Microstructures Evolution and Micromechanics Features of Ni-Cr-Si Coatings Deposited on Copper by Laser Cladding
Abstract
:1. Introduction
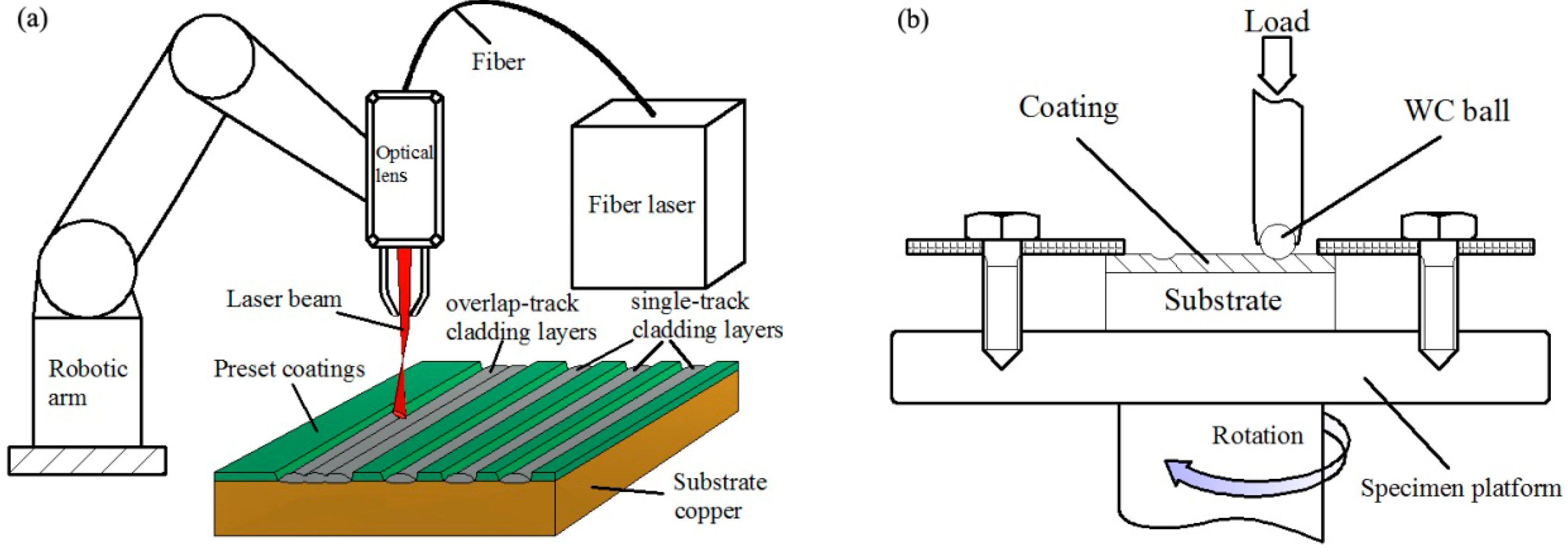
2. Materials and Methods
3. Results
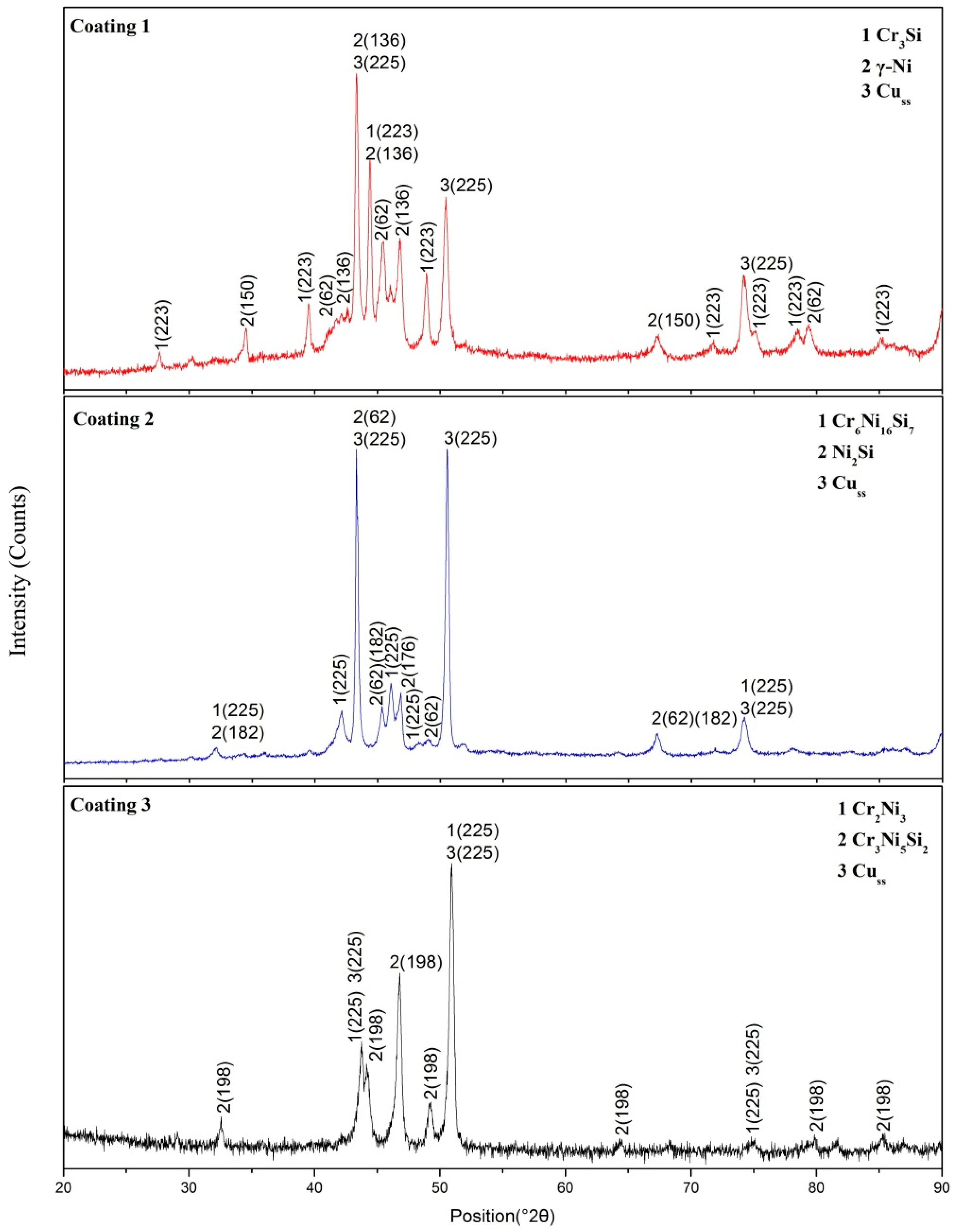
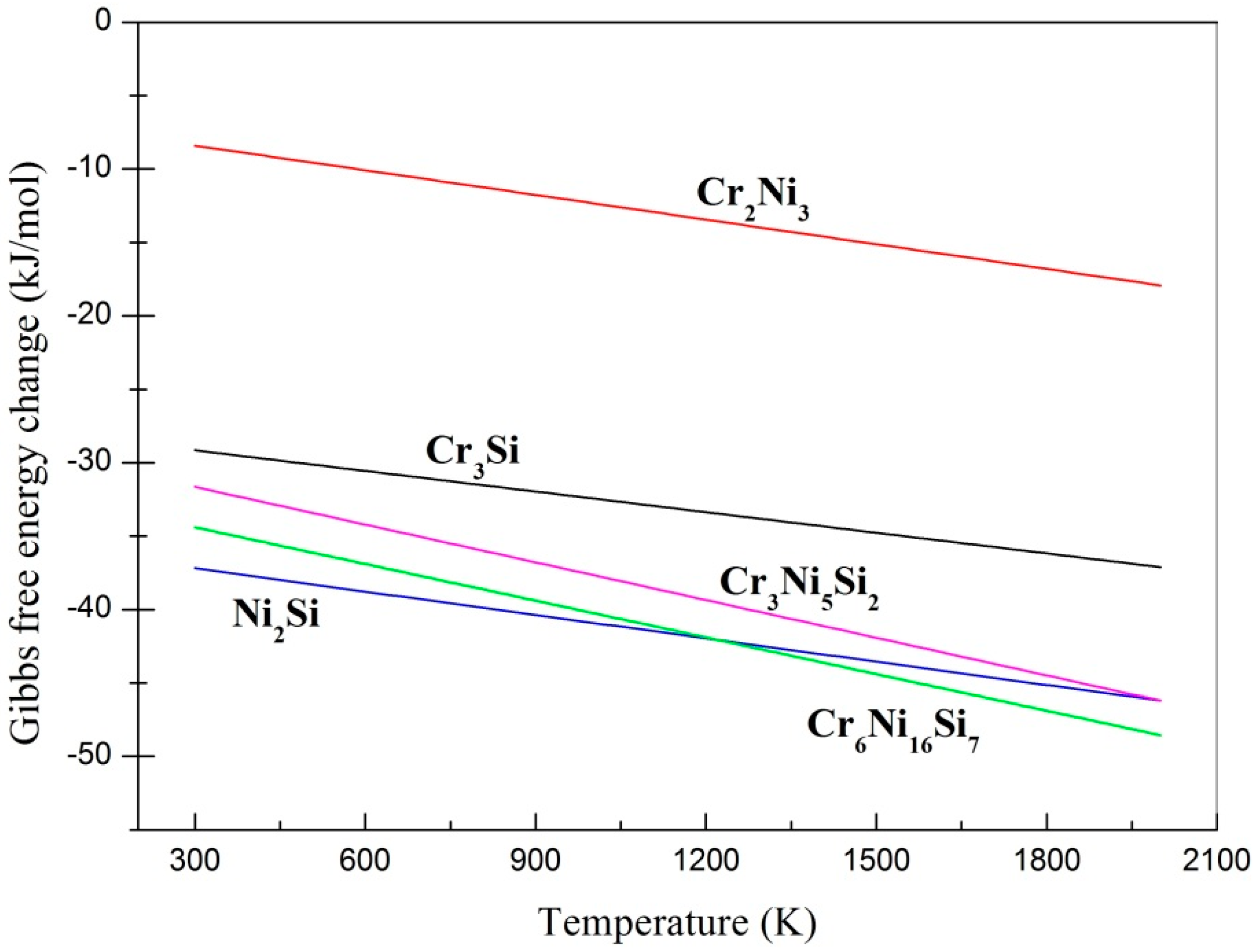
3.1. Phase Identification and Gibbs Free Energy Calculation
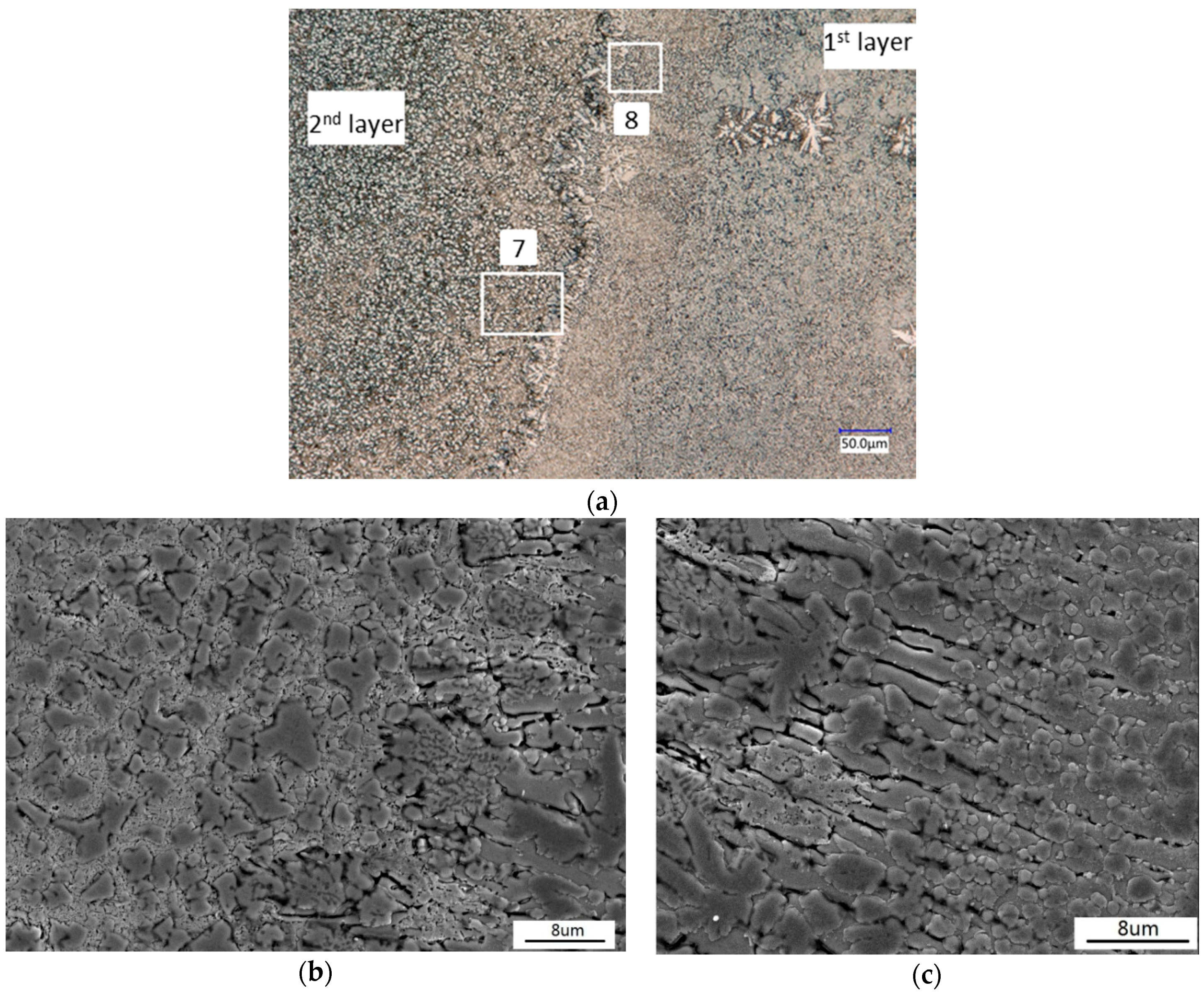
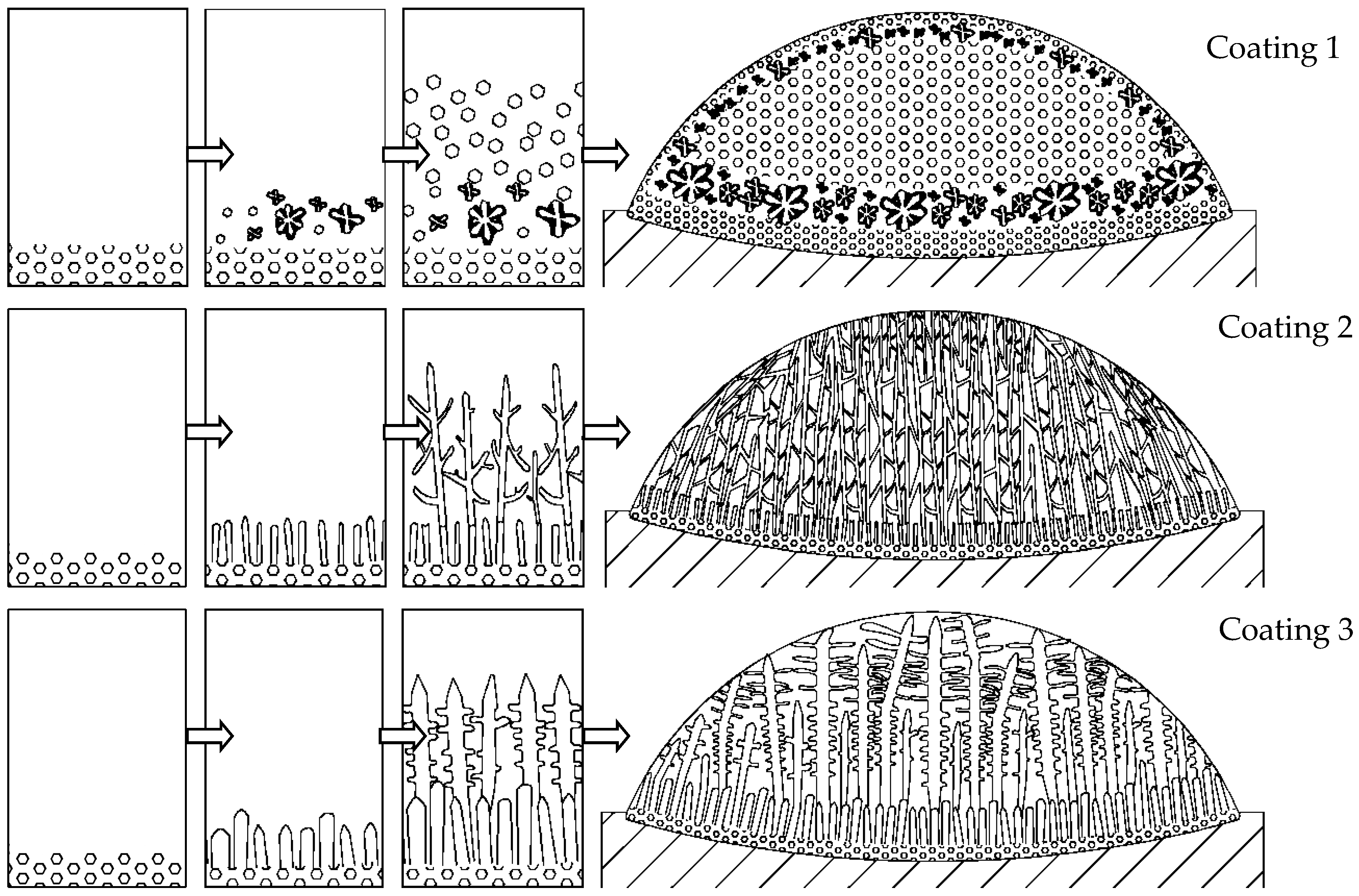
3.2. Microstructure Analysis
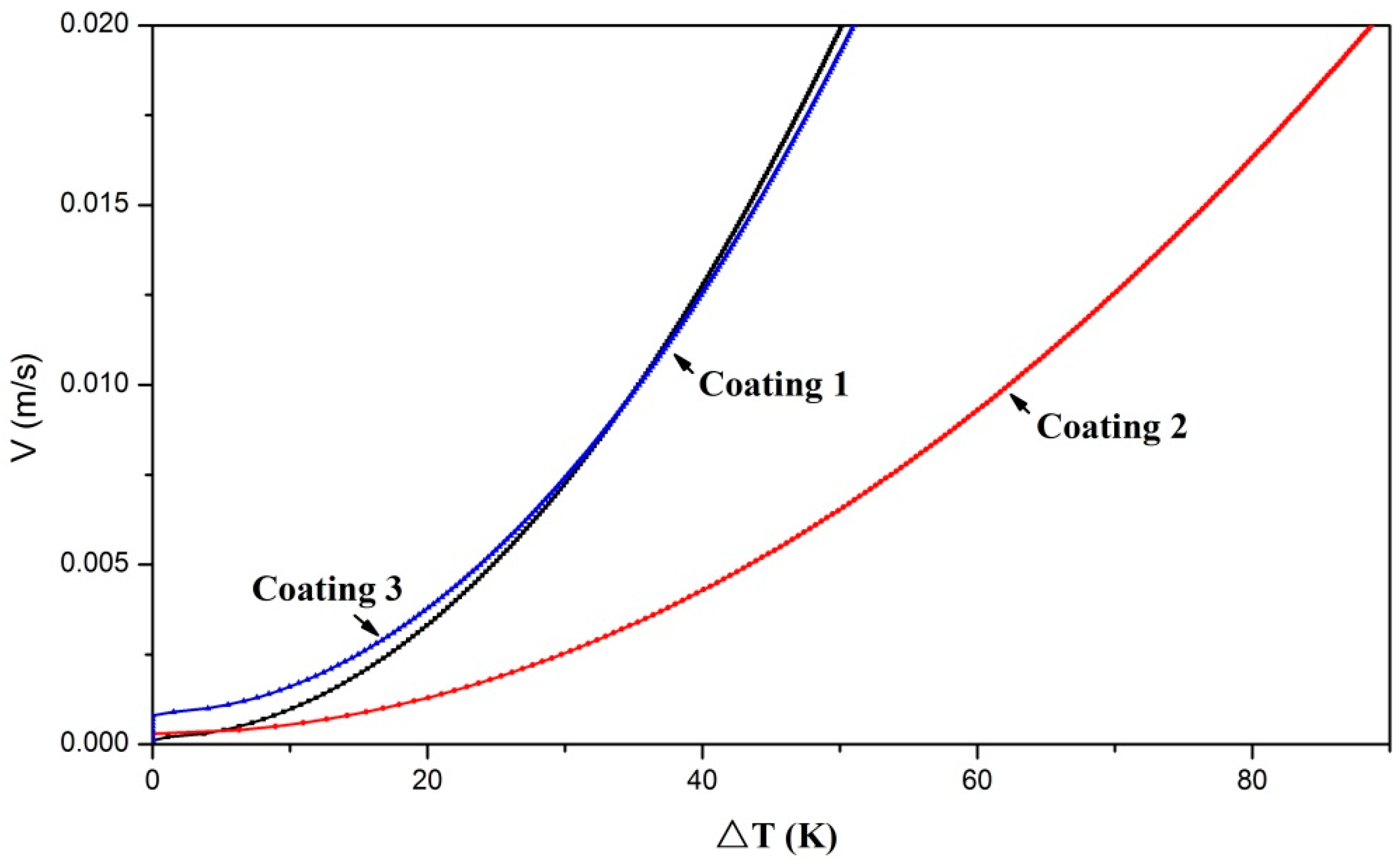
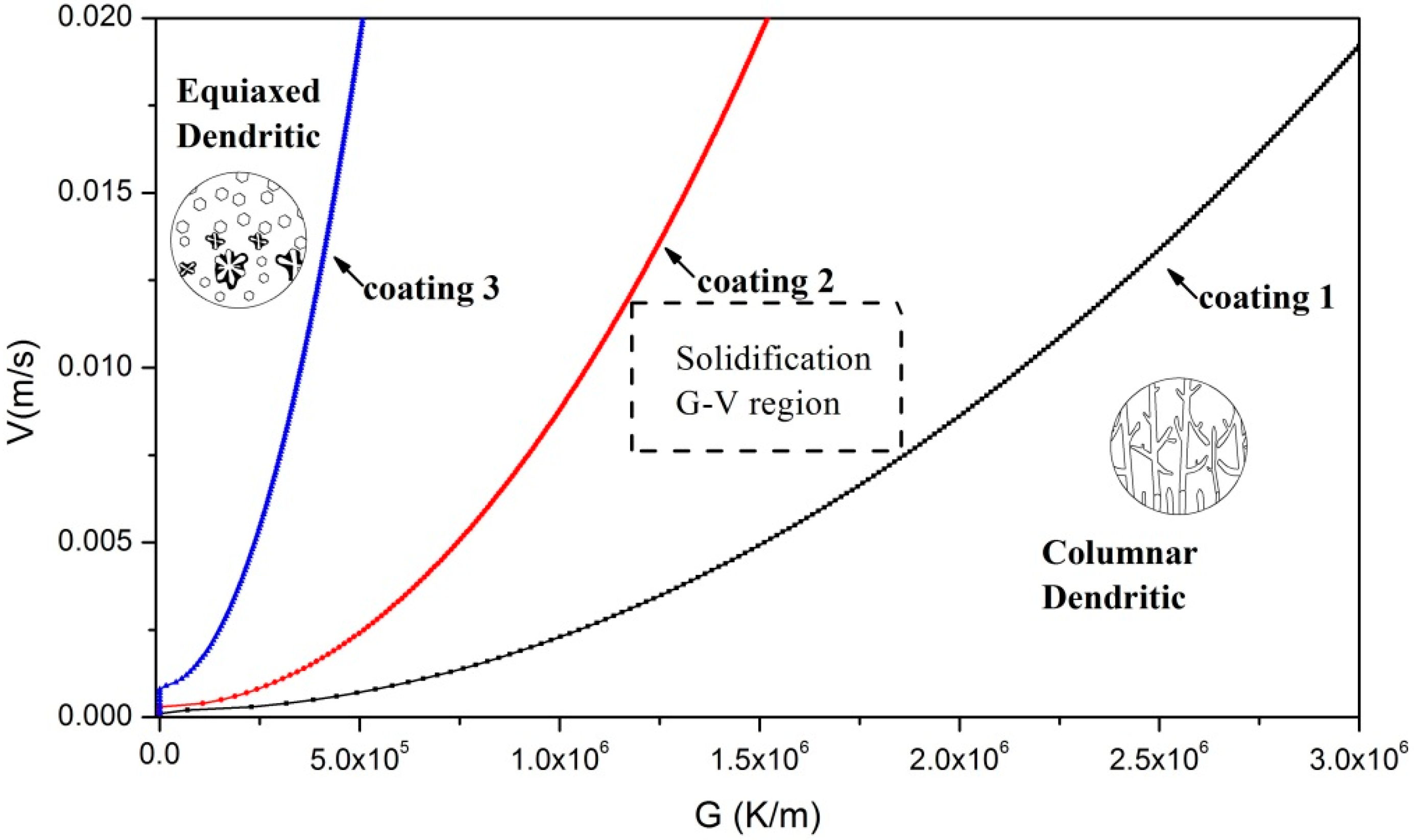
3.3. Analysis of the Solidification Process
3.4. Micromechanics Features
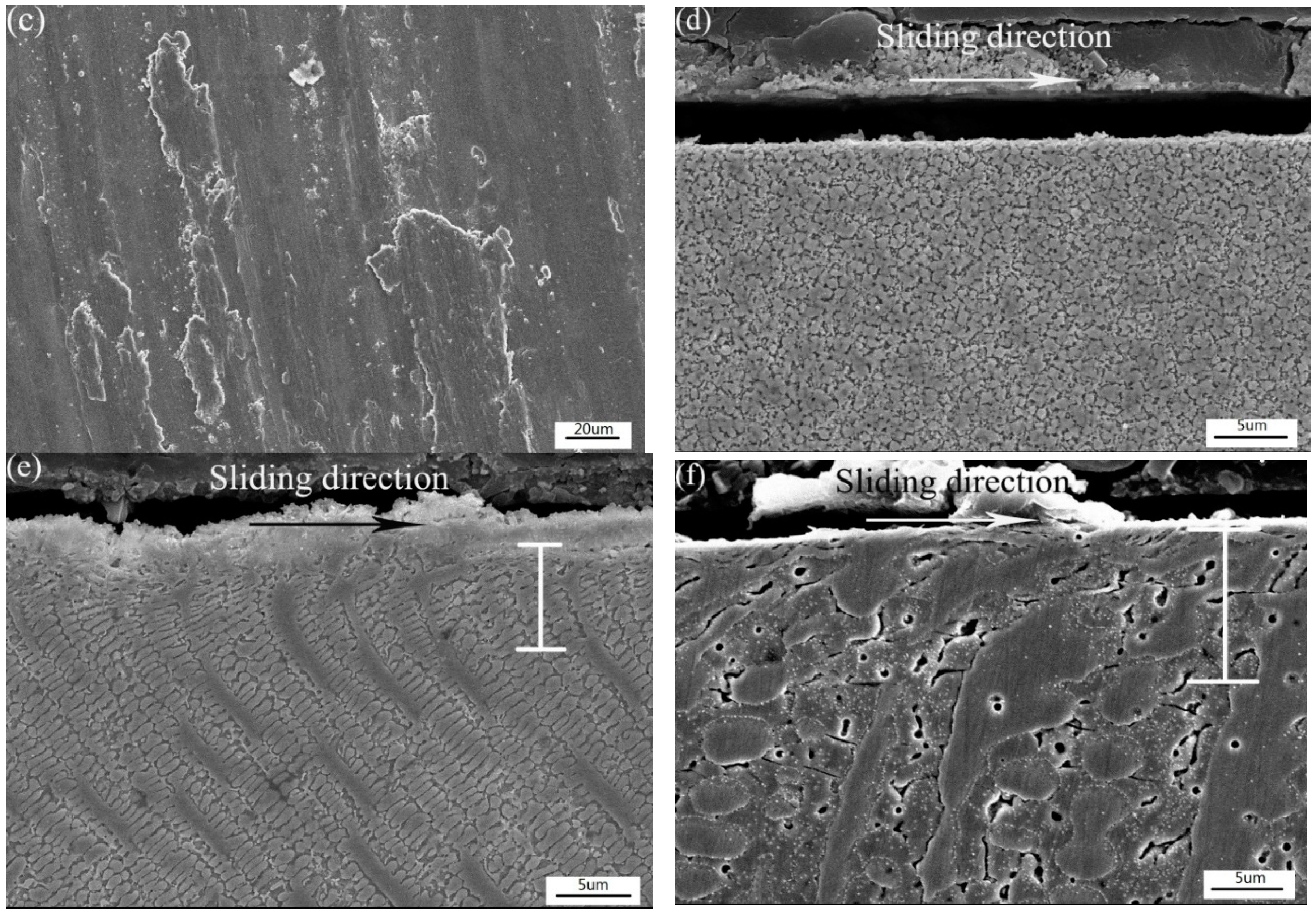
3.5. Tribological Behavior
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yan, H.; Zhang, P.L.; Yu, Z.S.; Lu, Q.H.; Yang, S.L.; Li, C.G. Microstructure and tribological properties of laser-clad Ni–Cr/TiB2 composite coatings on copper with the addition of CaF2. Surf. Coat. Technol. 2012, 206, 4046–4053. [Google Scholar] [CrossRef]
- Dehm, G.; Medres, B.; Shepeleva, L.; Scheua, C.; Mordikeb, B.L.; Mordikeb, S.; Rykc, G.; Halperinc, G.; Etsionc, I. Microstructure and tribological properties of Ni-based claddings on Cu substrates. Wear 1999, 225–229, 18–26. [Google Scholar] [CrossRef]
- Zhang, P.L.; Liu, X.P.; Lu, Y.L.; Yan, H.; Yu, Z.S.; Li, C.G.; Lu, Q.H. Microstructure and wear behavior of Cu–Mo–Si coatings by laser cladding. Appl. Surf. Sci. 2014, 311, 709–714. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Tu, Y.; Xi, M.Z.; Shi, L.K. CHARACTERIZATION on laser clad nickel based alloy coating on pure copper. Surf. Coat. Technol. 