Strengthening of Aluminum Wires Treated with A206/Alumina Nanocomposites
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Fabrication of a Master Al Matrix Nanocomposite
2.2. Wire Fabrication and Characterization
3. Results
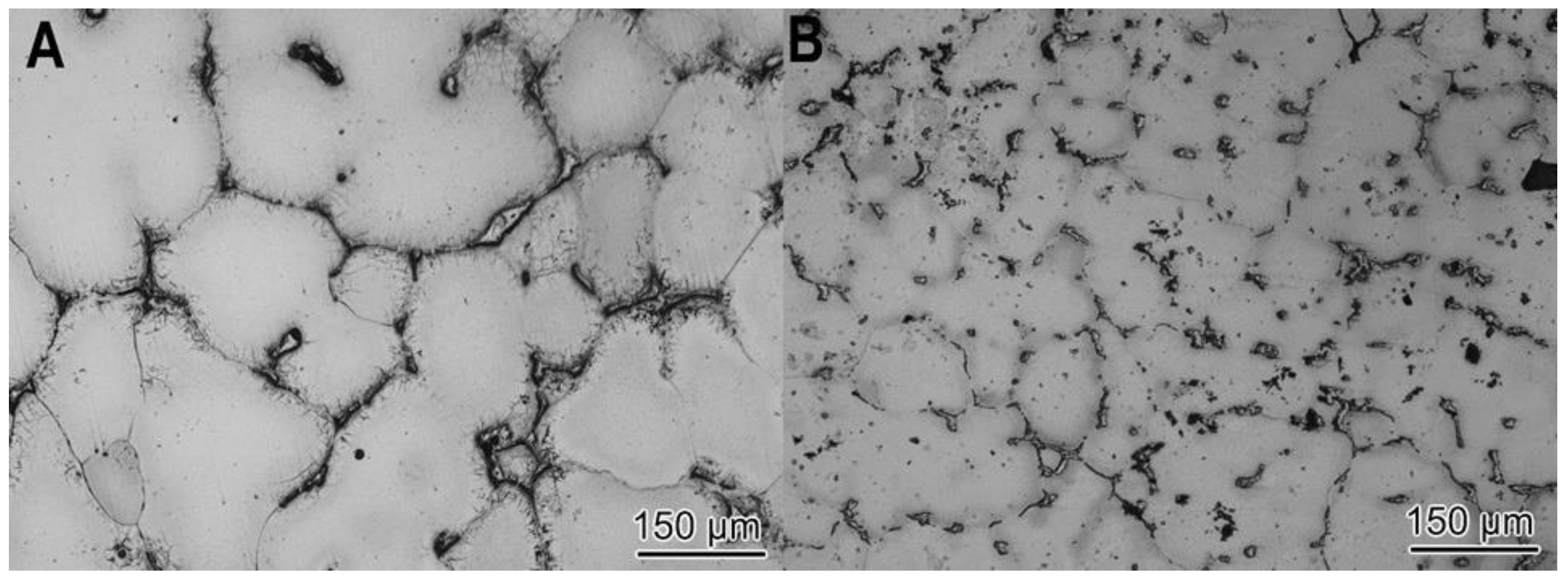
3.1. Master A206/γAl2O3 Composite Characterization
3.2. Wire Characterization
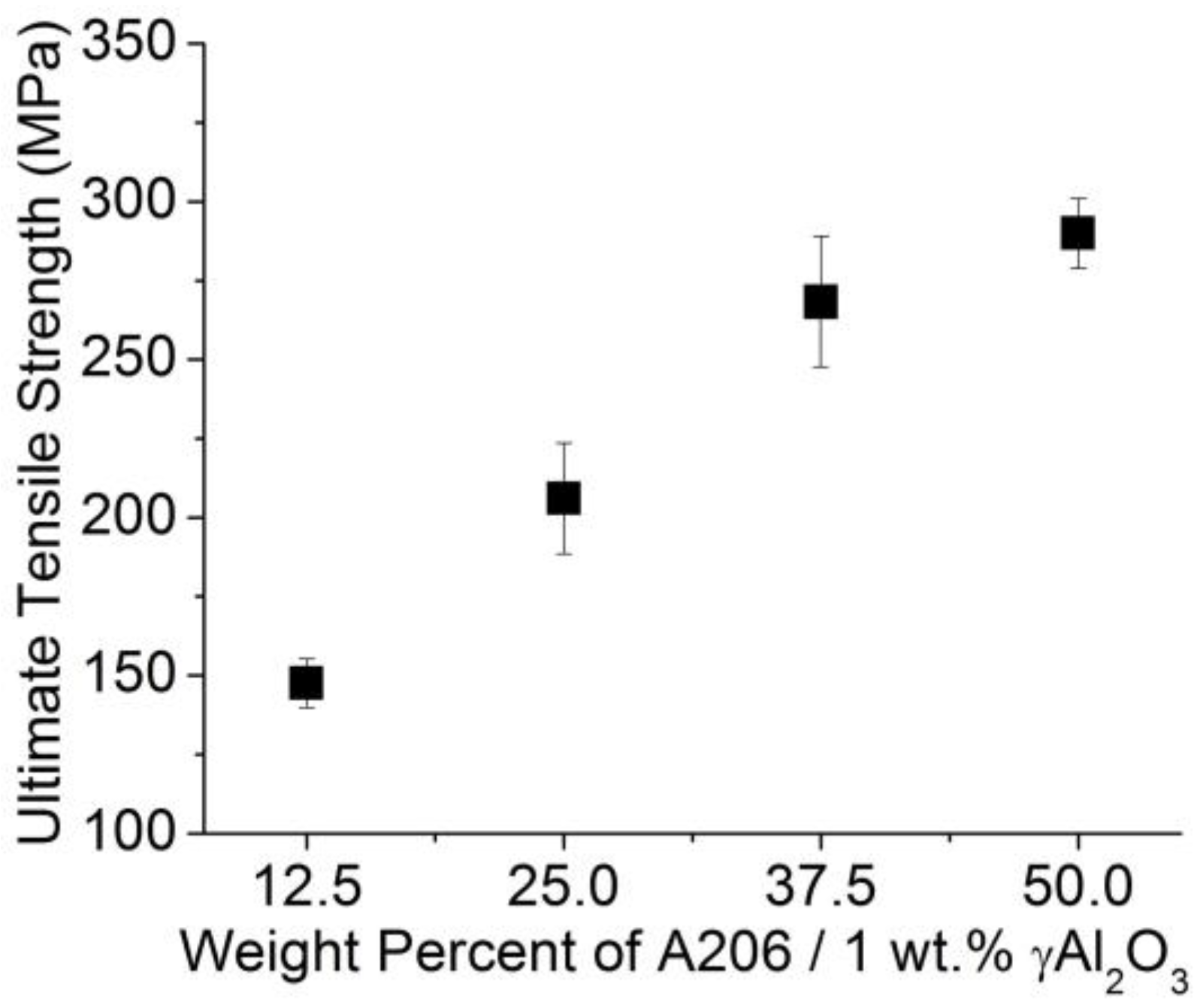
