1. Introduction
Selenium (Se) is a metalloid that can exist in a variety of valence states, including selenide (Se(-II)), elemental Se (Se(0)), selenite (Se(IV)) and selenate (Se(VI)) [
1,
2]. As a valuable element, Se can be widely used in photosensitive drums for copying machines, photoelectric devices, pigments, metallurgical additives, glass manufacturing and semi-conductors [
3,
4]. Its increasing usage has generated a considerable amount of selenium-contaminated wastewater, mainly containing selenate and selenite [
5]. Although Se is an essential trace element for human health at the concentration range from 0.8 to 1.7 µmol/L, excessive intake of Se could lead to serious health issues [
6,
7]. The United States Environmental Protection Agency (US EPA) has mandated the maximum contaminant level of Se in drinking water at 50 µg/L [
5]. The acute toxicity of Se(IV) is almost 10 times higher than that of Se(VI) and both species generally exist simultaneously in aerobic surface water. Therefore, there is an urgent need for developing efficient and feasible methods to remove Se(IV) from waste water [
1].
Using the green nanoparticles with a low cost and high adsorption capacity is a good approach to remove pollutants [
8]. Nanoscale zero-valent iron (NZVI) particles have been considered as one of the most promising permeable barrier materials applied in wastewater treatment because of their extremely small particle size, large specific surface area, excellent in-situ reactivity and high injectability into aqueous solutions [
9,
10,
11]. Previous reports have indicated that nanoscale zero-valent iron (NZVI) can remove a range of environmental contaminants, such as chlorinated solvents, organochlorine pesticides, polychlorinated biphenyls, organic dyes, and inorganic pollutants, tetracycline [
12,
13]. However, some technical issues, e.g., easy agglomeration, poor stability, shortage of durability and mechanical strength [
14,
15], prevent the practical application of NZVI particles. Furthermore, the aggregation is hard to be avoided due to the magnetic interaction among the NZVI particles [
8]. Previous researchers have shown that the aggregation of NZVI particles could be reduced when they were supported on the substrates. In this way, their dispersion ability and specific surface area were improved and their reactivity was also enhanced [
16,
17].
Reduced graphene oxide (rGO) has recently received an intensive attention due to its exceptional electron transport and mechanical properties as well as high surface area [
12,
18,
19]. And the synthesis of most rGO-based multifunctional composites stems from graphene oxide (GO) [
20,
21], which can be readily made from low-cost natural graphite in a large scale. GO and rGO were broadly used in the adsorption processes to remove the pollutants from wastewater [
22], such as heavy metal ions [
23,
24], radionuclides [
25], and many cationic or anionic dyes [
26]. NZVI particles supported on graphene do not only increase the dispersion ability and stability but also strengthen electron transfer and removal of pollutants by coupling the advantages of NZVI reactivity with graphene adsorption ability [
8]. Therefore, the composites based on rGO are significantly more applicable than those based on bare nanomaterials [
12].
The most important stage in a removal process is to improve a system and increase the efficiency of the process via modeling and optimization without increasing the costs [
27]. Because the removal processes involve interactions of variables and non-linear behavior, it is highly important that optimum experimental conditions are determined to obtain a maximum efficiency. Optimization techniques based on artificial intelligence (AI) approaches can be an effective solution in these processes [
28]. AI tools have broadly applied in various fields, e.g., image understanding, intelligent internet search, autonomous driving, pattern recognition, automatic programming, robotics, human-computer games and big data. As one of the AI primary tools, artificial neural network (ANN) inspired by biological neurons can well describe multivariate nonlinear problems with the suitable amount of data and the appropriate training algorithm applied [
29]. Optimization algorithms, e.g., genetic algorithm (GA) and particle swarm optimization (PSO), were inspired from social behavior or natural phenomena, which can be utilized to solve complex problems [
30]. Both ANN and GA play an important role in various fields of science and technology. Based on the principles of evolution through a natural selection, GA has been found to be suitable for finding the optimum solutions in some complex systems [
31]. PSO is a stochastic optimization technique motivated by the behavior of a bird’s flock [
32].
The objective of this study was to apply the reduced graphene oxide-supported nanoscale zero-valent iron (nZVI/rGO) composites in Se(IV) removal from aqueous solutions. Response surface methodology (RSM) as an experimental design tool was employed to examine the effect of process parameters (viz. temperature, contact time, initial pH and initial Se(IV) concentration) on removal efficiency, which was combined with AI techniques (including ANN-PSO and ANN-GA) applied for modeling the removal process of Se(IV) and predicting its maximum removal efficiency. Furthermore, the experimental data obtained were fitted to the adsorption isotherms (Langmuir, Freundlich and Redlich Peterson) and the removal kinetic models (pseudo-first-order, pseudo-second-order and intraparticle diffusion). Finally, the thermodynamic study and the XPS analysis were carried out to explore the mechanisms of Se(IV) removal process.
2. Materials and Methods
All commonly used chemicals of analytical grades (i.e., HCl, NaOH, Na
2SeO
3, FeSO
4·7H
2O, and NaBH
4) were applied without further purification in this study, and graphite powder (purity > 99.85%, particle size < 30 μm) was obtained from Sinopharm Chemical Reagent (Beijing, China). The Se(IV) stock solution (1000 mg/L) was prepared by dissolving an amount of Na
2SeO
3 in deionized water. Graphene oxide was synthesized using the modified Hummers method [
33], and nZVI/rGO magnetic composites were synthesized according to our previous reports [
34,
35].