2008, 202, 5924–5928. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, J.; Zhang, P.L.; Yu, Z.S.; Li, C.G.; Xu, P.Q.; Lu, Y.L. Laser cladding of Co-based alloy/TiC/CaF2 self-lubricating composite coatings on copper for continuous casting mold. Surf. Coat. Technol. 2013, 232, 362–369. [Google Scholar] [CrossRef]
- Yue, T.M.; Xie, H.; Lin, X. Solidification behaviour in laser cladding of AlCoCrCuFeNi high-entropy alloy on magnesium substrates. J. Alloy. Compd. 2014, 587, 588–593. [Google Scholar] [CrossRef]
- Dehm, G.; Bamberger, M. Laser cladding of Co-based hardfacing on Cu substrate. J. Mater. Sci. 2002, 37, 5345–5353. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.S.; Chen, S.Y.; Tao, X.Q.; Zhang, Y. Laser cladding Ni–Co duplex coating on copper substrate. Opt. Lasers Eng. 2010, 48, 792–799. [Google Scholar] [CrossRef]
- Ng, K.W.; Man, H.C.; Cheng, F.T.; Yue, T.M. Laser cladding of copper with molybdenum for wear resistance enhancement in electrical contacts. Appl. Surf. Sci. 2007, 253, 6236–6246. [Google Scholar] [CrossRef]
- Wang, H.M.; Duan, G. Microstructure and wear resistance of a laser clad reinforced Cr3Si metal silicide composite coating. Mater. Sci. Eng. 2002, A336, 117–123. [Google Scholar] [CrossRef]
- Raj, S.V. An evaluation of the properties of Cr3Si alloyed with Mo. Mater. Sci. Eng. A 1995, 201, 229–241. [Google Scholar] [CrossRef]
- Wang, H.M.; Duan, G. Wear and corrosion behavior of laser clad Cr3Si reinforced intermetallic composite coatings. Intermetallics 2003, 11, 755–762. [Google Scholar] [CrossRef]