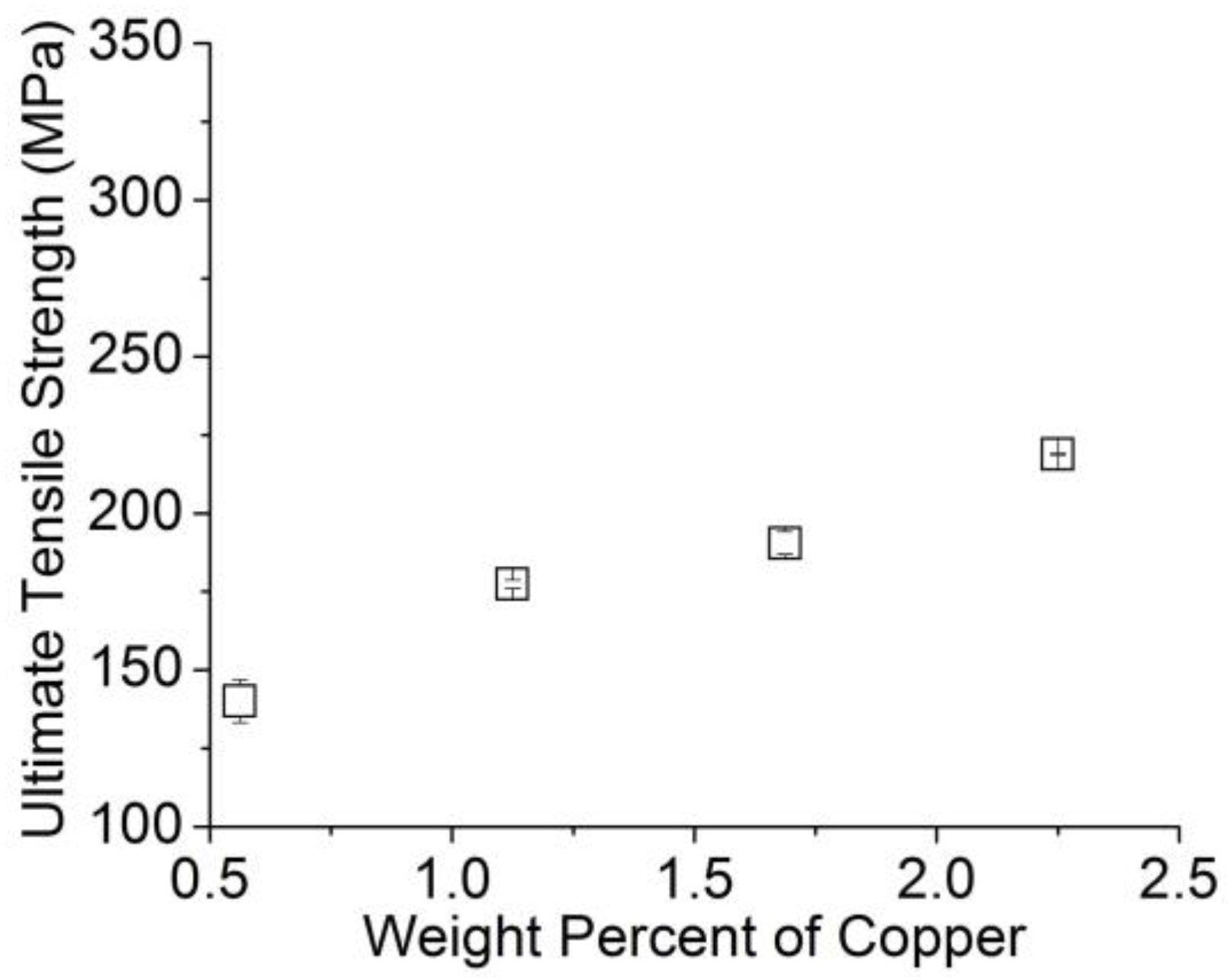
3.2.1. Tensile Test Results
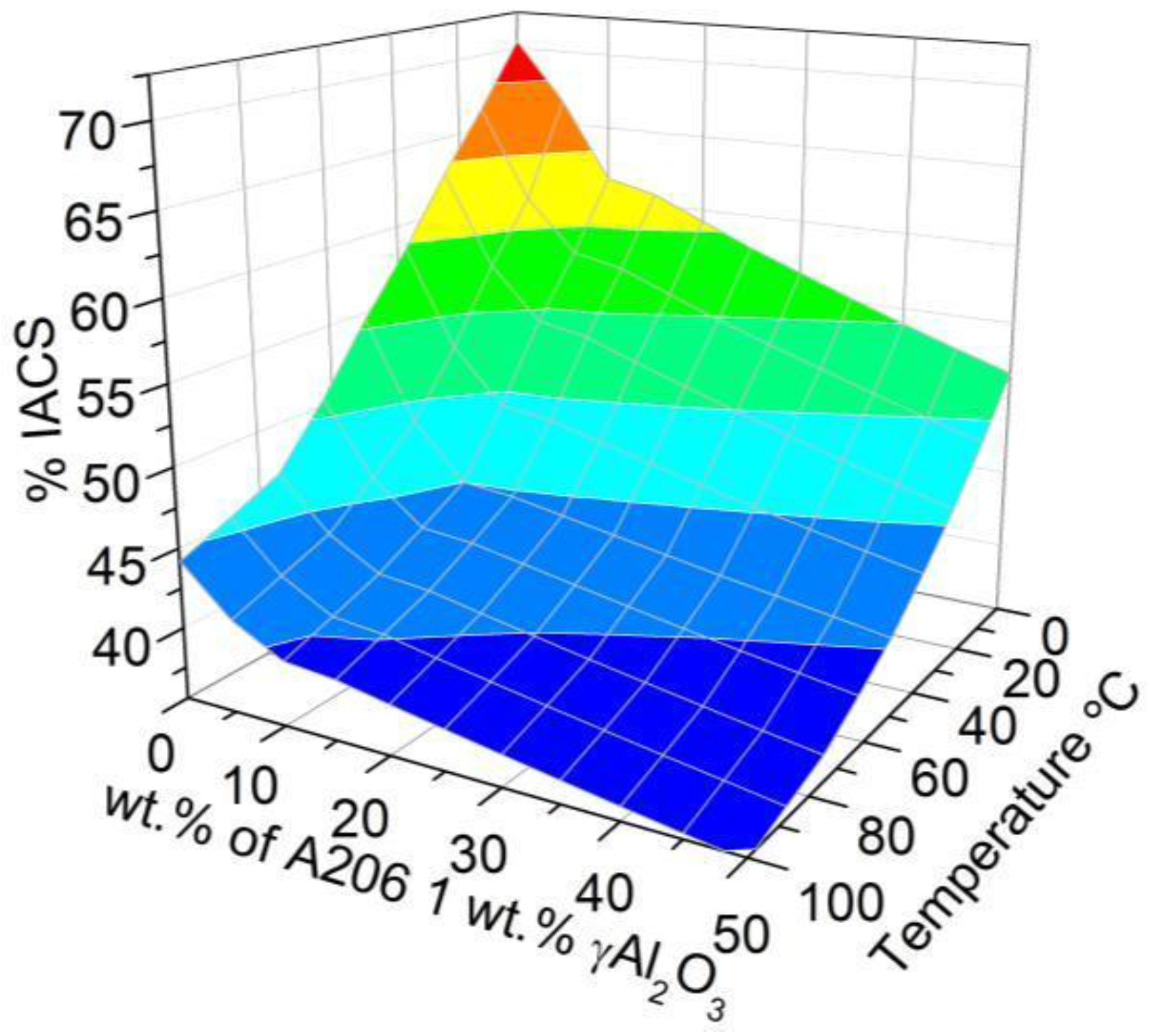
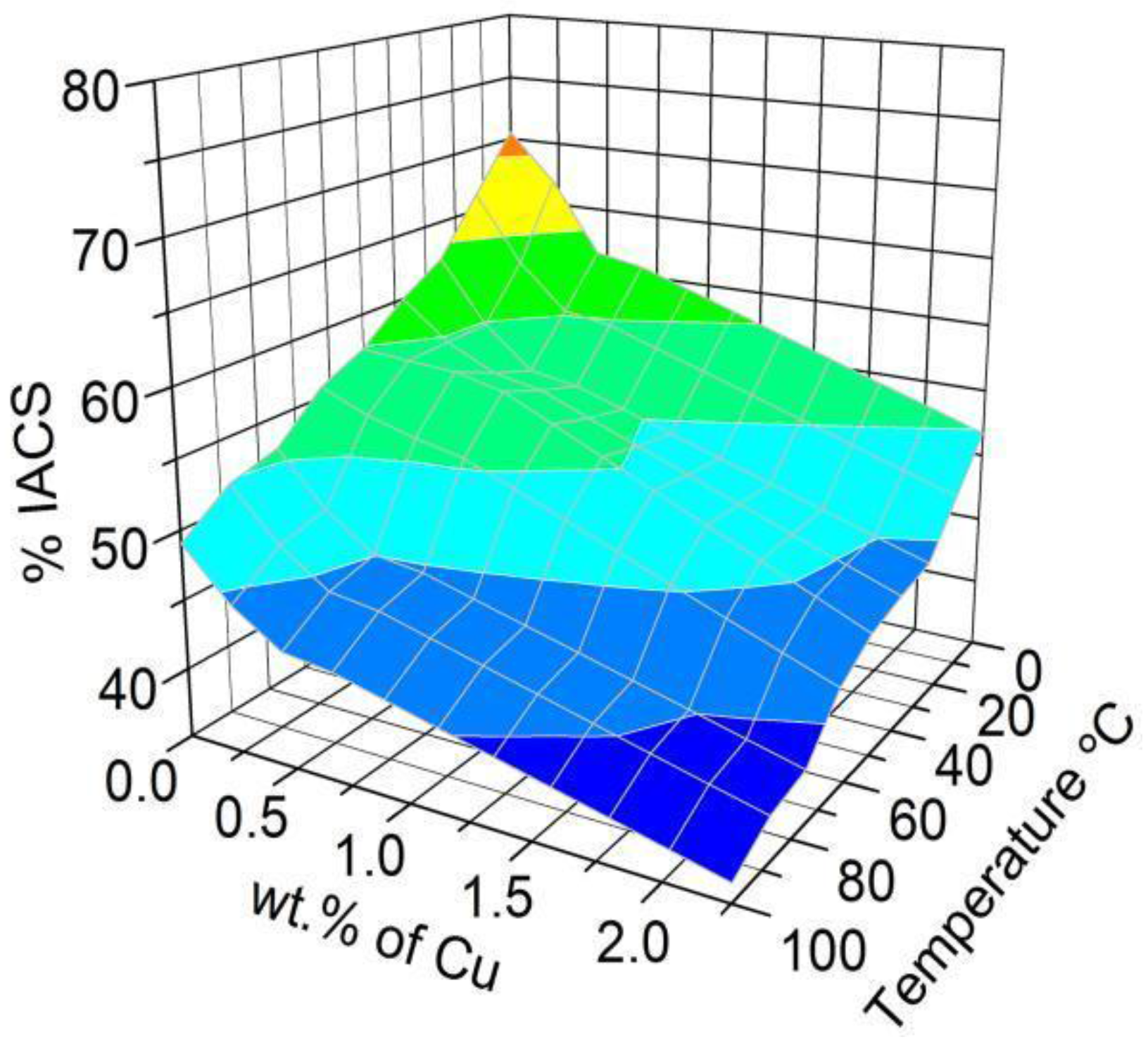