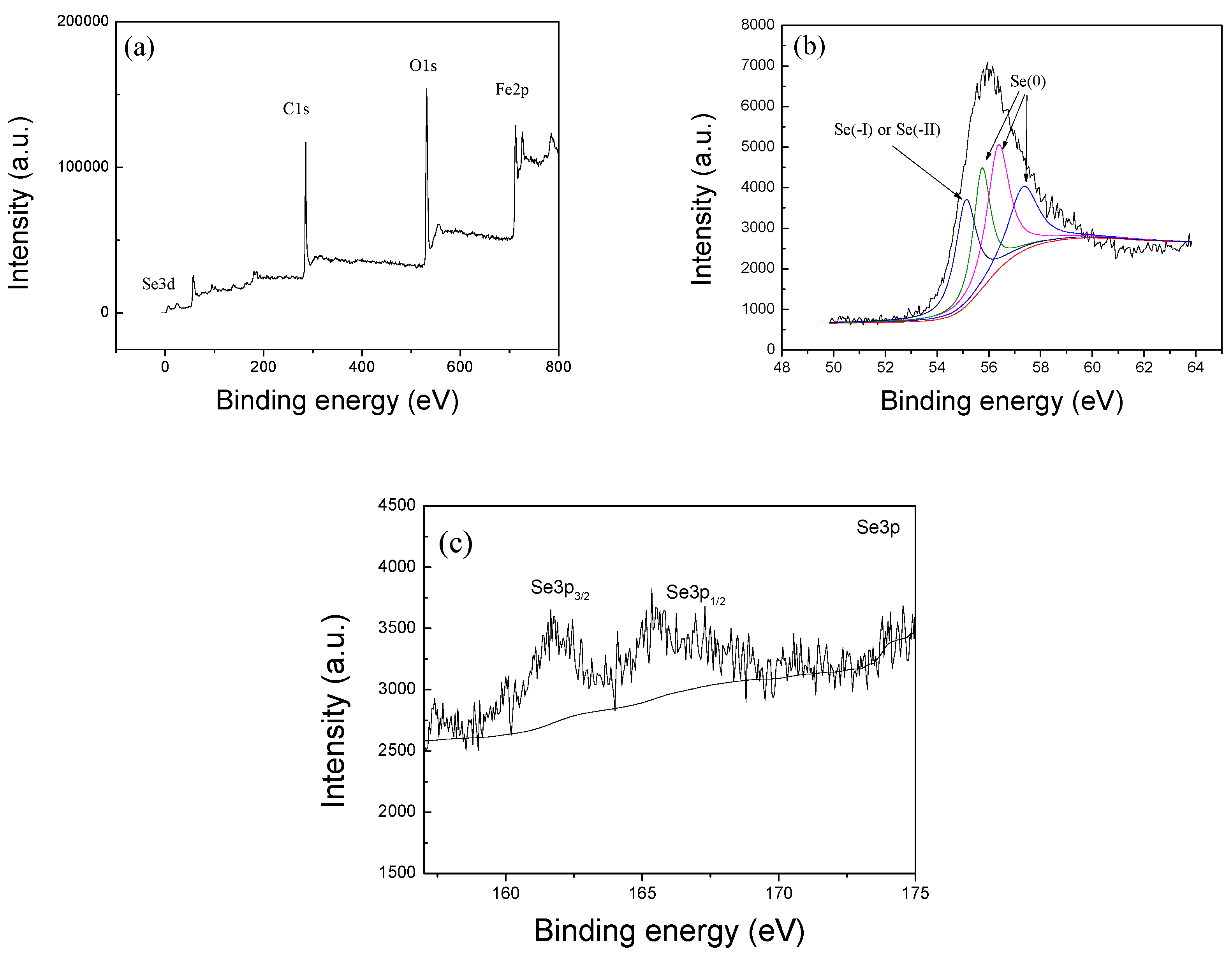
2.1. Characterization of nZVI/rGO Composites
The characterization (XRD, SEM, TEM, Raman, FTIR, and N
2 sorption) of nZVI/rGO composites was carried out in our earlier study [
34]. The
X-ray photoelectron spectroscopy (XPS) measurements were recorded on an ESCALAB 250Xi spectrometer using monochromatized Al Kα radiation (hν = 1486.6 eV), and all binding energies were corrected with the binding energy of C1s (hν = 284.8 eV) as a reference.
2.2. Adsorption Experiments
Removal of the Se(IV) solutions by nZVI/rGO composites was performed in a 100 mL conical flask. 30 mg of the nZVI/rGO composites was added to 50 mL of Se(IV) solution, which was shaken in a vibrator (HZQ-F160, HDL, Jingda Instrument Manufacturing Co. Ltd, Jiangsu, China) at 200 rpm. Batch removal experiments of Se(IV) (single factor experiments) were carried out to examine the effect of initial Se(IV) concentration (C), contact time (t), initial pH and operating temperature (T) on the removal efficiency of Se(IV), and the initial pH of Se(IV) solution was adjusted to the desired value by using 0.1 mol/L HCl or 0.1 mol/L NaOH. The nZVI/rGO composites were separated from aqueous solutions by a magnet after the removal experiment. The Se(IV) concentration in the sample solutions was determined through an inductively coupled plasma-optical emission spectroscopy (optima 5300 V, Perkin Elmer Corporation, Waltham, MA, USA). All experiments were carried out in triplicates and the average values of the results obtained were used for data analysis.
The removal quantity of Se(IV) by nZVI/rGO composites was calculated using the following equation (
qe) [
27]:
where
qe (mg·g
−1) stands for the removal quantity of the Se(IV) per unit mass of the adsorbent,
C0 (mg·L
−1) and
Ce (mg·L
−1) represent the initial and equilibrium concentrations of the Se(IV), respectively,
V (L) stands for the volume of the solution, and
m (g) is the dry weight of the nZVI/rGO composites. The removal percentage (R) of Se(IV) was calculated using the following equation [
36]:
where
C0 (mg·L
−1) and
Ct (mg·L
−1) represent the concentrations of initial and after time t, respectively.