- Duan, G.; Wang, H.M. High-temperature wear resistance of a laser-clad γ/Cr3Si metal silicide composite coating. Scr. Mater. 2002, 46, 107–111. [Google Scholar] [CrossRef]
- Gupta, K.P. The Cr-Ni-Si (Chromium-Nickel-Silicon) System. J. Phase Equilib. Diffus. 2006, 27, 523–528. [Google Scholar] [CrossRef]
- Tang, H.B.; Fang, Y.L.; Wang, H.M. Microstructure and dry sliding wear resistance of a Cr13Ni5Si2 ternary metal silicide alloy. Acta Mater. 2004, 52, 1773–1783. [Google Scholar] [CrossRef]
- Jian, L.N.; Wang, H.M. Microstructure and wear behaviours of laser-clad Cr13Ni5Si2-based metal-silicide coatings on a titanium alloy. Surf. Coat. Technol. 2005, 192, 305–310. [Google Scholar] [CrossRef]
- Lu, X.D.; Wang, H.M. Microstructure and dry sliding wear properties of laser clad Mo2Ni3Si/NiSi metal silicide composite coatings. J. Alloy. Compd. 2003, 359, 287–291. [Google Scholar] [CrossRef]
- Lu, X.D.; Wang, H.M. High-temperature sliding wear behaviors of laser clad Mo2Ni3Si/NiSi metal silicide composite coating. Appl. Surf. Sci. 2003, 214, 190–195. [Google Scholar] [CrossRef]
- Lu, X.D.; Wang, H.M. High-temperature phase stability and tribological properties of laser clad Mo2Ni3Si/NiSi metal silicide coatings. Acta Mater. 2004, 52, 5419–5426. [Google Scholar] [CrossRef]
- Lu, X.D.; Wang, H.M. Corrosive sliding wear behavior of laser clad Mo2Ni3Si/NiSi intermetallic coating. Appl. Surf. Sci. 2005, 245, 346–352. [Google Scholar] [CrossRef]
