Recyclable Aggregates of Mesoporous Titania Synthesized by Thermal Treatment of Amorphous or Peptized Precursors
Abstract
:1. Introduction
2. Experimental
3. Results
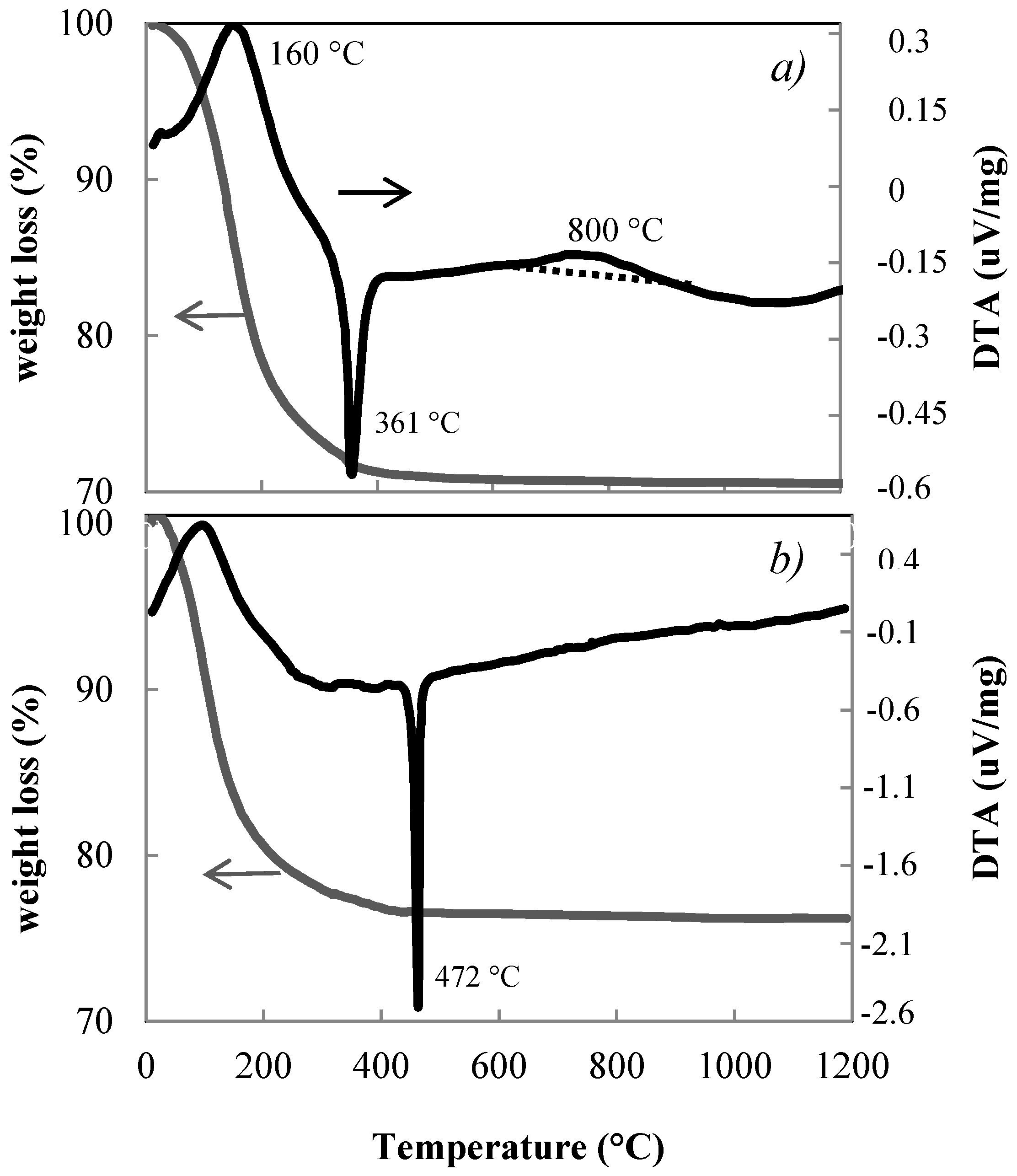
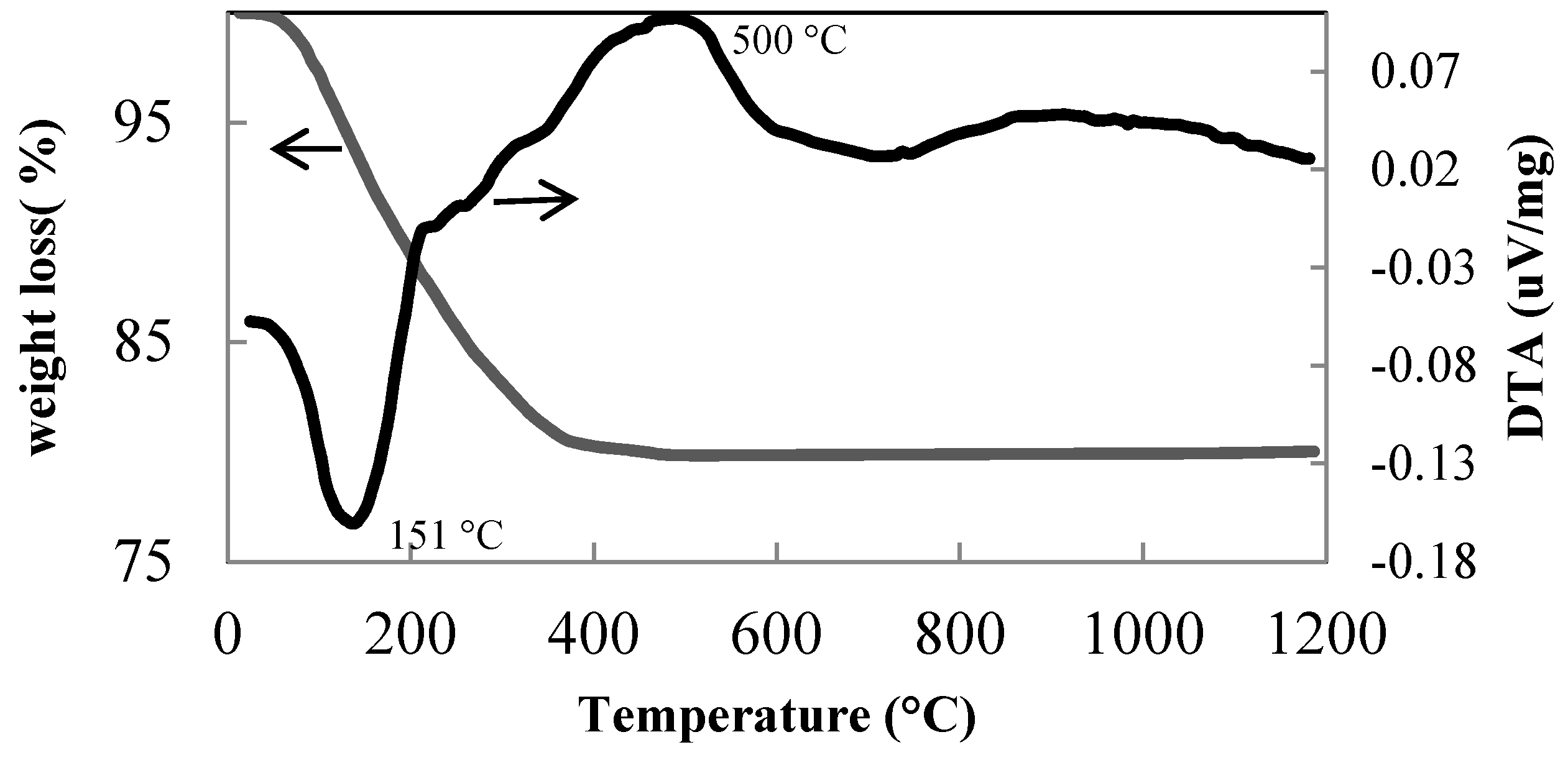
3.1. Thermal Behaviour of the Xerogels
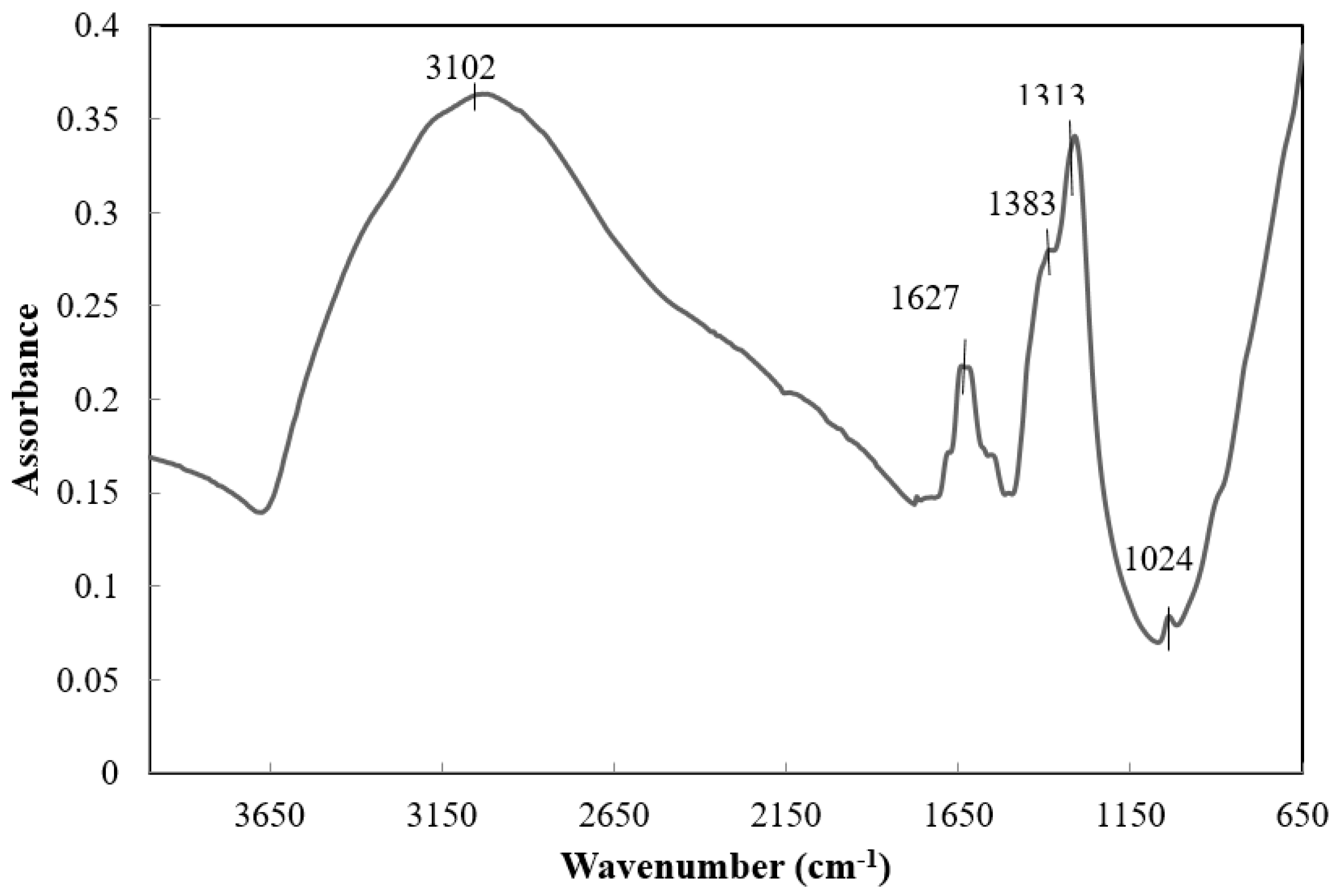
3.2. Peptization of TiO2 Xerogels with HNO3