2.3. Response Surface Methodology
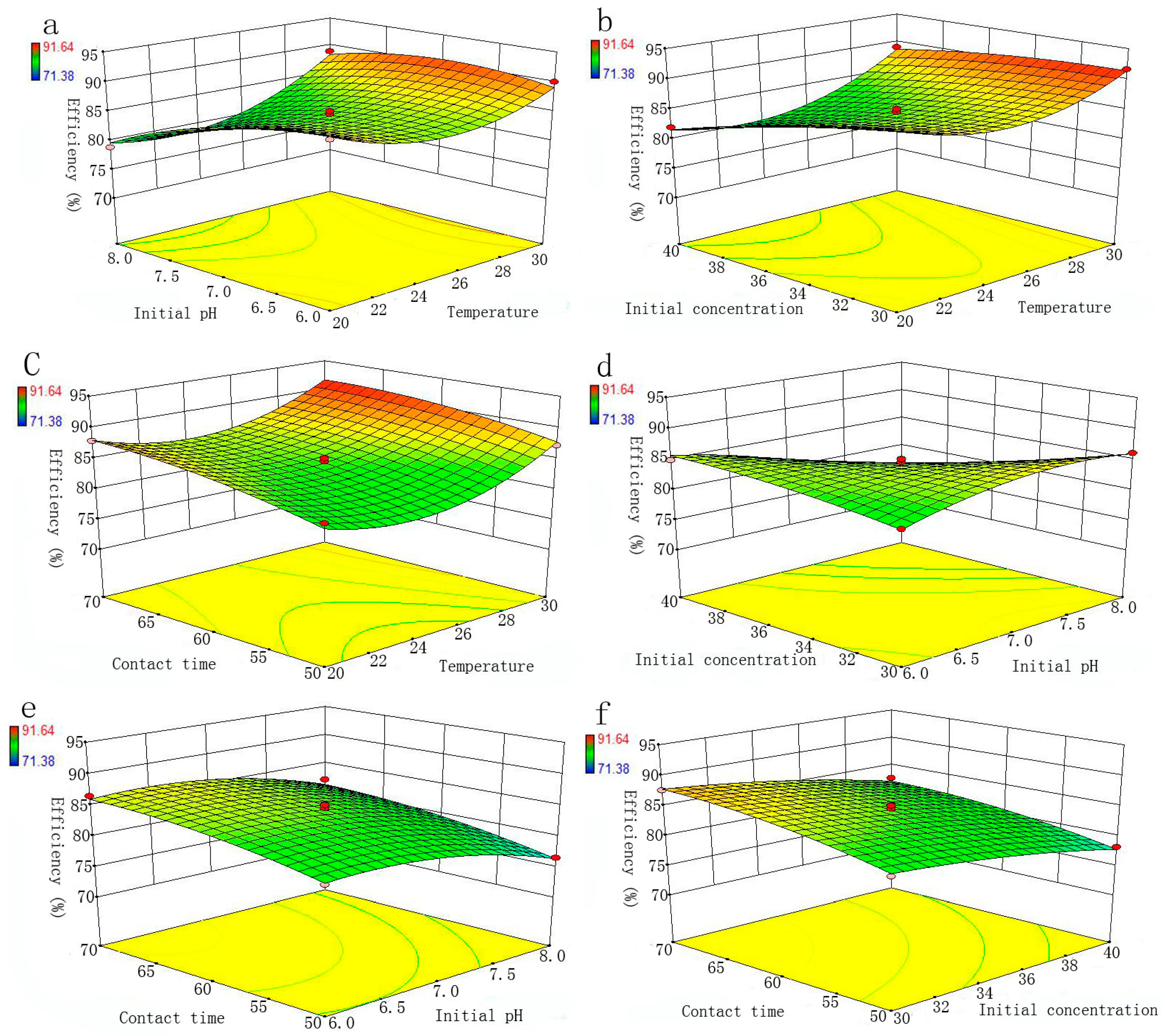
RSM is a main branch of experimental design, which is used to evaluate the effect of several factors and their interaction on the system response. This method is useful for developing and optimizing the independent variables and response, and providing the smaller number of experimental runs [
27]. The Box-Behnken design (BBD) model is a standard RSM [
37], which can be applied to evaluate the interactive effects of adsorption variables and optimize the removal process. Specifically, C (30–40 mg/L), initial pH (6–8), t (50–70 min) and T (20–30 °C) were selected as independent variables, and the removal efficiency of Se(IV) as the dependent variable. The experimental design involved four parameters, each at three levels, which were coded as shown in
Table 1. For the evaluation of experimental data of Se (IV) removal, the response variable was fitted by the quadratic polynomial equation given below:
where
Y stands for the removal efficiency of Se(IV) used as the dependent variable,
x1, x2, x3, and
x4 represent the independent variables, and
α0, α1, α2, α3, α4, α12, α13, α14, α23, α24, α34, α11, α22, α33 and
α44 represent the model coefficients, respectively.