- Lu, X.D.; Wang, H.M.; Zhou, Z.R. Reciprocating sliding wear behavior of laser-clad small amplitude Mo2Ni3Si/NiSi metal silicide composite coatings. Appl. Surf. Sci. 2005, 240, 432–440. [Google Scholar] [CrossRef]
- Lu, X.D.; Wang, H.M. Dry sliding wear behavior of laser clad Mo2Ni3Si/NiSi metal silicide composite coatings. Thin Solid Films 2005, 472, 297–301. [Google Scholar] [CrossRef]
- Xu, Y.W.; Wang, H.M. Oxidation behavior of γ/Mo2Ni3Si ternary metal silicide alloy. J. Alloy. Compd. 2008, 457, 239–243. [Google Scholar] [CrossRef]
- Gui, Y.L.; Song, C.Y.; Yang, L.; Qin, X.L. Microstructure and tribological properties of NiMo/Mo2Ni3Si intermetallic “in-situ” composites. J. Alloy. Compd. 2011, 509, 4987–4991. [Google Scholar] [CrossRef]
- Song, C.Y.; Gui, Y.L.; Kuang, S.B.; Wang, S.H.; Zhao, D.G. Microstructure and wear resistance of a novel Mo-Ni-Si system intermetallic composite with ductile Mo phase. Mater. Trans. 2016, 57, 721–725. [Google Scholar] [CrossRef]
- Huang, B.Y.; Song, C.Y.; Liu, Y.; Gui, Y.L. Microstructure characterization and wear-resistant properties evaluation of an intermetallic composite in Ni-Mo-Si system. Materials 2017, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Zhang, P.L.; Li, M.C.; Yan, H.; Yu, Z.S.; Lu, Q.H. Microstructure, Wear Resistance and Oxidation Behavior of Ni-Ti-Si Coatings Fabricated on Ti6Al4V by Laser Cladding. Materials 2017, 10, 1248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.L.; Liu, X.P.; Yan, H. Phase composition, microstructure evolution and wear behavior of Ni-Mn-Si coatings on copper by laser cladding. Surf. Coat. Technol. 2017, 332. [Google Scholar] [CrossRef]