3.2.1. Effect of the Concentration
3.2.2. Effect of Treatment Time of Peptization
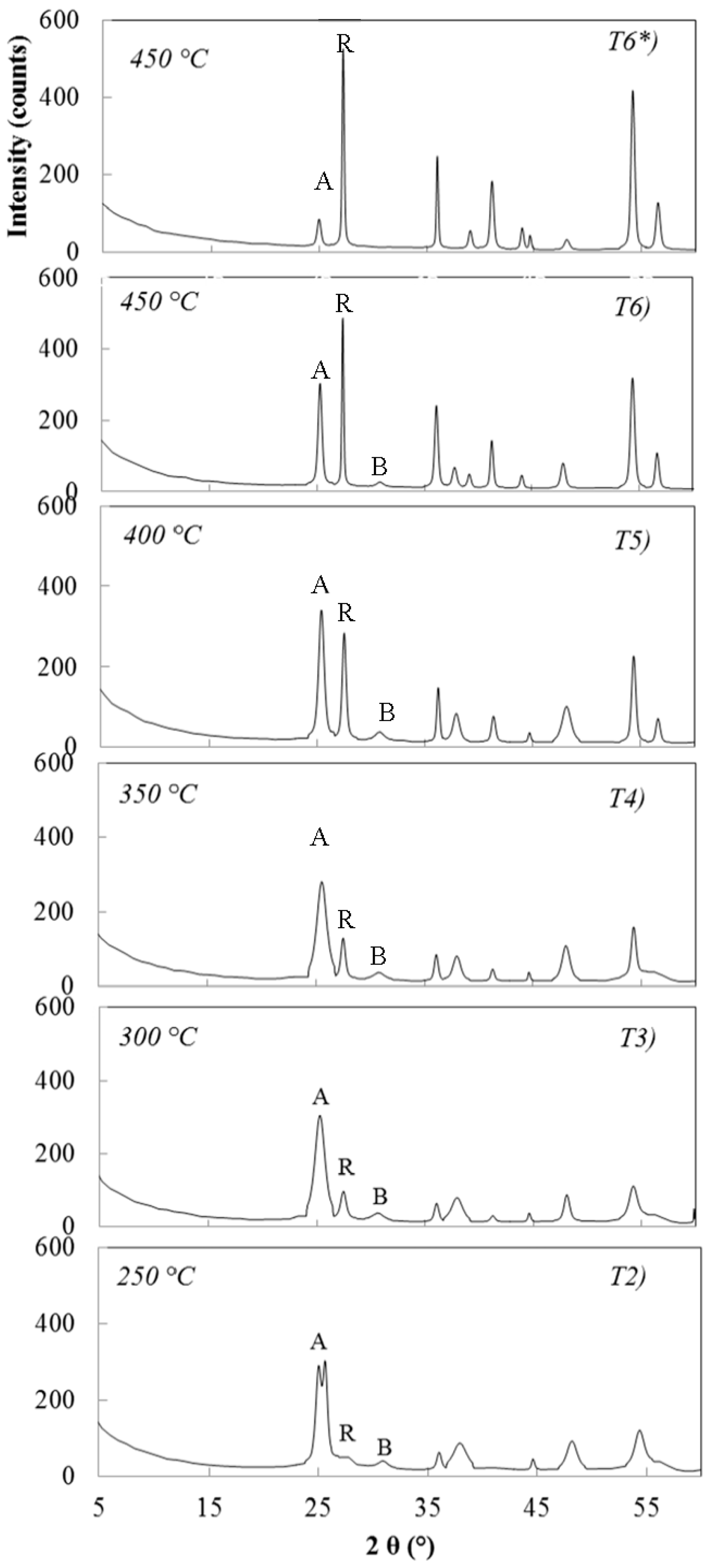
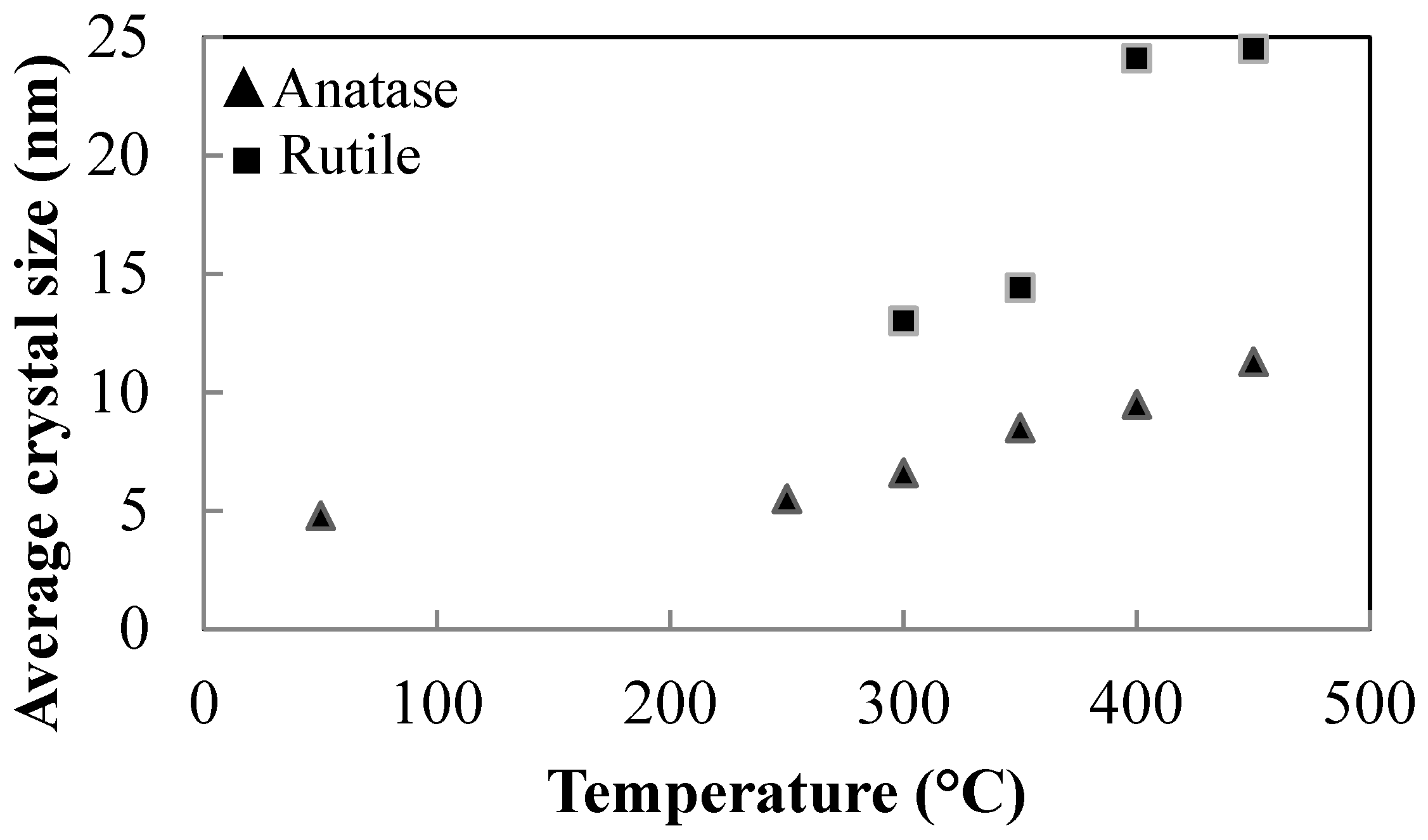
3.3. Thermal Treatments of Peptized Titania
3.3.1. Sample Hydrolysed with 0.07 M NH4OH and Peptized with 0.02 M HNO3
3.3.2. Samples Hydrolysed with 0.07 M NH4OH and Peptized with 0.1 M HNO3