3.2.2. Electrical Conductivity Measurements
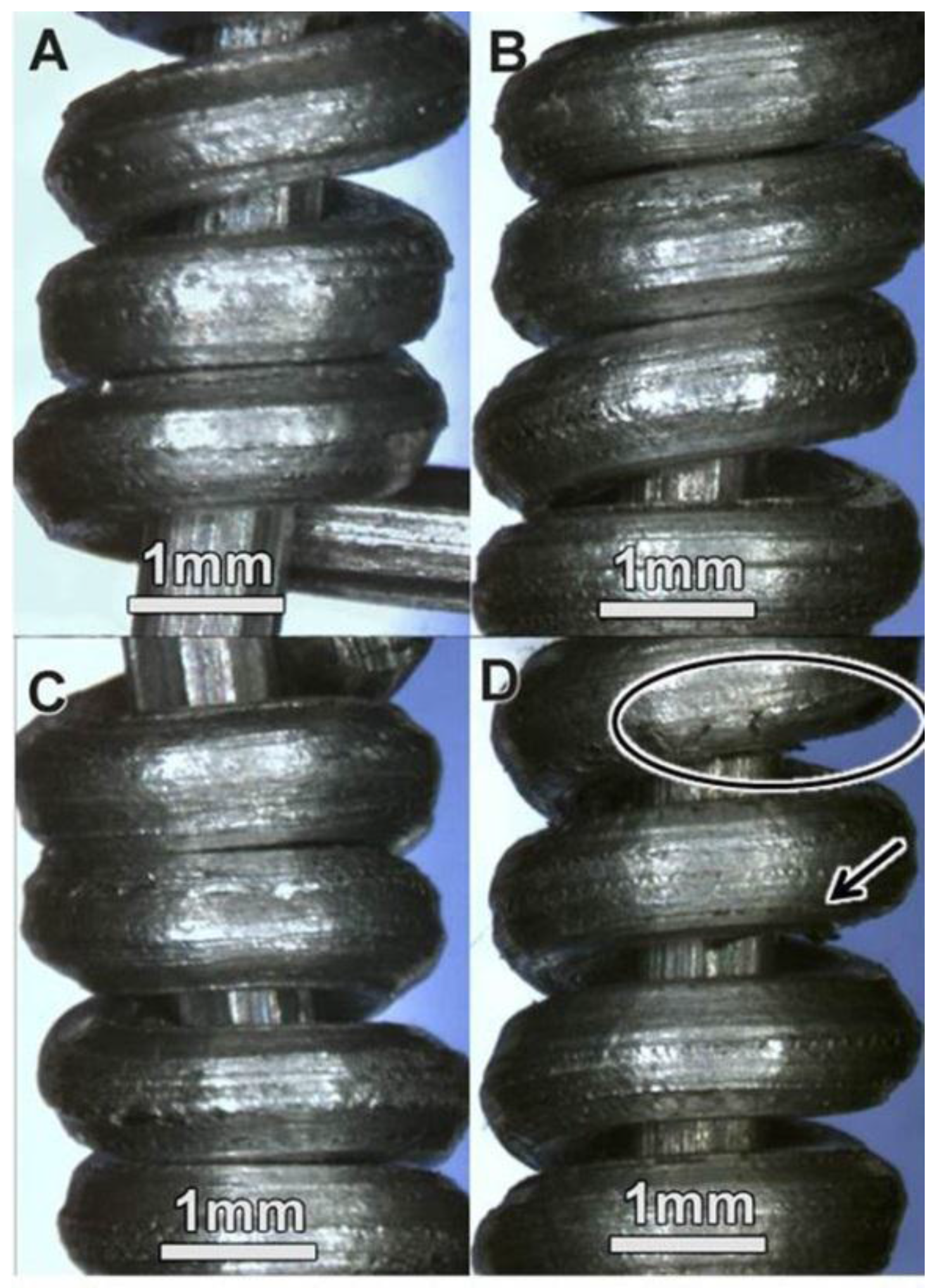
3.2.3. Bending (Looping) Properties
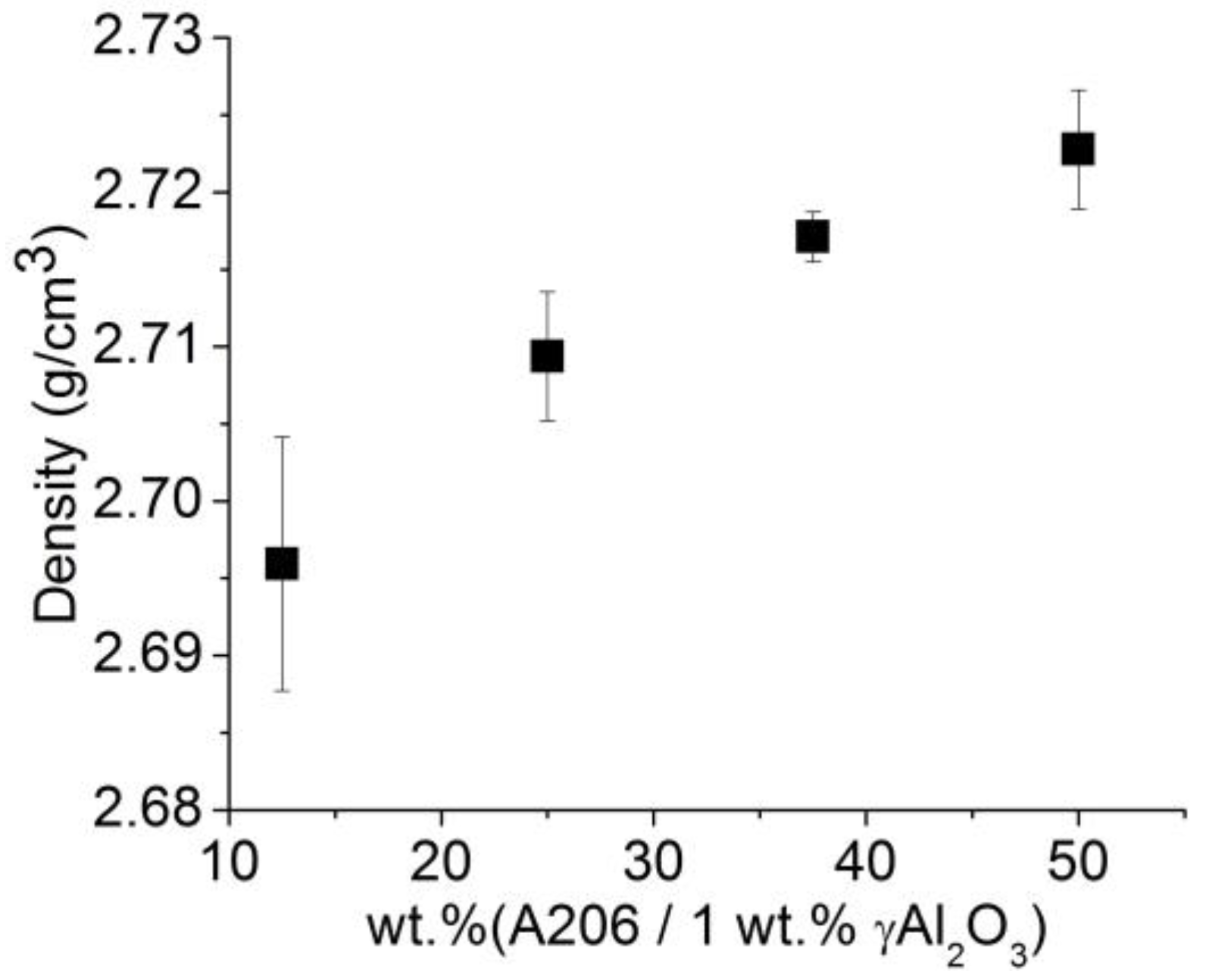
3.2.4. Density of Wires