2.4. Artificial Neural Network
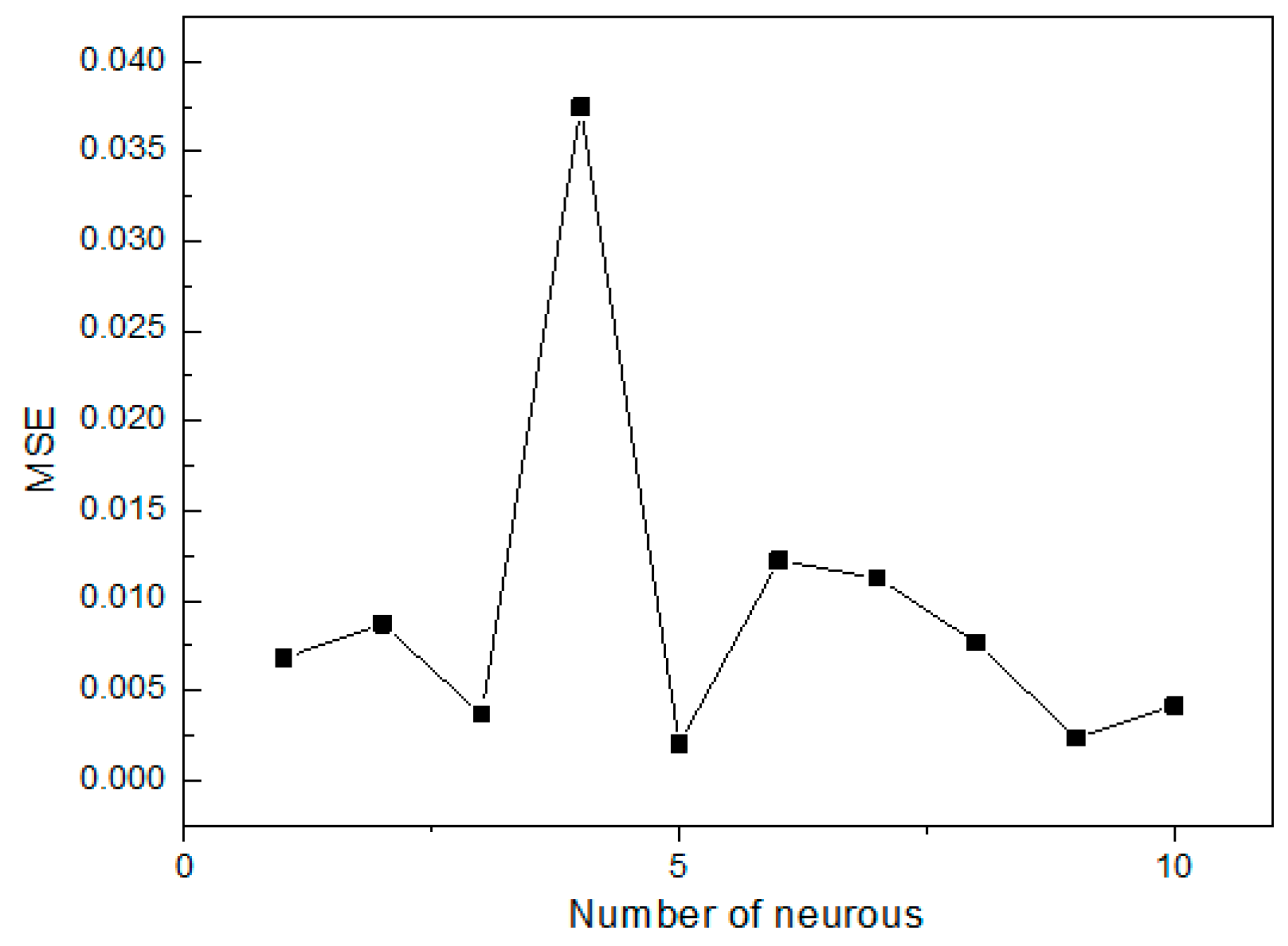
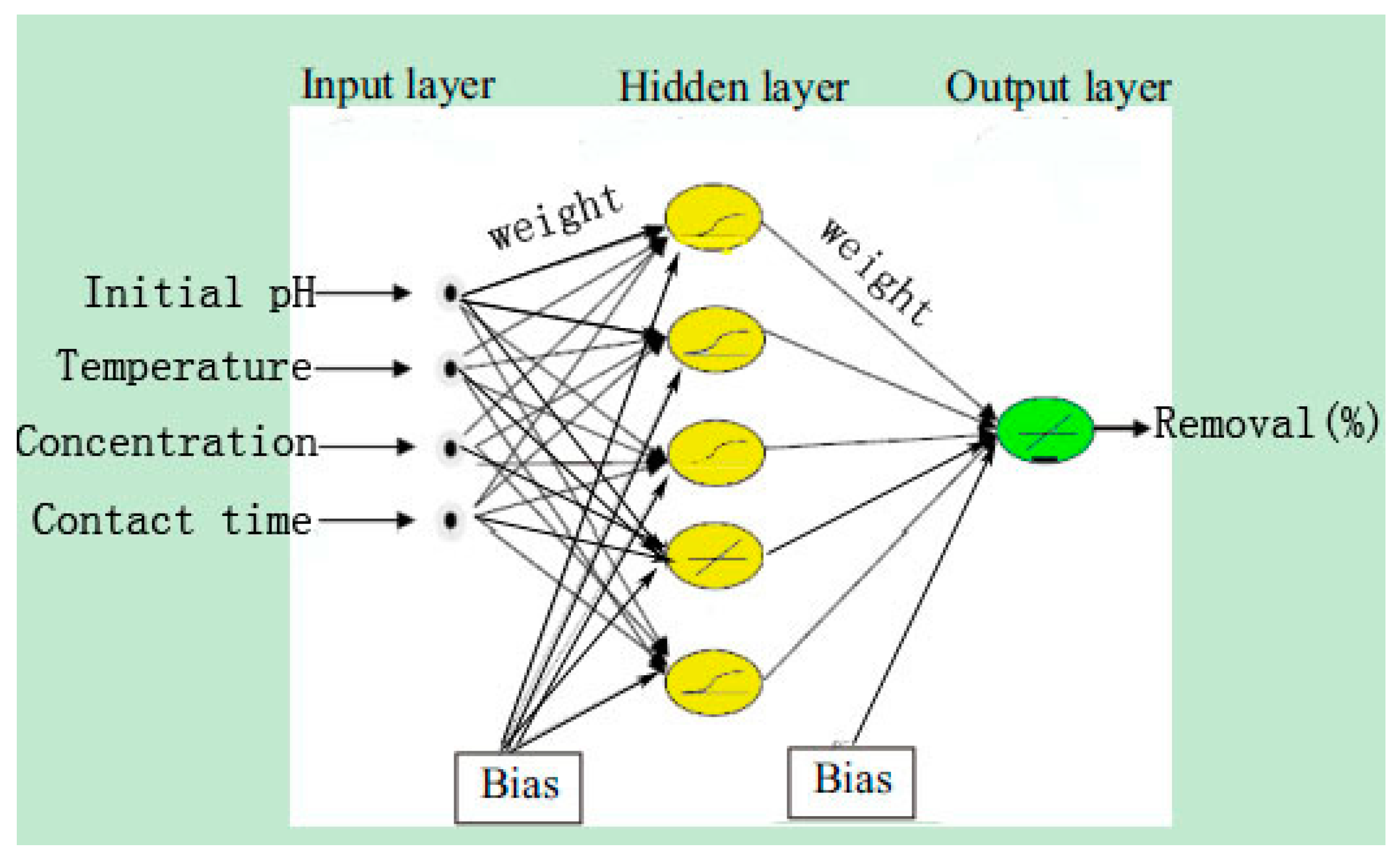
ANN is a good inspiration of human brain and nerve systems that are known for their superior ability to learn and classify the data, thus a feed-forward multilayer perception (MLP) ANN model was utilized in the present study with a back propagation (BP) algorithm, which contains an input layer, a hidden layer and an output layer [
27]. The initial Se(IV) concentration, initial pH, operating temperature and contact time were utilized as input parameters, while the removal efficiency of Se(IV) was used as the output parameter. Among the 29 datasets generated from RSM, 24 datasets were employed to train the network and remaining 5 datasets were used for testing of the ANN model [
38]. The performance of the ANN models was determined based on the criteria, such as mean squared error (
MSE) and the correlation coefficient (
R2), which can be shown as follows [
39]:
where
yprd,i stands for the predicted value by ANN model,
yexp,i represents the experimental value,
N stands for the number of data, and
ym represents the average of the experimental value.