3.3.3. Samples Hydrolysed with 1.0 M NH4OH and Peptized with 0.02 M HNO3
3.3.4. Sample Hydrolyzed with 1.0 M NH4OH and Peptized with 0.1 M HNO3
3.4. Hydrolysis, Peptization and Calcination of Titania in Mixture with Zirconia (5 mol %)
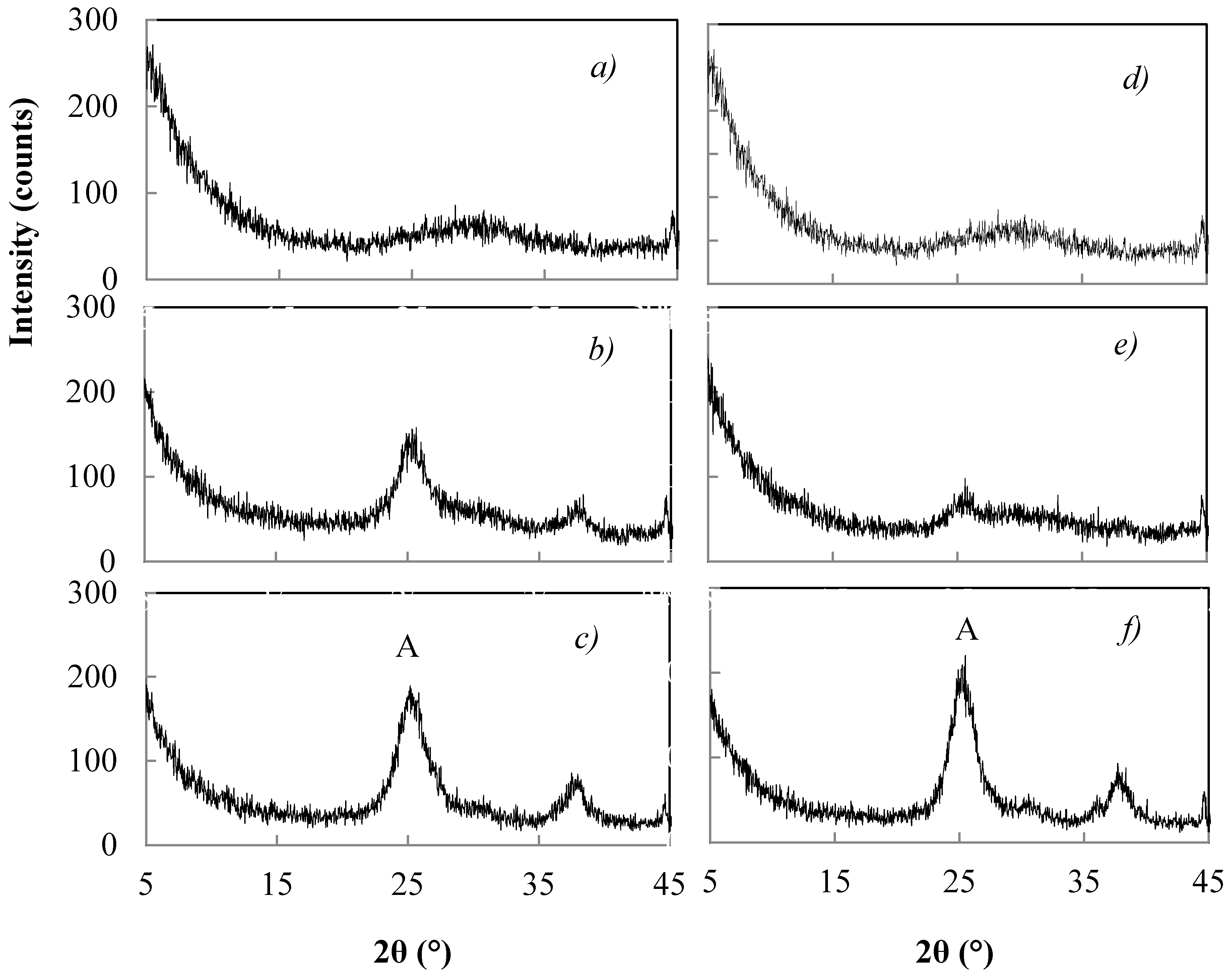
- The calcination of the unpeptized samples favours the crystallization of anatase with relatively larger crystals (samples E and F).