- Lu, Y.L.; Zhang, P.L.; Ma, K.; Yu, Z.S.; Yan, H.; Li, C.G. Microstructure and properties of laser alloying Ni-W-Si composite coating. Rare Metal Mater. Eng. 2016, 45, 375–380. [Google Scholar]
- Yan, H.; Zhang, P.L.; Yu, Z.S.; Li, C.G.; Li, R.D. Development and characterization of laser surface cladding (Ti,W)C reinforced Ni–30Cu alloy composite coating on copper. Opt. Laser Technol. 2012, 44, 1351–1358. [Google Scholar] [CrossRef]
- Tang, R.Z.; Tian, R.Z. Binary Alloy Phase Diagrams and Crystal Structure of Intermediate Phase; Central South University Press: Changsha, China, 2009; Volume 443, ISBN 978-7-81105-831-4. [Google Scholar]
- Villars, P.; Prince, A.; Okamoto, H. Handbook of Ternary Alloy Phase Diagrams; ASM: New York, NY, USA, 1995; ISBN 0871705346. [Google Scholar]
- Xia, M.X.; Zhang, S.G.; Li, J.G.; Ma, C.L. Evaluation of glass-forming ability for metallic glasses based on order-disorder competition. Appl. Phys. Lett. 2006, 89, 091917. [Google Scholar] [CrossRef]
- Slater, J.C. Atomic radii in crystals. J. Chem. Phys. 1964, 41, 3199–3204. [Google Scholar] [CrossRef]
- Kurz, W.; Giovanola, B.; Trivedi, R. Theory of microstructural development during rapid solidification. Acta Mater. 1986, 34, 823–830. [Google Scholar] [CrossRef]
- Lipton, J.; Kurz, W.; Trivedi, R. Rapid dendrite growth in undercooled alloys. Acta Mater. 1987, 35, 957–964. [Google Scholar] [CrossRef]
- Heard, D.W.; Gauvin, R.; Brochu, M. Non-equilibrium solute partitioning in a laser re-melted Al–Li–Cu alloy. Acta Mater. 2013, 61, 7432–7436. [Google Scholar] [CrossRef]
