e.g., Limestone, Dolostone, Granite, Quartzite) |
|---|---|---|---|---|
| 13.8 | 28.0 | 3.9 | 10.5 | 43.8 |
| Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Alkali | TiO2 | LOI | I.R. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| K2O | Na2O | ||||||||||
| Cement | 20.06 | 4.77 | 2.98 | 61.29 | 1.79 | 3.01 | 1.14 | 0.15 | 0.45 | 2.98 | 0.99 |
| Mineral Component | Content (%) |
|---|---|
| Cement | 50.8 |
| Quartz | 25.4 |
| Potassium feldspar | 0.4 |
| Plagioclase | 0.2 |
| Chalcedony-LF | 7.2 |
| Muscovite | 0.6 |
| Calcite | 4.4 |
| Glaukonite | 11.0 |
| Open Porosity (%) | Apparent Density (g·cm−3) |
|---|---|
| 10 | 2.26 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czapik, P. Degradation of Glaukonite Sandstone as a Result of Alkali-Silica Reactions in Cement Mortar. Materials 2018, 11, 924. https://doi.org/10.3390/ma11060924
Czapik P. Degradation of Glaukonite Sandstone as a Result of Alkali-Silica Reactions in Cement Mortar. Materials. 2018; 11(6):924. https://doi.org/10.3390/ma11060924
Chicago/Turabian StyleCzapik, Przemysław. 2018. "Degradation of Glaukonite Sandstone as a Result of Alkali-Silica Reactions in Cement Mortar" Materials 11, no. 6: 924. https://doi.org/10.3390/ma11060924
APA StyleCzapik, P. (2018). Degradation of Glaukonite Sandstone as a Result of Alkali-Silica Reactions in Cement Mortar. Materials, 11(6), 924. https://doi.org/10.3390/ma11060924