- Upon calcination, the peptized samples form anatase with crystals that are relatively smaller in size (samples G, H, I, J, L, M).
- The peptizing treatment with increasing concentration of HNO3 reduces the anatase crystal size for samples G, I and L, which were subjected to the same peptizing time of 1 h and to the same heat treatment for 2 h at 450 °C.
- The heat rate in calcination significantly affects the crystal size of anatase. Comparing sample L, heated at 2 °C/min, with sample N heat treated at 30 °C/min, very different crystal sizes have been detected. For sample L, the average crystal size was 6.3 nm, while for sample N it was 17.0 nm.
- After calcination at either 450 °C or 600 °C, no anatase-to-rutile phase transformation has been detected. This confirms the inhibitory effect of zirconia on the anatase-to-rutile phase transition observed in the literature [26].
- Larger anatase crystal sizes result for products thermally treated at 600 °C, compared with those treated at 450 °C (Samples E, F and L, M).
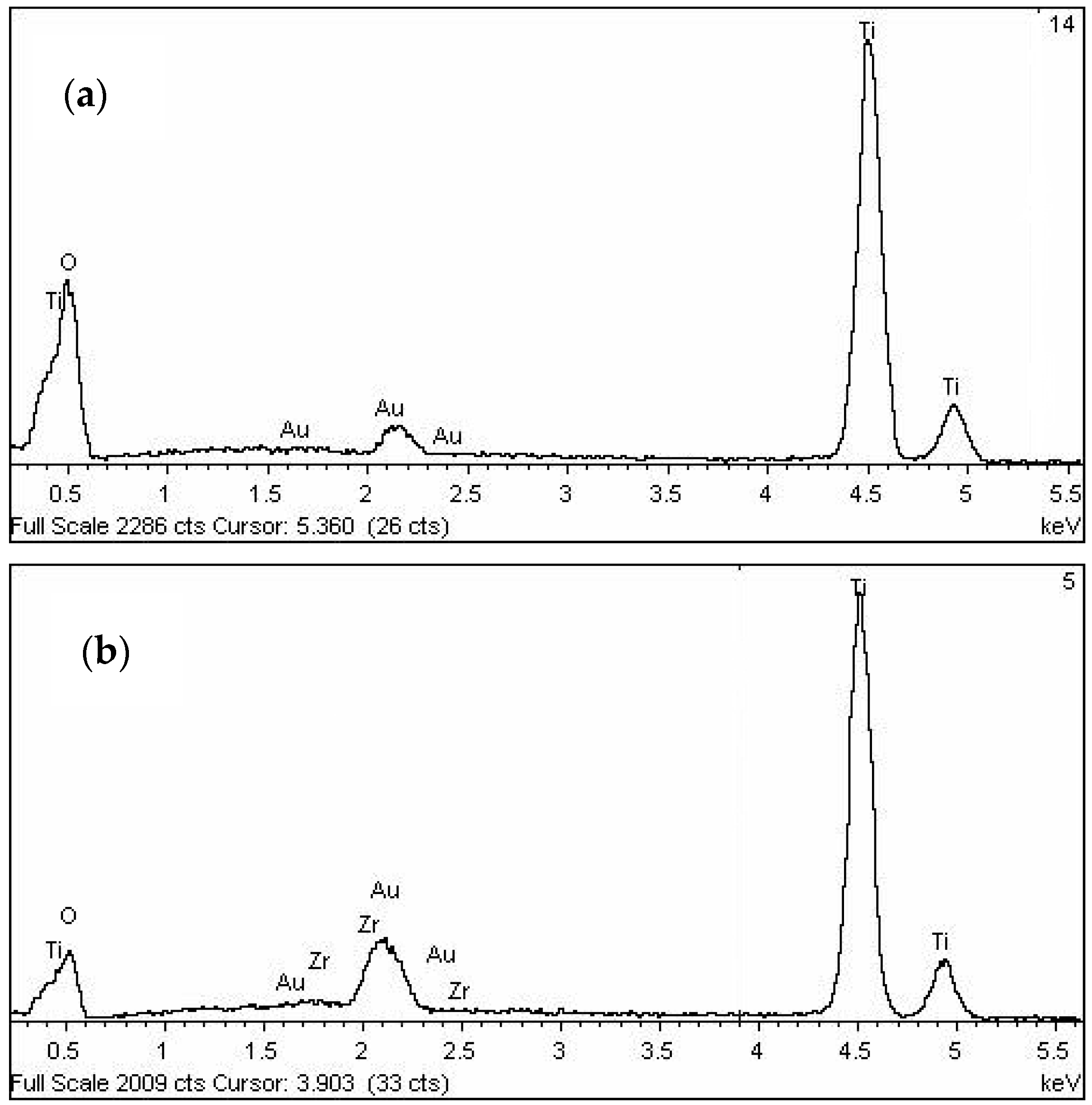
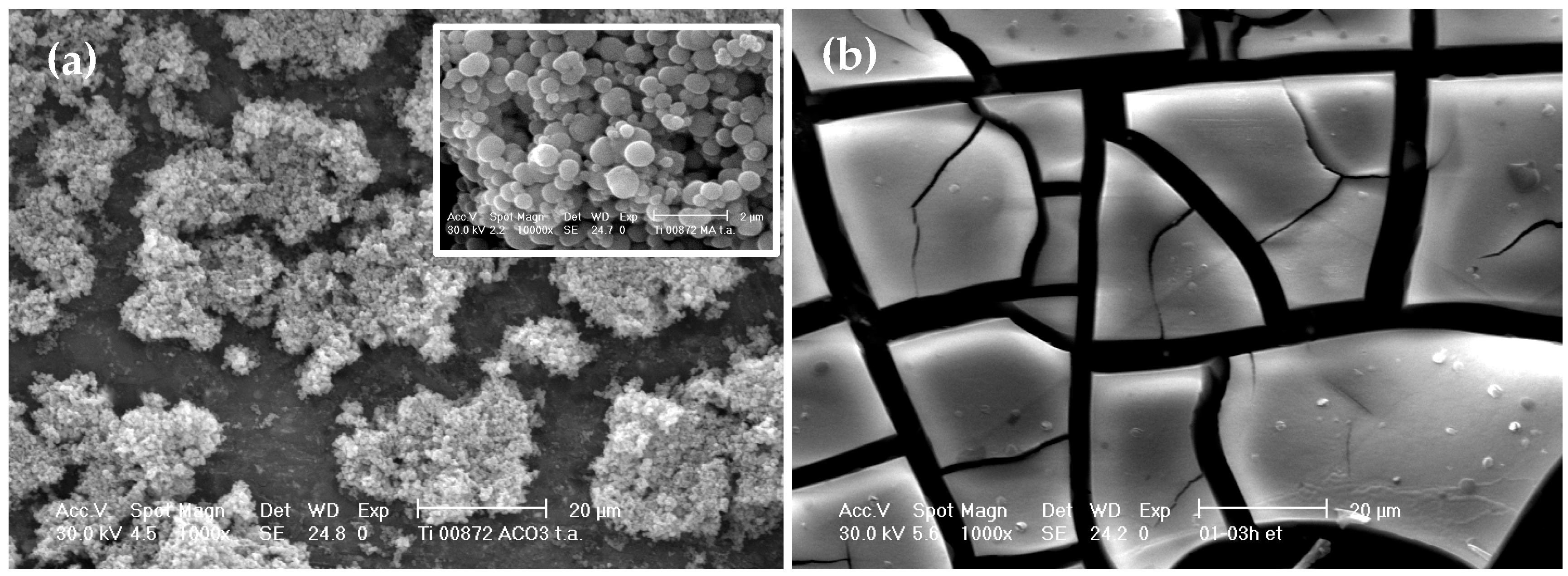
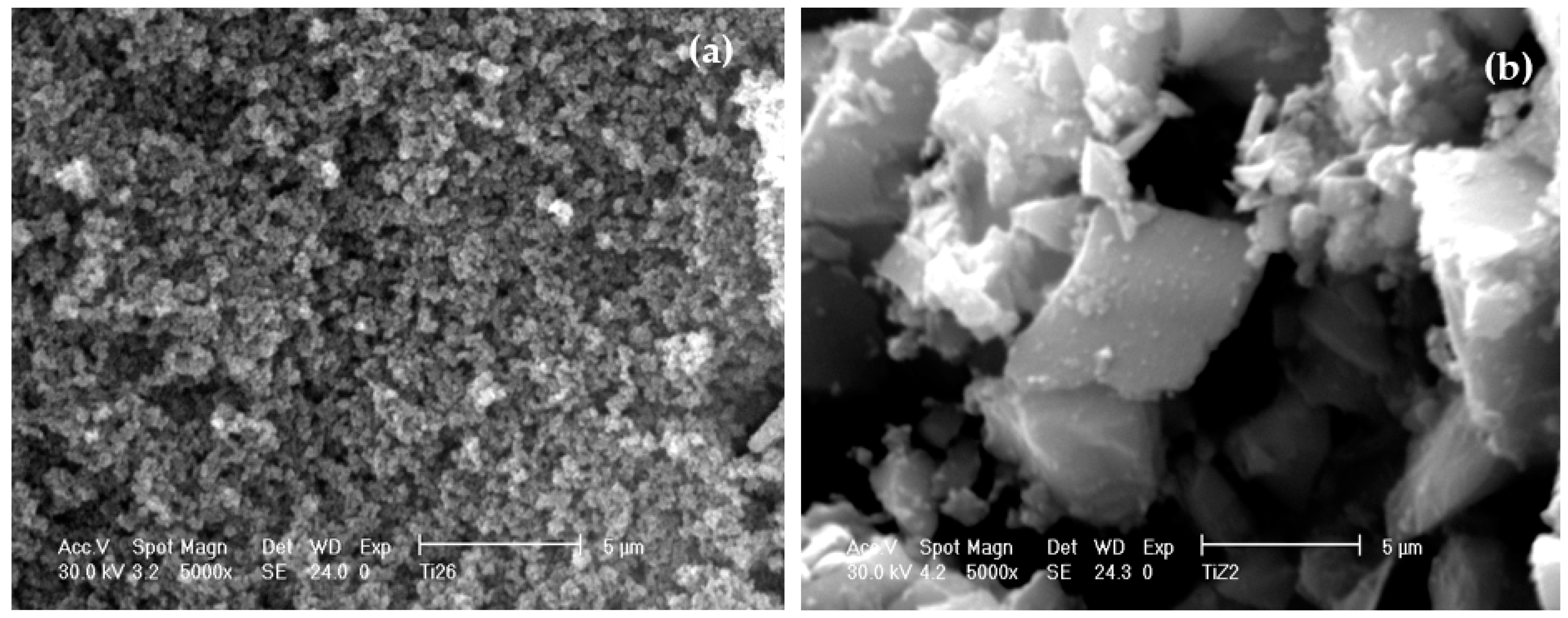
3.5. Morphology of Un-Peptized, Peptized and Calcined Products
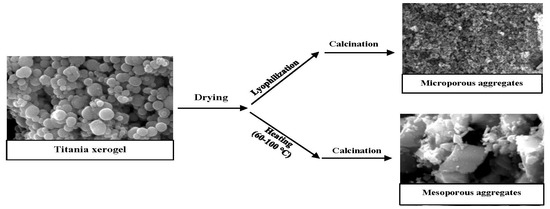
3.6. Effect of Drying Method on the Aggregation of Titania Particles
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Nah, Y.C.; Paramasivam, I.; Schmuki, P. Doped TiO2 and TiO2 nanotubes: Synthesis and applications. Chem. Phys. Chem. 2010, 11, 2698–2713. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.A.; Burkett, S.L.; Mendelson, N.H.; Mann, S. Bacterial templating of ordered macrostructures in silica and silica-surfactant mesophases. Nature 1997, 385, 420–423. [Google Scholar] [CrossRef]
- Chae, W.S.; Land, S.W.; Kim, Y.R. Templating route to mesoporous nanocrystalline titania nanofibers. Chem. Mater. 2005, 17, 3072–3074. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Mesoporous titania photocatalysts: Preparation, characterization and reaction mechanisms. J. Mater. Chem. 2011, 21, 11686–11707. [Google Scholar] [CrossRef]