- Heard, D.W.; Boselli, J.; Rioja, R.; Marquis, E.A.; Gauvin, R.; Brochu, M. Interfacial morphology development and solute trapping behavior during rapid solidification of an Al–Li–Cu alloy. Acta Mater. 2013, 61, 1571–1580. [Google Scholar] [CrossRef]
- Eckler, K.; Cochrane, R.F.; Herlach, D.M.; Feuerbacher, B.; Jurisch, M. Evidence for a transition from diffusion-controlled to thermally controlled solidification in metallic alloys. Am. Phys. Soc. 1992, 45, 5019–5022. [Google Scholar] [CrossRef]
- Liu, F.C.; Lin, X.; Leng, H.; Cao, J.; Liu, Q.; Huang, C.H.; Huang, W.D. Microstructural changes in a laser solid forming Inconel 718 superalloy thin wall in the deposition direction. Opt. Laser Technol. 2013, 45, 330–335. [Google Scholar] [CrossRef]
- Gaumann, M.; Bezencon, C.; Canalis, P. Single-Crystal laser deposition of superalloys: Processing–microstructure maps. Acta Mater. 2001, 49, 1051–1062. [Google Scholar] [CrossRef]
- Cai, L.X.; Wang, C.M. Laser cladding for wear-resistant Cr-alloyed Ni2Si-NiSi intermetallic composite coatings. Mater. Lett. 2003, 57, 2914–2918. [Google Scholar] [CrossRef]
- Rappaz, M.; David, S.A.; Vitek, J.M.; Boatner, L.A. Analysis of solidification microstructures in Fe-Ni-Cr Single-Crystal welds. Metall. Trans. A 1990, 21A, 1767–1781. [Google Scholar] [CrossRef]
- Hunt, J.D. Steady state columnar and equiaxed growth of dendrites and eutectic. Mater. Sci. Eng. 1984, 65, 75–83. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]







| Coating Number | Powder Composition | Laser Parameters | ||||