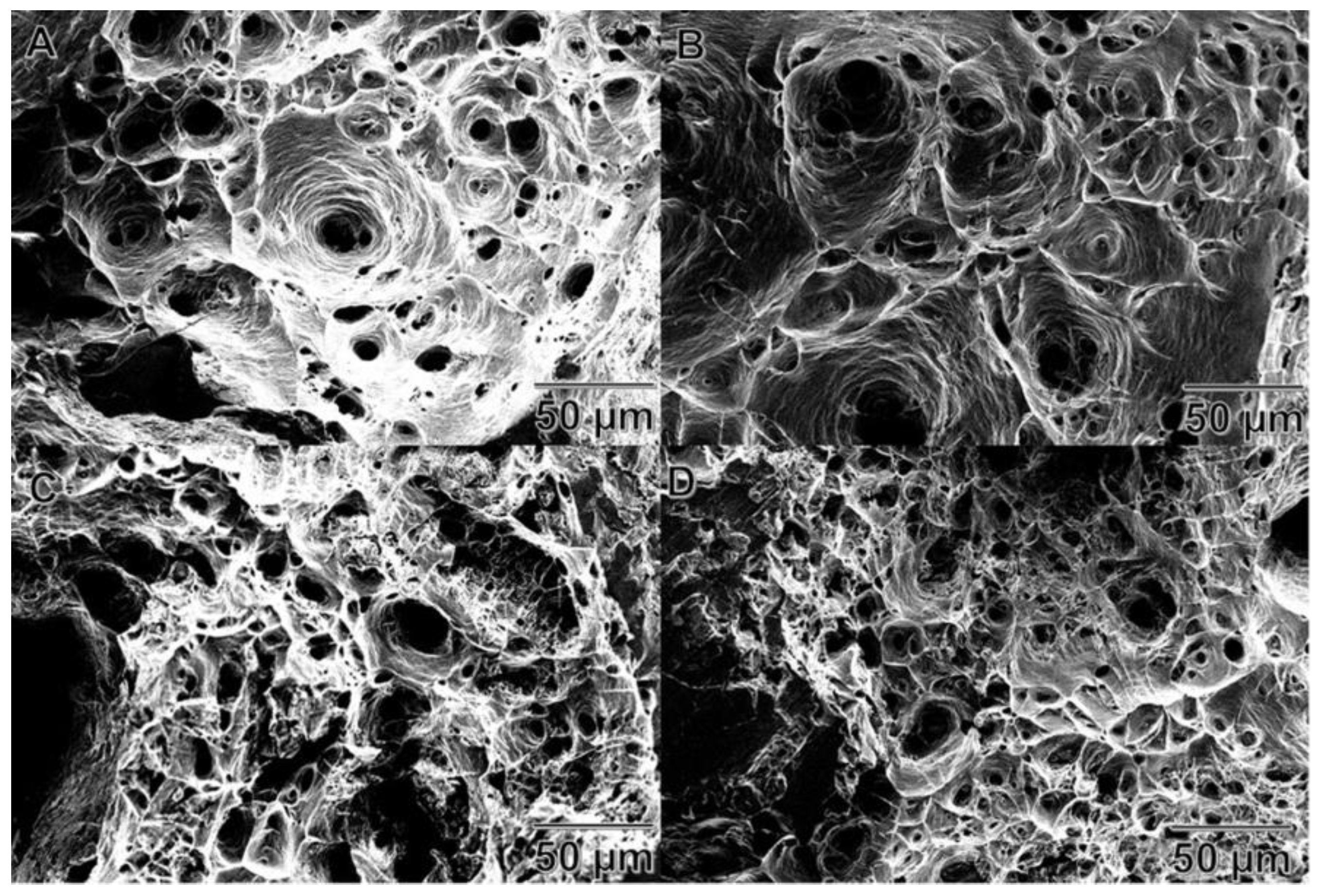
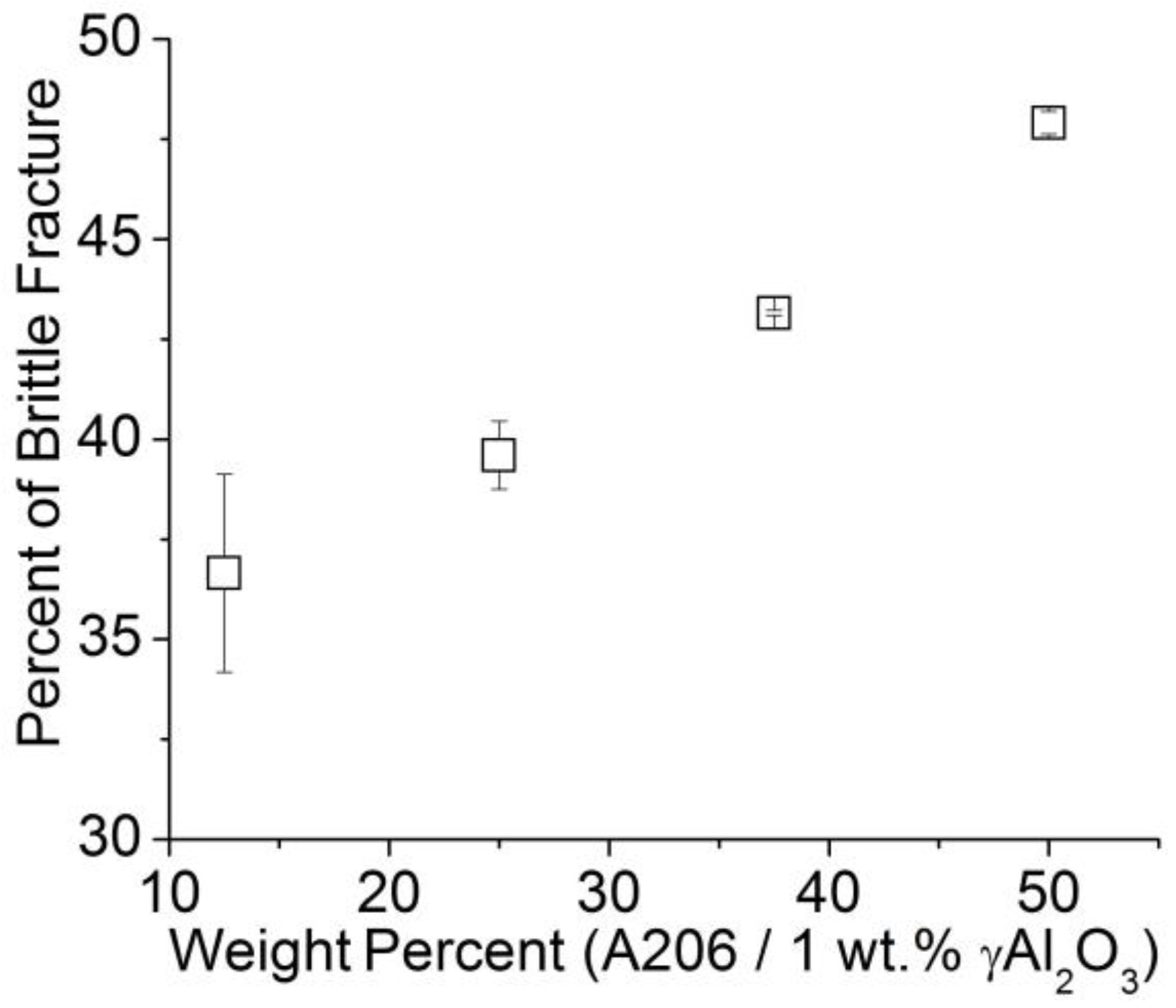
3.2.5. Fractographic Study of Wires
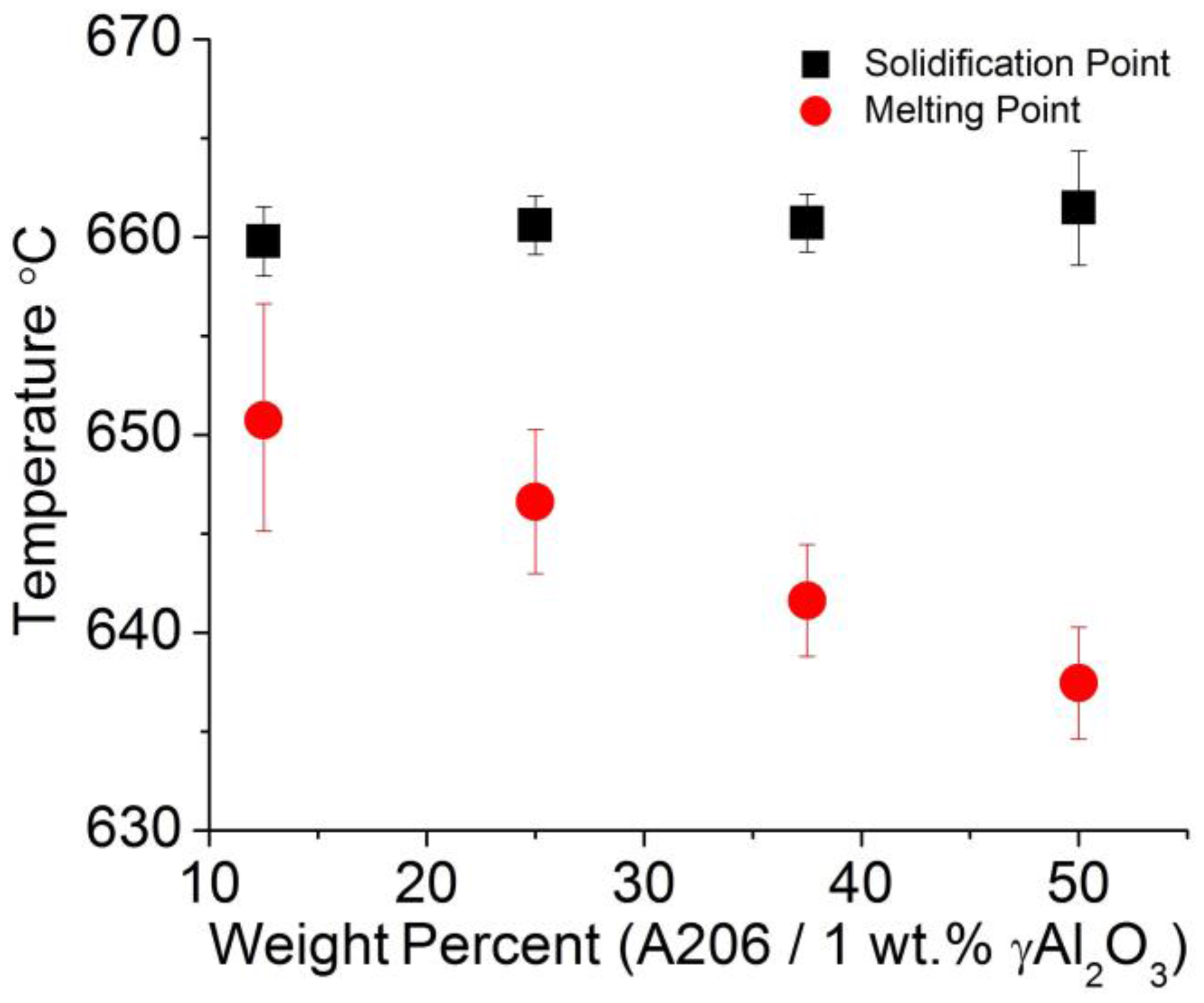
3.2.6. Wires Thermal Analysis
4. Discussion
5. Conclusions
- ▪
- The γAl2O3 nanoparticles can be successfully added to molten A206 to fabricate an A206/1 wt % γAl2O3 nanocomposite by semi-solid mixing and ultrasonic processing.