- Tammaro, M.; Fiandra, V.; Mascolo, M.C.; Salluzzo, A.; Riccio, C.; Lancia, A. Photocatalytic degradation of atenolol in aqueous suspension of new recyclable catalysts based on titanium dioxide. J. Environ. Chem. Eng. 2017, 5, 3224–3234. [Google Scholar] [CrossRef]
- Lu, P.J.; Huang, S.C.; Chen, Y.P.; Chiueh, L.; ChihShih, D. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Atabaev, T.S.; Hossain, M.A.; Lee, D.; Kim, H.K.; Hwang, Y.H. Pt-coated TiO2 nanorods for photoelectrochemical water splitting applications. Results Phys. 2016, 6, 373–376. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, L.; Ferronato, C.; Yang, X.; Salvador, A.; Zeng, C.; Chovelon, J. Photocatalytic degradation of atenolol in aqueous titanium dioxide suspensions: Kinetics, intermediates and degradation pathways. J. Photochem. Photobiol. A Chem. 2016, 254, 35–44. [Google Scholar] [CrossRef]
- Chemseddine, A.; Moritz, T. Nanostructuring Titania: Control over Nanocrystal Structure, Size, Shape, and Organization. Eur. J. Inorg. Chem. 1999, 1999, 235–245. [Google Scholar] [CrossRef]
- Buonsanti, R.; Grillo, V.; Carlino, E.; Giannini, C.; Kipp, T.; Cingolani, R.; Cozzoli, P.D. Nonhydrolytic Synthesis of High-Quality Anisotropically Shaped Brookite TiO2 Nanocrystals. J. Am. Chem. Soc. 2008, 130, 11223–11233. [Google Scholar] [CrossRef] [PubMed]
- Likodimos, V.; Chrysi, A.; Calamiotou, M.; Fernández-Rodríguez, C.; Doña-Rodríguez, J.M.; Dionysiou, D.D.; Falaras, P. Microstructure and charge trapping assessment in highly reactive mixed phase TiO2 photocatalysts. Appl. Catal. B Environ. 2016, 192, 242–252. [Google Scholar] [CrossRef]
- Puddu, V.; Choi, H.; Dionysiou, D.D.; Puma, G.L. TiO2 photocatalyst for indoor air remediation: Influence of crystallinity, crystal phase, and UV radiation intensity on trichloroethylene degradation. Appl. Catal. B Environ. 2010, 94, 211–218. [Google Scholar] [CrossRef]