Figure 1 is a flow diagram of ANN training:
Sensitivity analysis was carried out to explore the connection weights of the trained ANN [
29]. Analysis based on the magnitude of weights are based exclusively on the values stored in the static matrix of weights in order to determine the relative influence of each input variable on each one of the network outputs [
40]. The equation below is proposed by Garson for this type of analysis [
40,
41]:
where
Gik stands for the percentage of influence of the dependent variable
xi on the independent variable
Yk,
w represents the connection weight,
i,
j and
k stand for the number of neurons in the input layer, hidden layer and output layer, respectively.
2.5. Genetic Algorithm
GA has been proven to be a successful method for solving the optimization problems by mimicking the principle of biological evolution [
42]. GA based optimization processes can be executed using trained ANN models as the fitness functions to give a global optimum solution [
43], which applies crossover, mutation, and selecting operators to a population of encoded variables space. [
44,
45]. The values of GA-specific parameters used in the optimization technique were as follows: the number of variables = 4, maximum generation = 100, size of population = 20, crossover probability = 0.8 and mutation probability = 0.01, bounds (C: 30–40 mg/L, initial pH: 6–8, t: 50–70 min and T: 20–30 °C). The flow diagram of genetic algorithm is shown in
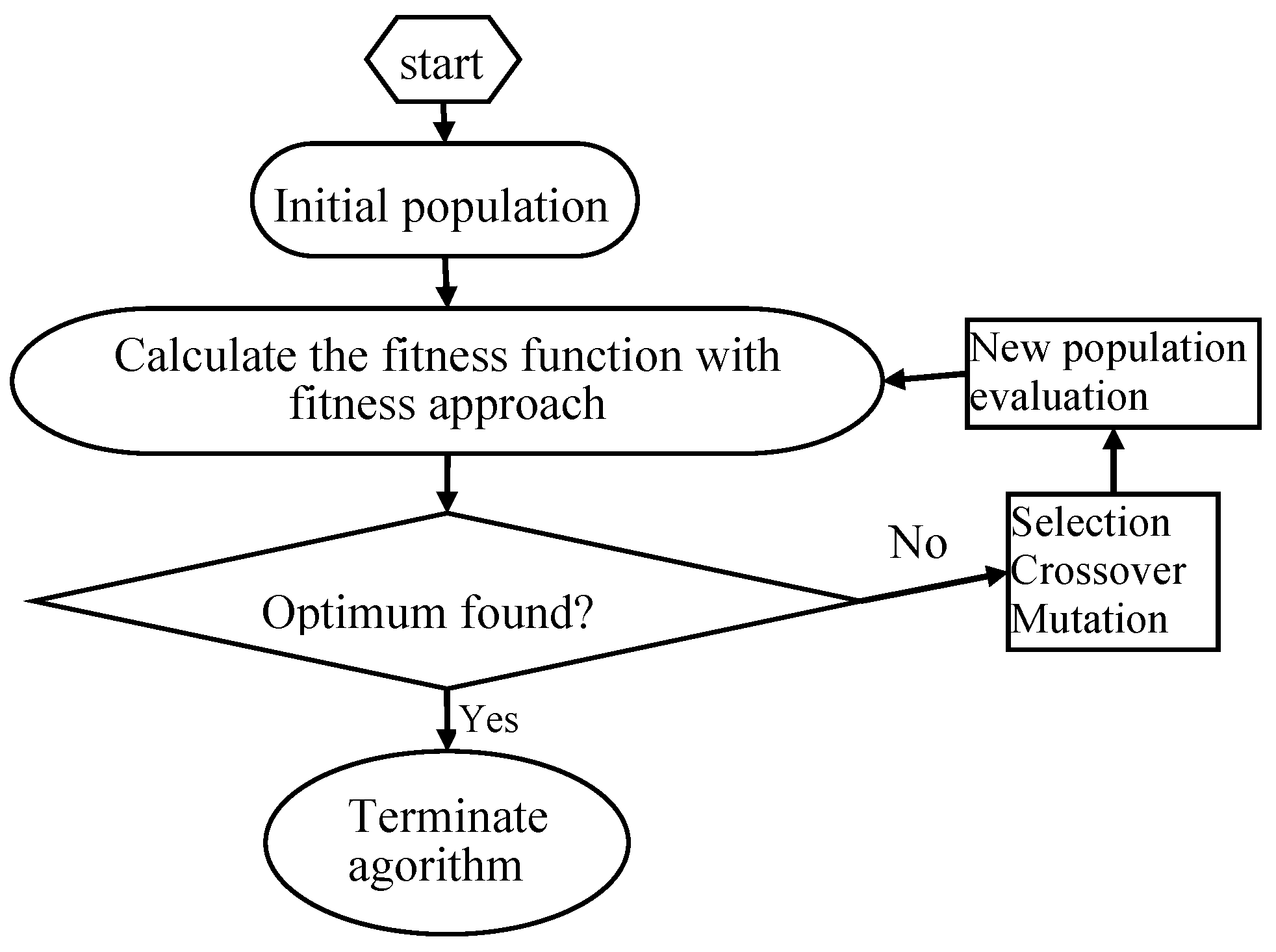
Figure 2. Specific steps were as follows [
46]: (1) initial population was chosen randomly; (2) every individual of the population was evaluated; (3) the population with the lowest MSE value (best individual) was selected for the next generation (elitism); (4) the best parents were chosen to generate the children using the GA operators; (5) the same procedure was repeated for the second generation and the algorithm was run for a defined number of cycles. (6) after finishing the GA optimization, the BP algorithm started from the solution provided by GA.