- ▪
- The ultimate tensile strength of the wires can be increased by increasing the amount of γAl2O3 nanoparticles and Cu added to the aluminum melt.
- ▪
- Increasing the levels of γAl2O3 nanoparticles and Cu lowers the electrical conductivity and melting point of the wires.
- ▪
- A fractography study revealed that an increment of A206/1 wt % γAl2O3 nanocomposite in the aluminum matrix leads to more brittleness of the wires.
- ▪
- All those results are also corroborated via statistical analysis. They evince the feasibility of using this new material as a filler in aluminum welding.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Z.D.; Liu, L.M.; Song, G. Welding characteristics of AZ31B magnesium alloy using DC-PMIG welding. Trans. Nonferr. Met. Soc. China Engl. Ed. 2013, 23, 315–322. [Google Scholar] [CrossRef]
- Gao, M.; Tang, H.-G.; Chen, X.-F.; Zeng, X.-Y. High power fiber laser arc hybrid welding of AZ31B magnesium alloy. Mater. Des. 2012, 42, 46–54. [Google Scholar] [CrossRef]
- Seshagiri, P.C.; Nair, B.S.; Reddy, G.M.; Rao, K.S.; Bhattacharya, S.S.; Rao, K.P. Improvement of mechanical properties of aluminum–copper alloy (AA2219) GTA welds by Sc addition. Sci. Technol. Weld. Join. 2008, 13, 146–158. [Google Scholar] [CrossRef]
- Nie, J.F. Preface to viewpoint set on: Phase transformations and deformation in magnesium alloys. Scr. Mater. 2003, 48, 981–984. [Google Scholar] [CrossRef]
- Jun, J.-H. Microstructure and Damping Capacity of Mg2Si/Mg-Al-Si-(Bi) Composites. Mater. Trans. 2012, 53, 2064–2066. [Google Scholar] [CrossRef]
- Fauzi, M.N.A.; Uday, M.B.; Zuhailawati, H.; Ismail, A.B. Microstructure and mechanical properties of alumina-6061 aluminum alloy joined by friction welding. Mater. Des. 2010, 31, 670–676. [Google Scholar] [CrossRef]
- Qin, Q.D.; Huang, B.W.; Li, W.; Zeng, Z.Y. Preparation and wear resistance of aluminum composites reinforced with in situ formed TiO/Al2O3. J. Mater. Eng. Perform. 2016, 25, 2029–2036. [Google Scholar] [CrossRef]
- Hoseini, M.; Meratian, M. Tensile properties of in-situ aluminum–alumina composites. Mater. Lett. 2005, 59, 3414–3418. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, N.; Zhu, D. Friction and wear properties of in-situ synthesized Al2O3 reinforced aluminum composites. Trans. Nonferr. Met. Soc. China 2014, 24, 2352–2358. [Google Scholar] [CrossRef]
- Belhouchet, H.; Garnier, V.; Fantozzi, G.; Franc, J. Control of the γ-alumina to α-alumina phase transformation for an optimized alumina densification. Bol. Soc. Esp. Cerám. Vidrio 2016, 56, 4–11. [Google Scholar]
- Yalamaç, E.; Trapani, A.; Akkurt, S. Sintering and microstructural investigation of gamma e alpha alumina powders. Eng. Sci. Technol. Int. J. 2014, 17, 2–7. [Google Scholar] [CrossRef]
- Dorri, A.; Omrani, E.; Lopez, H.; Zhou, L.; Sohn, Y.; Rohatgi, P.K. Strengthening in hybrid alumina-titanium diboride aluminum matrix composites synthesized by ultrasonic assisted reactive mechanical mixing. Mater. Sci. Eng. A 2017, 702, 312–321. [Google Scholar] [CrossRef]
- ASM Handbook Properties and Selection: Nonferrous Alloys and Special-Purpose Materials, 10th ed.; ASM International: Geauga County, OH, USA, 1990; Volume 2, pp. 78–79.
- Sreekumar, V.M.; Babu, N.H.; Eskin, D.G. Potential of an Al-Ti-MgAl2O4 master alloy and ultrasonic cavitation in the grain refinement of a cast aluminum alloy. Metall. Mater. Trans. B 2017, 48, 208–219. [Google Scholar] [CrossRef]
- Choi, H.; Cho, W.H.; Konishi, H.; Kou, S.; Li, X. Nanoparticle-induced superior hot tearing resistance of A206 alloy. Metall. Mater. Trans. A 2013, 44, 1897–1907. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, S.; Li, X. Ultrasonic attenuation based inspection method for scale-up production of A206-Al2O3 metal matrix nanocomposites. J. Manuf. Sci. Eng. 2014, 137, 1–10. [Google Scholar]
- Chen, L.Y.; Weiss, D.; Morrow, J.; Xu, J.Q.; Li, X.C. A novel manufacturing route for production of high-performance metal matrix nanocomposites. Manuf. Lett. 2013, 1, 62–65. [Google Scholar] [CrossRef]
- Florián-Algarín, D.; Marrero, R.; Padilla, A.; Suárez, O.M. Strengthening of Al and Al- Mg alloy wires by melt inoculation with Al/MgB2 nanocomposite. J. Mech. Behav. Mater. 2015, 24, 207–212. [Google Scholar] [CrossRef]
- Florián-Algarín, D.; Padilla, A.; López, N.N.; Suárez, O.M. Fabrication of aluminum wires treated with nanocomposite pellets. Sci. Eng. Compos. Mater. 2014, 22, 485–490. [Google Scholar] [CrossRef]
- B557-06 ASTM. Standard Test Methods for Tension Testing Wrought and Cast Aluminum-and Magnesium-Alloy Products; ASM International: Geauga County, OH, USA, 2010; pp. 1–15.