- Cargnello, M.; Gordon, T.R.; Murray, C.B. Solution-Phase Synthesis of Titanium Dioxide Nanoparticles and Nanocrystals. Chem. Rev. 2014, 114, 9319–9345. [Google Scholar] [CrossRef] [PubMed]
- Linsebigler, A.L.; Guangquan, L.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Byrne, J.A.; Eggins, B.R.; Brown, N.M.D.; McKinney, B.; Rouse, M. Immobilisation of TiO2 powder for the treatment of polluted water. Appl. Catal. B Environ. 1998, 17, 25–36. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, X.; Lu, G.Q.; Yue, P.; Greenfield, P.F. Novel Silica Gel Supported TiO2 Photocatalyst Synthesized by CVD Method. Langmuir 2000, 16, 6216–6222. [Google Scholar] [CrossRef]
- Mascolo, M.C.; Pe, Y.I.; Ring, T.A. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large pH Window with Different Bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, M.C.; Dell’Agli, G.; Mascolo, G. Mesoporous aggregates of ZrO2-doped (5 mol %) titania by interconn ection of primary nano-particles. Microporous Mesoporous Mater. 2010, 132, 196–200. [Google Scholar] [CrossRef]
- Mascolo, M.C. Synthesis of wide spectrum of mesoporous titania materials by forced co-hydrolysis of Zr–Ti alkoxides. Microporous Mesoporous Mater. 2013, 181, 160–165. [Google Scholar] [CrossRef]
- Li, G.; Yu, J.C.; Zhu, J.; Cao, Y. Hierarchical mesoporous grape-like titania with superior recyclability and photoactivity. Microporous Mesoporous Mater. 2007, 106, 278–283. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.A.; O’shea, K.; et al. A Review on the Visible Light Active Titanium Dioxide Photocatalysts for Environmental Applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T., Jr. Surface Science Studies of the Photoactivation of TiO2—New Photochemical Processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef] [PubMed]