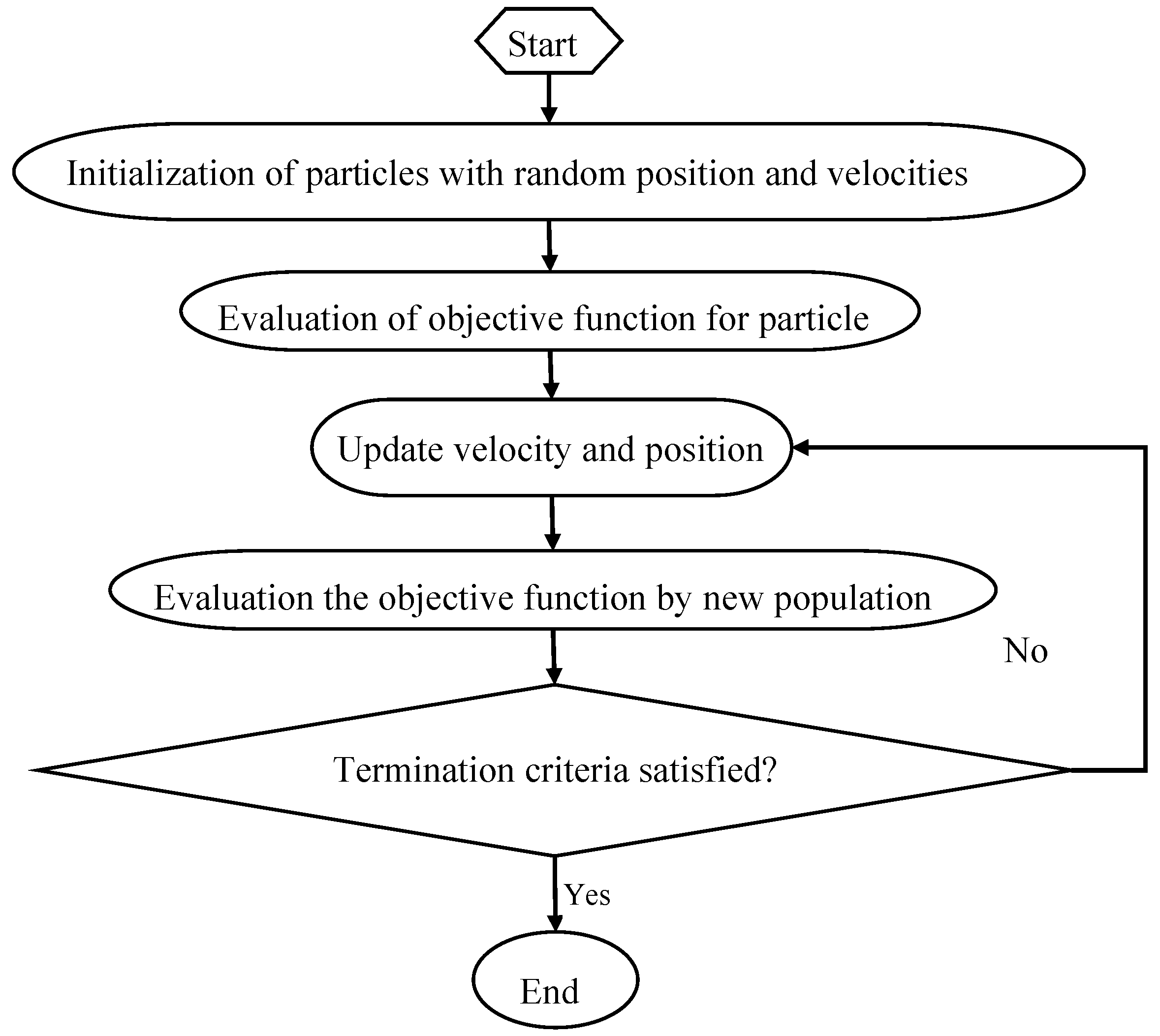
2.6. Particle Swarm Optimization
PSO is an evolutionary computational algorithm proposed by Kennedy and Eberhart in 1995, which is based on the simulation of natural habits of bird flocking [
32]. PSO algorithm uses a population of particles that has the ability to evolve during the search for an optimal solution. The system is initialized randomly and the particles fly through the multi-dimensional search space at a certain velocity [
47]. PSO computes the particles based on a fitness function and finds a particle with a good position. If a better position is obtained, the previous value will be replaced by the current value. No two particles are the same and each still learns the attributes of others that will help improve their fitness [
48]. PSO consists of different control parameters, including acceleration coefficients (c1: personal learning coefficient, c2: global learning coefficient), swarm size, and inertia weight. Wrong initialization of these parameters may lead to divergent or cyclic behavior. The minimum inertia weight, maximum inertia weight, c1, c2, swarm size and maximum iteration number were set as: 0.3, 0.9, 2, 2, 20 and 50. The flow chart of PSO is shown in
Figure 3:
2.7. Equilibrium Adsorption Studies
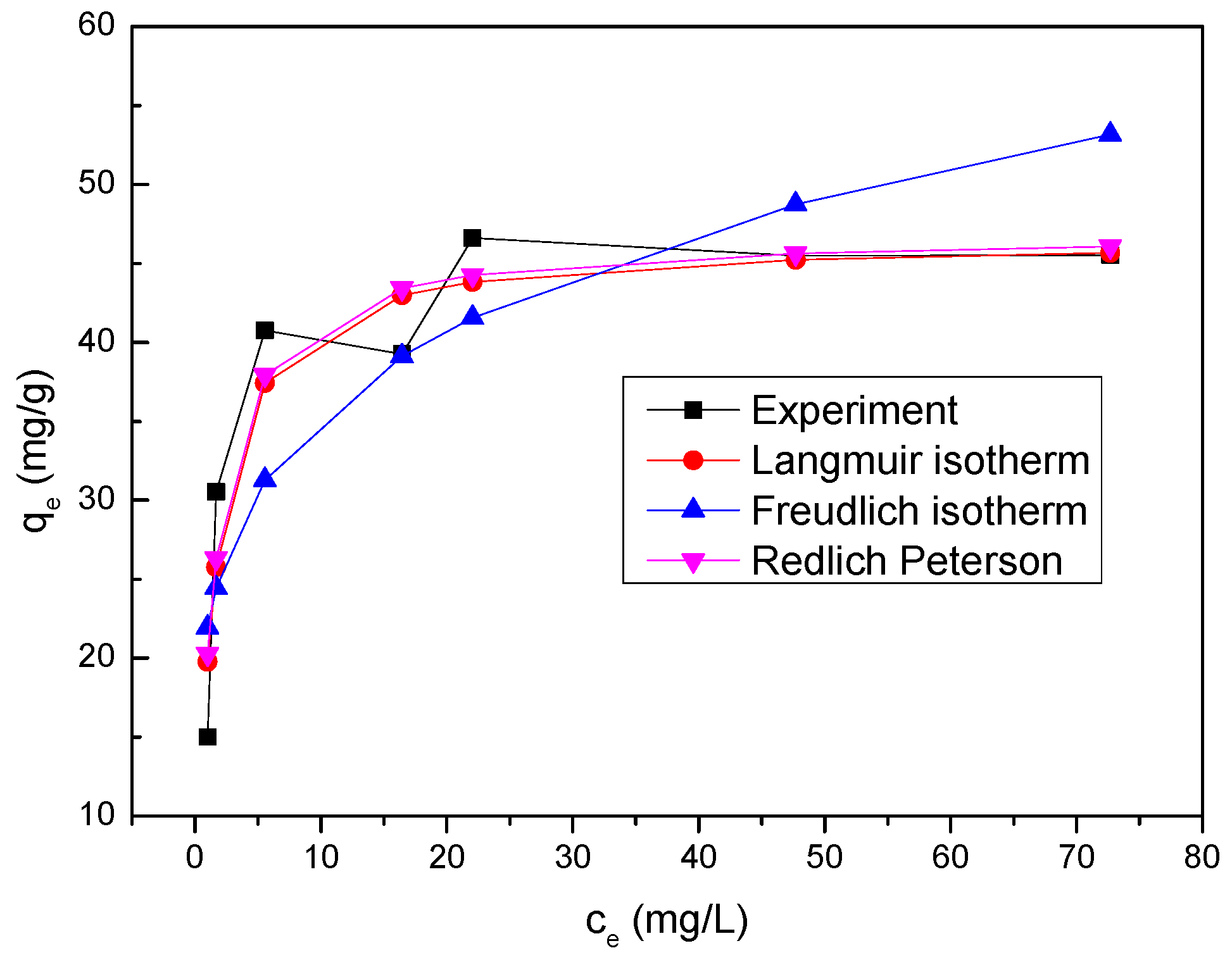