- Suárez, O.M.; Stone, D.S.; Kailhofer, C.J. Measurement of electrical resistivity in metals and alloys using a commercial data acquisition software. J. Mater. Educ. 1999, 20, 341–356. [Google Scholar]
- Stratton, S.W. U.S. National Bureau of Standards Copper Wire Tattles, 3rd ed.; Washington Government Printing Office: Washington, DC, USA, 1914. [Google Scholar]
- Dieringa, H. Properties of magnesium alloys reinforced with nanoparticles and carbon nanotubes: A review. J. Mater. Sci. 2010, 46, 289–306. [Google Scholar] [CrossRef]
- ASM Handbook Properties and Selection: Nonferrous Alloys and Special-Purpose Materials, 10th ed.; ASM International: Geauga County, OH, USA, 1990; Volume 2, p. 17.
- ASTM B230/B230M. Standard Specification for Aluminum 1350—H19 Wire for Electrical Purposes 1; ASM International: Geauga County, OH, USA, 1975; pp. 1–4.
- Corchado, M.; Reyes, F.; Suárez, O.M. Effects of AlB2 Particles and zinc on the absorbed impact energy of gravity cast aluminum matrix composites. JOM 2014, 66, 926–934. [Google Scholar] [CrossRef]
- Yan, X.-Y.; Chang, Y.; Xie, F.-Y.; Chen, S.-L.; Zhang, F.; Daniel, S. Calculated phase diagrams of aluminum alloys from binary Al–Cu to multicomponent commercial alloys. J. Alloys Compd. 2001, 320, 151–160. [Google Scholar] [CrossRef]
- Suarez, O.M.; Perepezko, J.H. Microstructural observation of active nucleants in Al-Ti-B master alloys. Light Met. 1992, 3, 851–859. [Google Scholar]
- Suárez, O.M. A study of the role of diborides in the heterogeneous nucleation of aluminum. Rev. Met. 2004, 40, 173–181. [Google Scholar]
- Yang, L.; Xia, M.; Li, J.G. Epitaxial growth in heterogeneous nucleation of pure aluminum. Mater. Lett. 2014, 132, 52–54. [Google Scholar] [CrossRef]
- Aksöz, S.; Ocak, Y.; Maraşlı, N.; Çadirli, E.; Kaya, H.; Böyük, U. Dependency of the thermal and electrical conductivity on the temperature and composition of Cu in the Al based Al–Cu alloys. Exp. Therm. Fluid Sci. 2010, 34, 1507–1516. [Google Scholar] [CrossRef]



| Alloy | wt % Cu | wt % Mn | wt % Mg | wt % Ti |
|---|---|---|---|---|
| A206.0 | 4.5 | 0.3 | 0.25 | 0.22 |
| Wires | wt % Al | wt % γAl2O3 | wt % Cu | wt % Mn | wt % Mg | wt % Ti |
|---|---|---|---|---|---|---|
| Al-12.5 wt % (A206/1 wt % γAl2O3) | 99.218 | 0.125 | 0.562 | 0.037 | 0.031 | 0.027 |
| Al-25.0 wt % (A206/1 wt % γAl2O3) | 98.433 | 0.250 | 1.125 | 0.075 | 0.062 | 0.055 |
| Al-37.5 wt % (A206/1 wt % γAl2O3) | 97.651 | 0.375 | 1.687 | 0.112 | 0.093 | 0.082 |
| Al-50.0 wt % (A206/1 wt % γAl2O3) | 96.865 | 0.500 | 2.250 | 0.150 | 0.125 | 0.110 |
| Parameter | Value | Standard Error of the Coefficient | p-Value |
|---|---|---|---|
| Constant | 70.124 | 8.319 | 0.000 |
| wt % Al2O3 | 595.989 | 62.778 | 0.000 |
| wt % Cu | 30.763 | 5.762 | 0.000 |
| wt % Cu·wt % Al2O3 | −143.890 | 32.424 | 0.001 |
| Parameter | Value | Standard Error of the Coefficient | p-Value |
|---|---|---|---|
| Constant | 64.624 | 1.387 | 0.000 |
| wt % Al2O3 | −26.958 | 9.301 | 0.000 |
| wt % Cu | −7.456 | 0.874 | 0.000 |
| Temperature | −0.125 | 0.009 | 0.000 |
| wt % Cu·wt % Al2O3 | 8.837 | 4.787 | 0.072 |
| Parameter | Value | Standard Error of the Coefficient | p-Value |
|---|---|---|---|
| Constant | 656.221 | 0.66204 | 0.000 |
| wt % Al2O3 | −11.315 | 1.57952 | 0.000 |
| wt % Cu | −5.987 | 0.46425 | 0.000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florián-Algarín, D.; Marrero, R.; Li, X.; Choi, H.; Suárez, O.M. Strengthening of Aluminum Wires Treated with A206/Alumina Nanocomposites. Materials 2018, 11, 413. https://doi.org/10.3390/ma11030413
Florián-Algarín D, Marrero R, Li X, Choi H, Suárez OM. Strengthening of Aluminum Wires Treated with A206/Alumina Nanocomposites. Materials. 2018; 11(3):413. https://doi.org/10.3390/ma11030413
Chicago/Turabian StyleFlorián-Algarín, David, Raúl Marrero, Xiaochun Li, Hongseok Choi, and Oscar Marcelo Suárez. 2018. "Strengthening of Aluminum Wires Treated with A206/Alumina Nanocomposites" Materials 11, no. 3: 413. https://doi.org/10.3390/ma11030413
APA StyleFlorián-Algarín, D., Marrero, R., Li, X., Choi, H., & Suárez, O. M. (2018). Strengthening of Aluminum Wires Treated with A206/Alumina Nanocomposites. Materials, 11(3), 413. https://doi.org/10.3390/ma11030413