- Colón, G.; Sánchez-España, J.M.; Hidalgo, M.C.; Navío, J.A. Effect of TiO2 acidic pre-treatment on the photocatalytic properties for phenol degradation. J. Photochem. Photobiol. A Chem. 2006, 179, 220–227. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Iida, Y.; Ozaki, S. Grain Growth and Phase Transformation of Titanium Oxide During Calcination. J. Am. Ceram Soc. 1961, 44, 120–128. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Reller, A. Phase transformation and grain growth of doped nanosized titania. Mater. Sci. Eng. C 2002, 19, 323–326. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A. Recent advances on TiO2-ZrO2 mixed oxides as catalysts and catalyst supports. Catal. Rev. Sci. Eng. 2005, 47, 257–296. [Google Scholar] [CrossRef]
- Neppolian, B.; Wang, Q.; Yamashita, H.; Choi, H. Synthesis and characterization of ZrO2-TiO2 binary oxide semiconductor nanoparticles: Application and interparticle electron transfer process. Appl. Catal. A 2007, 333, 264–271. [Google Scholar] [CrossRef]
- Ovenstone, J.; Yanagisawa, K. Effect of Hydrothermal Treatment of Amorphous Titania on the Phase Change from Anatase to Rutile during Calcination. Chem. Mater. 1999, 11, 2770–2774. [Google Scholar] [CrossRef]
- Li, H.; Hao, Y.; Lu, H.; Liang, L.; Wang, Y.; Qiu, J.; Shi, X.; Wang, Y.; Yao, J. A systematic study on visible-light N-doped TiO2 photocatalyst obtained from ethylenediamine by sol–gel method. Appl. Surf. Sci. 2015, 344, 112–118. [Google Scholar] [CrossRef]
- Peng, F.; Cai, L.; Yu, H.; Wang, H.; Yang, J. Synthesis and characterization of substitutional and interstitial nitrogen-doped titanium dioxides with visible light photocatalytic activity. J. Solid State Chem. 2008, 181, 130–136. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New Insights into the Mechanism of Visible Light Photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, X.; Chen, F.; Zhang, J. Effects of Calcination on the Physical and Photocatalytic Properties of TiO2 Powders Prepared by Sol–Gel Template Method. J. Sol-Gel Sci. Technol. 2005, 34, 181–187. [Google Scholar] [CrossRef]