The equilibrium adsorption isotherm is employed to give useful information concerning properties, tendency and mechanism of adsorption of Se(IV) onto nZVI/rGO composites. The data obtained from the equilibrium adsorption study were fitted to different adsorption isotherm equations such as Langmuir, Freundlich and the Redlich Peterson models to discuss the adsorption characteristics [
36].
Based on monolayer adsorption onto homogeneous adsorbent surface, the Langmuir isotherm is expressed as follows [
39]:
where
Ce (mg·L
−1) stands for the equilibrium Se(IV) concentration in the solution,
qmax (mg·g
−1) demonstrates the maximum adsorption capacity,
qe (mg·g
−1) represents the equilibrium amount of Se(IV) adsorbed per unit mass of sorbent,
KL (L·mg
−1) stands for the Langmuir constant related to adsorption energy. The favorability of Langmuir isotherm was judged based on the dimensionless equilibrium parameter (
RL) [
39]:
where
RL indicates the type of isotherm to be favorable (0 <
RL < 1), irreversible (
RL = 0), linear (
RL = 1) or unfavorable (
RL > 1),
C0 (mg·L
−1) stands for the initial Se(IV) concentration and
KL represents the Langmuir constant.
The Freundlich isotherm is generally employed to investigate the behavior and mechanism of adsorption base on heterogeneous surface. The equation of Freundlich isotherm can be represented as below [
39]:
where
KF [(mg·g
−1)/(mg·L
−1)
1/n] stands for the Freundlich constant give useful knowledge about adsorption capacity and n is a coefficient related to adsorption intensity.