|---|---|---|---|---|---|---|
| Ni (at %) | Cr (at %) | Si (at %) | Power (W) | Scan Speed (mm/min) | Diameter of Laser Beam (mm) | |
| 1 | 45 | 26 | 29 | 4500 | 800 | 3.5 |
| 2 | 60 | 10 | 30 | 4500 | 800 | 3.5 |
| 3 | 55 | 29 | 16 | 4500 | 800 | 3.5 |
| Mixing Enthalpy | Ni | Cr | Si | Atomic Radius/pm |
|---|---|---|---|---|
| Ni | - | −7 | −40 | 135 |
| Cr | - | - | −37 | 140 |
| Si | - | - | - | 110 |
| Region | Element Composition (at %) | |||
|---|---|---|---|---|
| Ni | Cr | Si | Cu | |
| a | 3.11 | 69.32 | 27.57 | — |
| b | 41.18 | 10.57 | 26.69 | 21.56 |
| c | 17.77 | 2.86 | 8.65 | 70.73 |
| d | 6.92 | 61.96 | 26.43 | 4.69 |
| e | 33.8 | 25.79 | 22.15 | 18.25 |
| f | 52.49 | 18.03 | 20.17 | 9.3 |
| g | 56.29 | 7.13 | 27.25 | 9.33 |
| h | 46.24 | 26.23 | 18.51 | 9.02 |
| i | 47.82 | 25.73 | 9.42 | 17.03 |
| Parameter | Value | Ref. |
|---|---|---|
| Liquidus temperature of Coating 1, Tm1 | 2040 K | Obtained using CALPHAD |
| Liquidus temperature of Coating 2, Tm2 | 1650 K | Obtained using CALPHAD |
| Liquidus temperature of Coating 3, Tm3 | 1580 K | Obtained using CALPHAD |
| Slope of liquidus surface with respect to chromium concentration, mCr1, mCr2, mCr3 | −3.63, −3.63, 5.87 K/(at %) | Obtained using CALPHAD |
| Slope of liquidus surface with respect to nickel concentration, mNi1, mNi2, mNi3 | −10.34, −12.62, −10.72 K/(at %) | Obtained using CALPHAD |
| Equilibrium partition coefficient for chromium, kCr1, kCr2, kCr3 | 0.264, 0.264, 0.2044 | Obtained using CALPHAD |