The experimental data of Se(IV) removal were also fitted to the Redlich Peterson isotherm, which can be expressed as follows [
49]:
where
A (L·g
−1) stands for the Redlich Peterson isotherm constant, and
B (L·mg
(1/A)−1) represents the Redlich Peterson isotherm constant. In addition, the statistical analysis was performed to investigate the validity of isotherms on the basis of two parameters, e.g., chi square test (
x2) and the sum of absolute errors (
SAE), which were evaluated using the following equations [
50]:
where
qe,exp and
qe,cal stand for the experimental and calculated adsorption capacity (mg·g
−1) and
n represents the number of measurements.
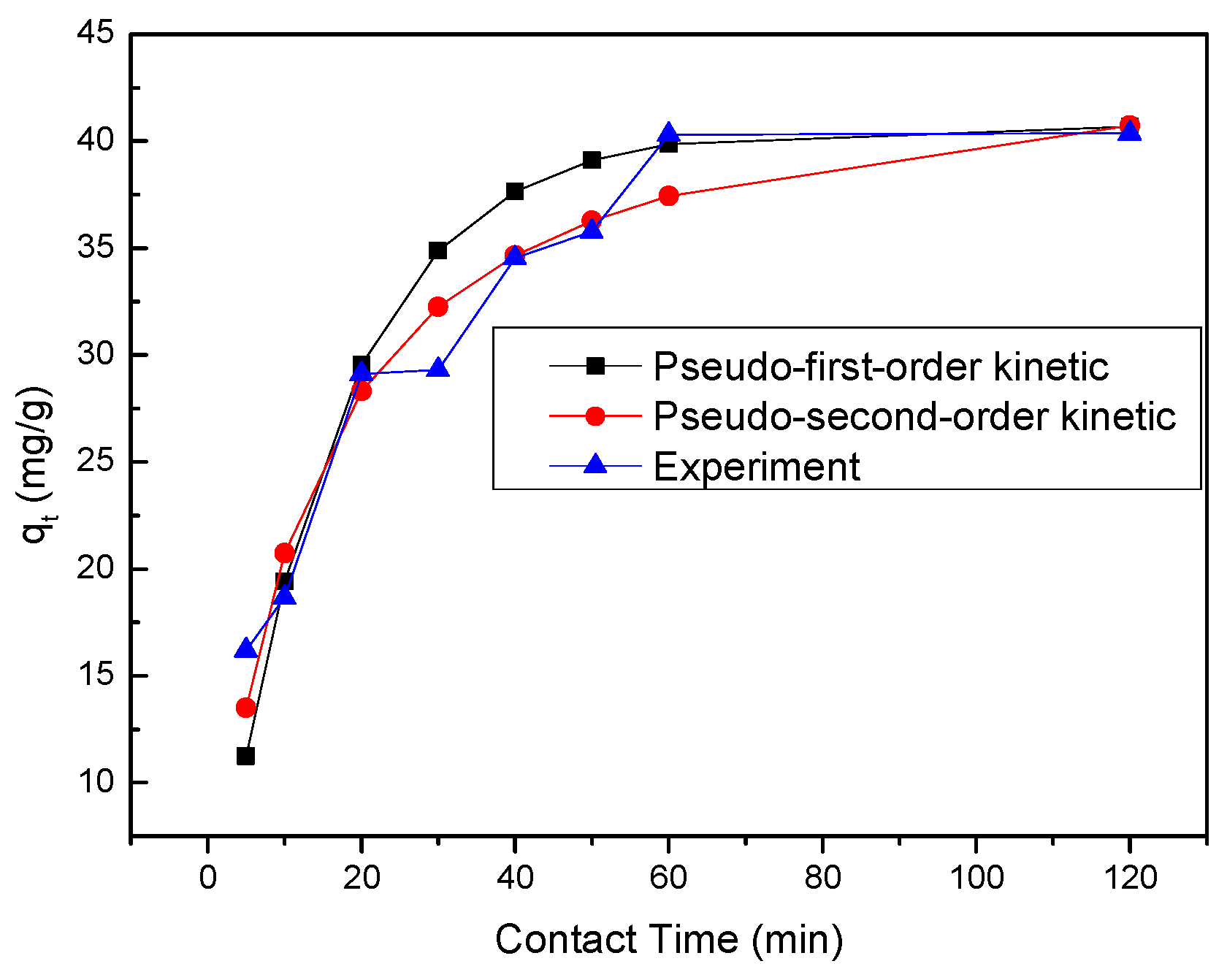
2.8. Kinetic Studies
Kinetic study was performed to investigate the removal rate that is important in design and modeling of the process. The pseudo-first-order (Equation (13), pseudo-second-order (Equation (14)) and intraparticle diffusion (Equation (15)) were used to perform the kinetic study [
36,
51,
52].
where
qe (mg·g
−1) and
qt (mg·g
−1) stands for the removal quantity of Se(IV) at equilibrium and at time
t,
k1 (1·min
−1) represents the rate constant of pseudo-first-order removal process,
k2 (g·mg
−1 min
−1) is the pseudo-second-order rate constant of removal,
kd (mg·g
−1·min
−0.5) represents the intraparticle diffusion constant, and
C as the intercept (mg·g
−1·min
−0.5).
2.9. Thermodynamic Study
Thermodynamics of Se(IV) adsorption onto nZVI/rGO was employed to evaluate the exothermic, spontaneity or endothermic nature of the removal process at the adsorbate–adsorbent interface [
36]. Thermodynamic parameters, viz. Gibbs’ free energy change (
ΔG0), enthalpy change (
ΔH0) and entropy change (
ΔS0) were investigated through the following equations:
where
KT stands for the distribution coefficient,
R (8.314 J·mol
−1 K
−1) represents the gas constant,
T (K) represents the temperature,
C0 (mg·L
−1) and
Ce (mg·L
−1) represent the initial and equilibrium concentrations of Se(IV), respectively.