| Equilibrium partition coefficient for nickel, kNi1, kNi2, kNi3 | 0.243, 0.295, 0.305 | Obtained using CALPHAD |
| Pre-exponential diffusion coefficient for chromium, DCr0 | 2.67 × 10−7 m2/s | [40] |
| Pre-exponential diffusion coefficient for nickel, DNi0 | 4.92 × 10−7 m2/s | [40] |
| Activation energy for diffusion for chromium, QCr | 6.69 × 104 J/mole | [40] |
| Activation energy for diffusion for nickel, Qni | 6.77 × 104 J/mole | [40] |
| Length scale for solute trapping, a0 | 5 × 10−9 m | [40] |
| Gibbs–Thomson coefficient, Γ | 2.47 × 10−7 Km | [41] |
| Linear kinetic coefficient, μk | 4.696 m/s K | [41] |
| Alloy parameter, n | 3.4 | [43] |
| The number of nucleation sites, N0 | 2 × 1015/m3 | [43] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Li, M.; Yu, Z. Microstructures Evolution and Micromechanics Features of Ni-Cr-Si Coatings Deposited on Copper by Laser Cladding. Materials 2018, 11, 875. https://doi.org/10.3390/ma11060875
Zhang P, Li M, Yu Z. Microstructures Evolution and Micromechanics Features of Ni-Cr-Si Coatings Deposited on Copper by Laser Cladding. Materials. 2018; 11(6):875. https://doi.org/10.3390/ma11060875
Chicago/Turabian StyleZhang, Peilei, Mingchuan Li, and Zhishui Yu. 2018. "Microstructures Evolution and Micromechanics Features of Ni-Cr-Si Coatings Deposited on Copper by Laser Cladding" Materials 11, no. 6: 875. https://doi.org/10.3390/ma11060875
APA StyleZhang, P., Li, M., & Yu, Z. (2018). Microstructures Evolution and Micromechanics Features of Ni-Cr-Si Coatings Deposited on Copper by Laser Cladding. Materials, 11(6), 875. https://doi.org/10.3390/ma11060875