- Gribb, A.A.; Banfiel, J.F. Particle size effects on transformation kinetics and phase stability in nanocrystalline TiO2. Am. Mineral. 1997, 82, 717–728. [Google Scholar] [CrossRef]
- Hu, Y.; Tsai, H.L.; Huang, C.L. Effect of brookite phase on the anatase–rutile transition in titania nanoparticles. J. Eur. Ceram. Soc. 2003, 23, 691–696. [Google Scholar] [CrossRef]

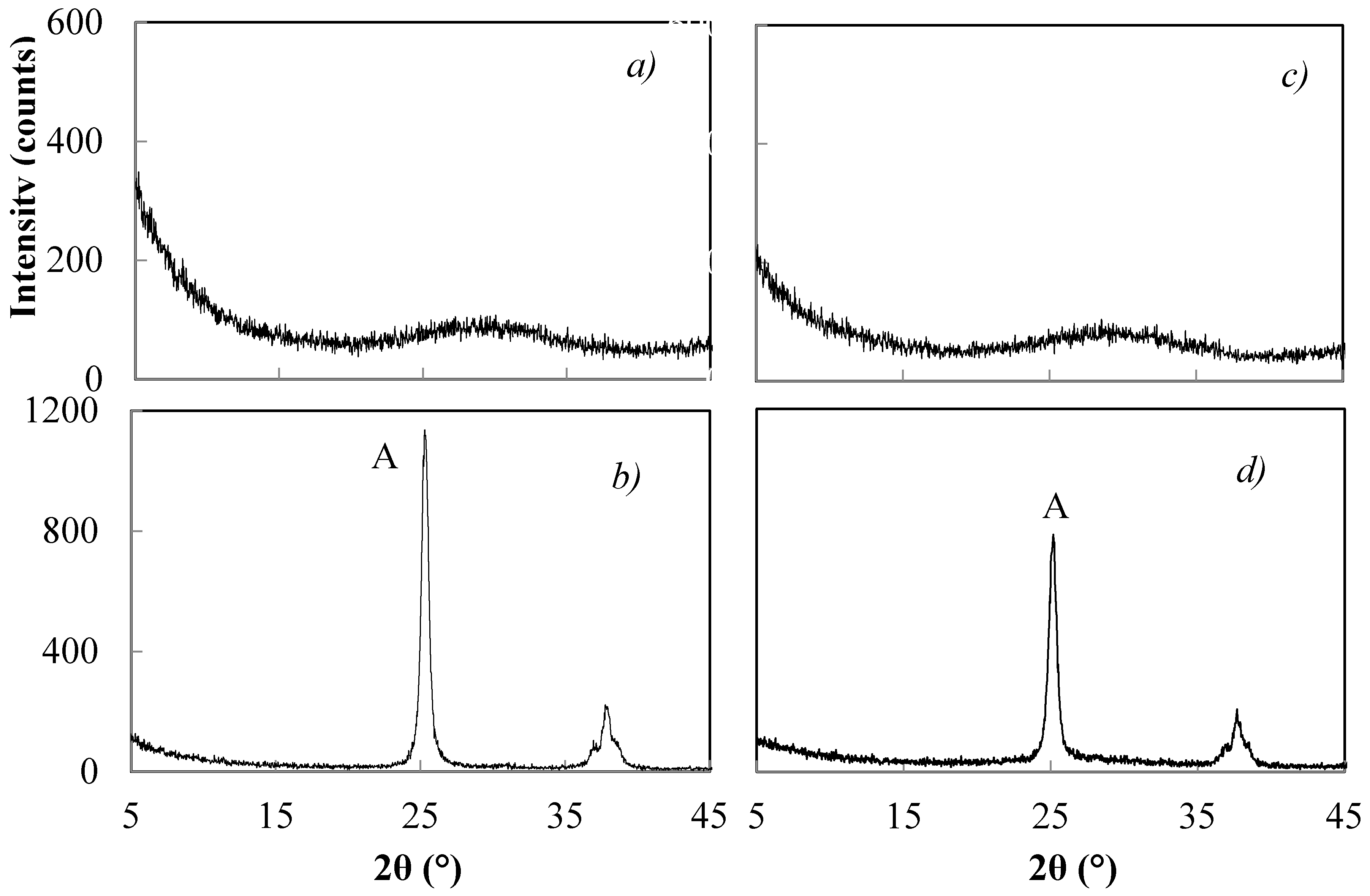
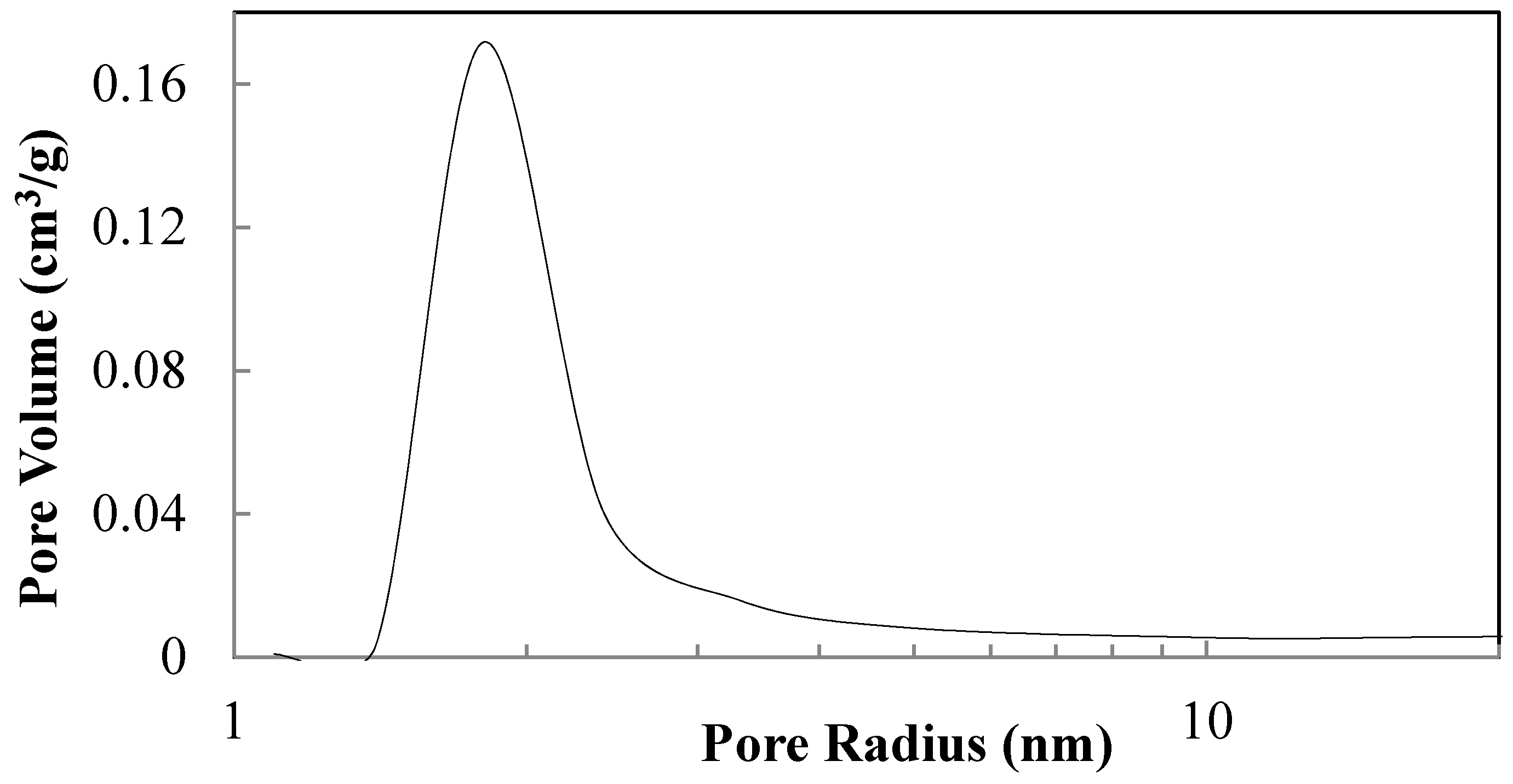
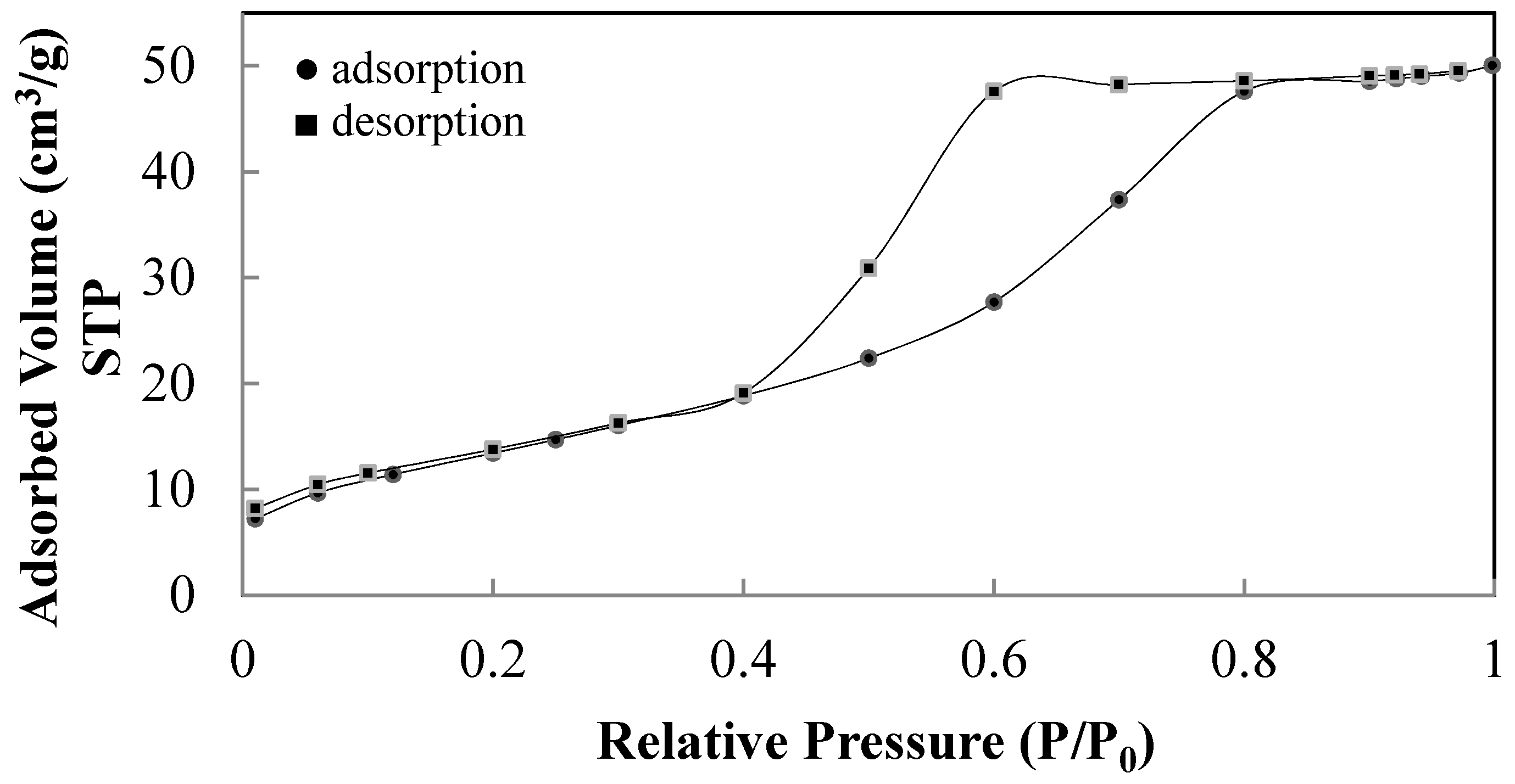
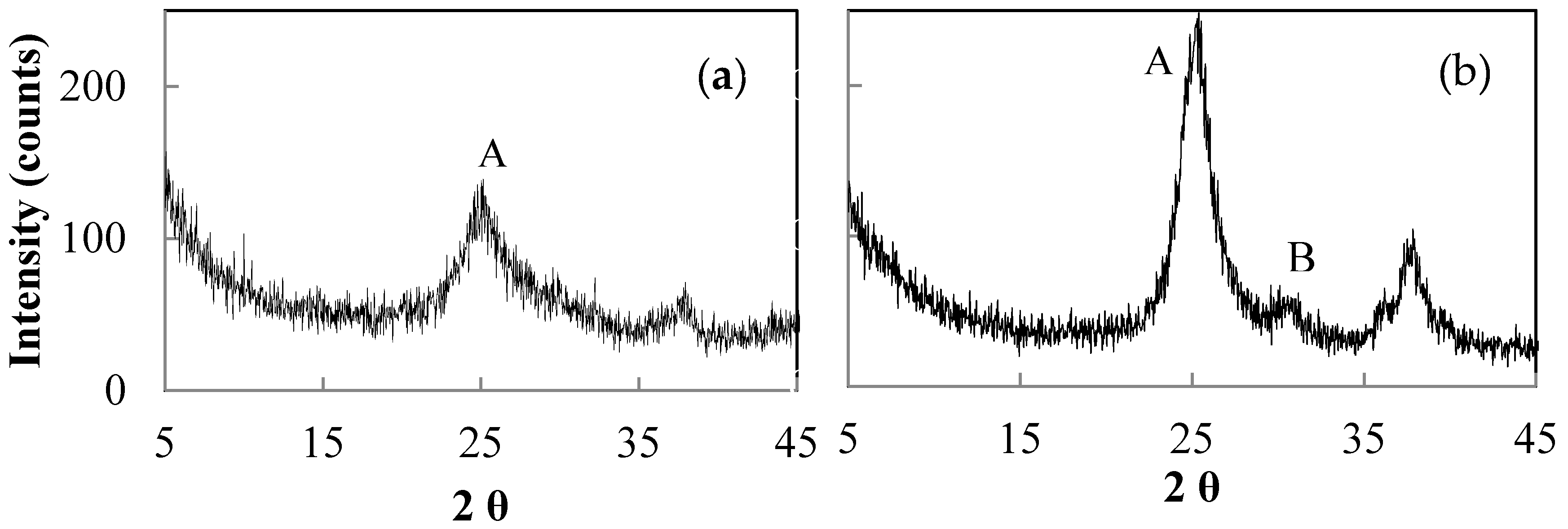
| Sample | NH4OH Concentration/Treatment Time (M/h) | HNO3 Concentration/Treatment Time (M/h) | Crystal Size (nm) by XRD | Polymorphic Phases A (Anatase), B (Brookite) |
|---|---|---|---|---|
| A | 0.07/1 | 0.1/1 | 4.8 | A, B (traces) |
| B | 0.07/1 | 0.1/3 | 4.7 | A, B (traces) |
| C | 1.0/1 | 0.1/1 | 4.7 | A, B (traces) |
| D | 1.0/1 | 0.1/3 | 4.6 | A, B (traces) |
| Sample | NH4OH Concentration/Treatment Time (M/h) | HNO3 Concentration/Treatment Time (M/h) | Calcination Temperature/ Heating Rate (°C/°C min−1) | BET Surface Area (m2/g) | Average Pore Diameter (nm) | Crystal Size by XRD (nm) | Polymorphic Phases (%) A (anatase) R (rutile) |
|---|---|---|---|---|---|---|---|
| T1 | 0.07/1 | 0.02/1 | 450/2 | 39.4 | 6.6 | 21.9 | 100 % (A) |
| T2 | 0.07/1 | 0.1/1 | 250/2 | - | - | 5.6 | 100 % (A) |
| T3 | 0.07/1 | 0.1/1 | 300/2 | - | - | 6.6 13.0 | 74.8 (A) 25.2 (R) |
| T4 | 0.07/1 | 0.1/1 | 350/2 | - | - | 8.5 14.4 | 67.5 (A) 32.5 (R) |
| T5 | 0.07/1 | 0.1/1 | 400/2 | - | - | 9.5 24.1 | 64.8 (A) 35.2 (R) |
| T6 | 0.07/1 | 0.1/1 | 450/2 | - | - | 11.3 24.5 | 30.1 (A) 69.9 (R) |
| T6* | 0.07/1 | 0.1/1 | 450/2 | - | - | 15.1 26.4 | A(9.0) R(91.0) |
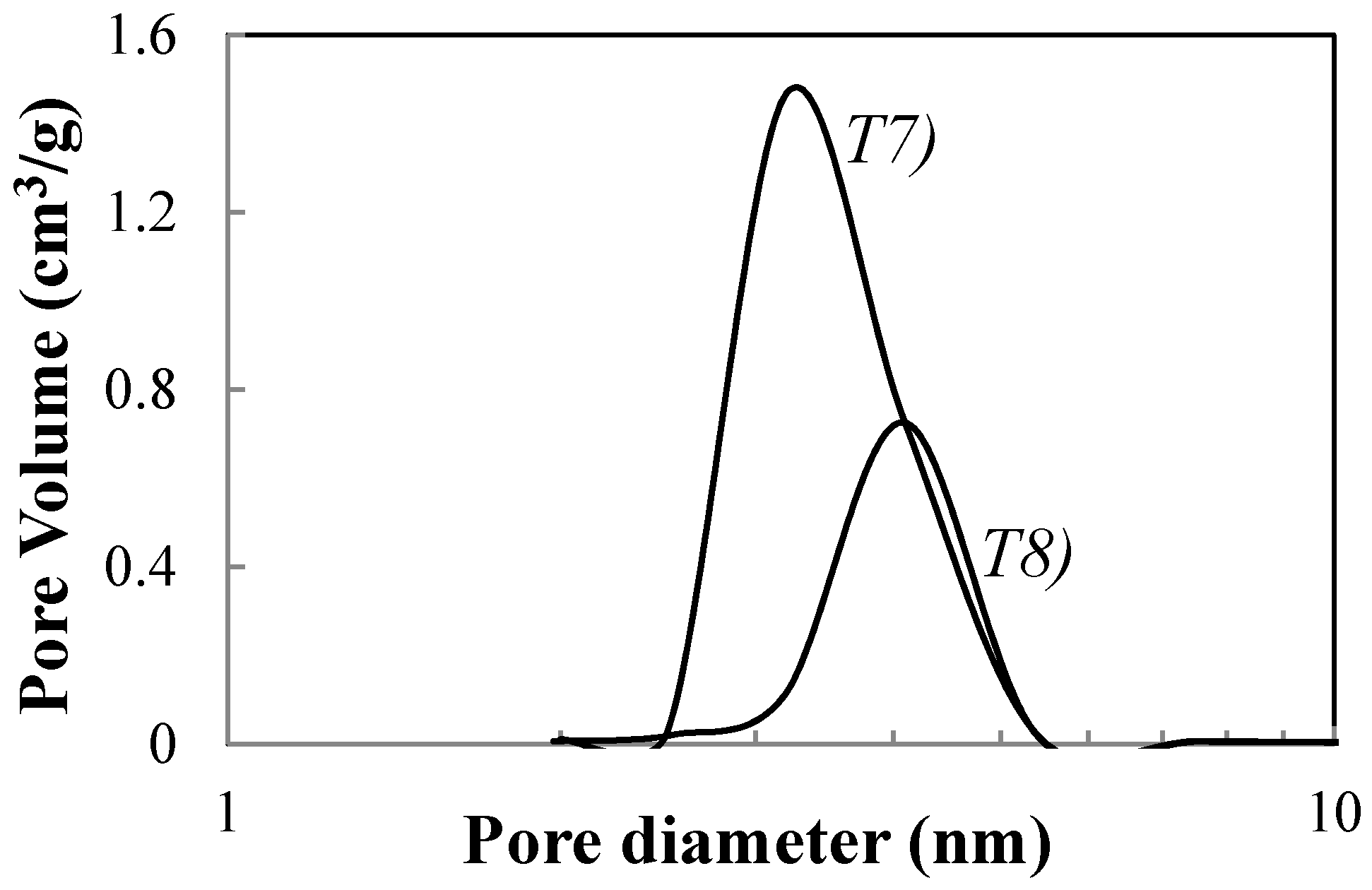
| T7 | 1.0/1 | 0.02/1 | 450/2 | 70.5 | 3.4 | 13.8 | 100 (A) |
| T8 | 1.0/1 | 0.02/1 | 450/30 | 65.5 | 4.0 | 19.8 | 100 (A) |
| T9 | 1.0/1 | 0.02/3 | 450/2 | 62.7 | 5.2 | 20.4 | 100 (A) |
| T10 | 1.0/1 | 0.02/3 | 450/30 | - | - | 26.3 | 100 (A) |
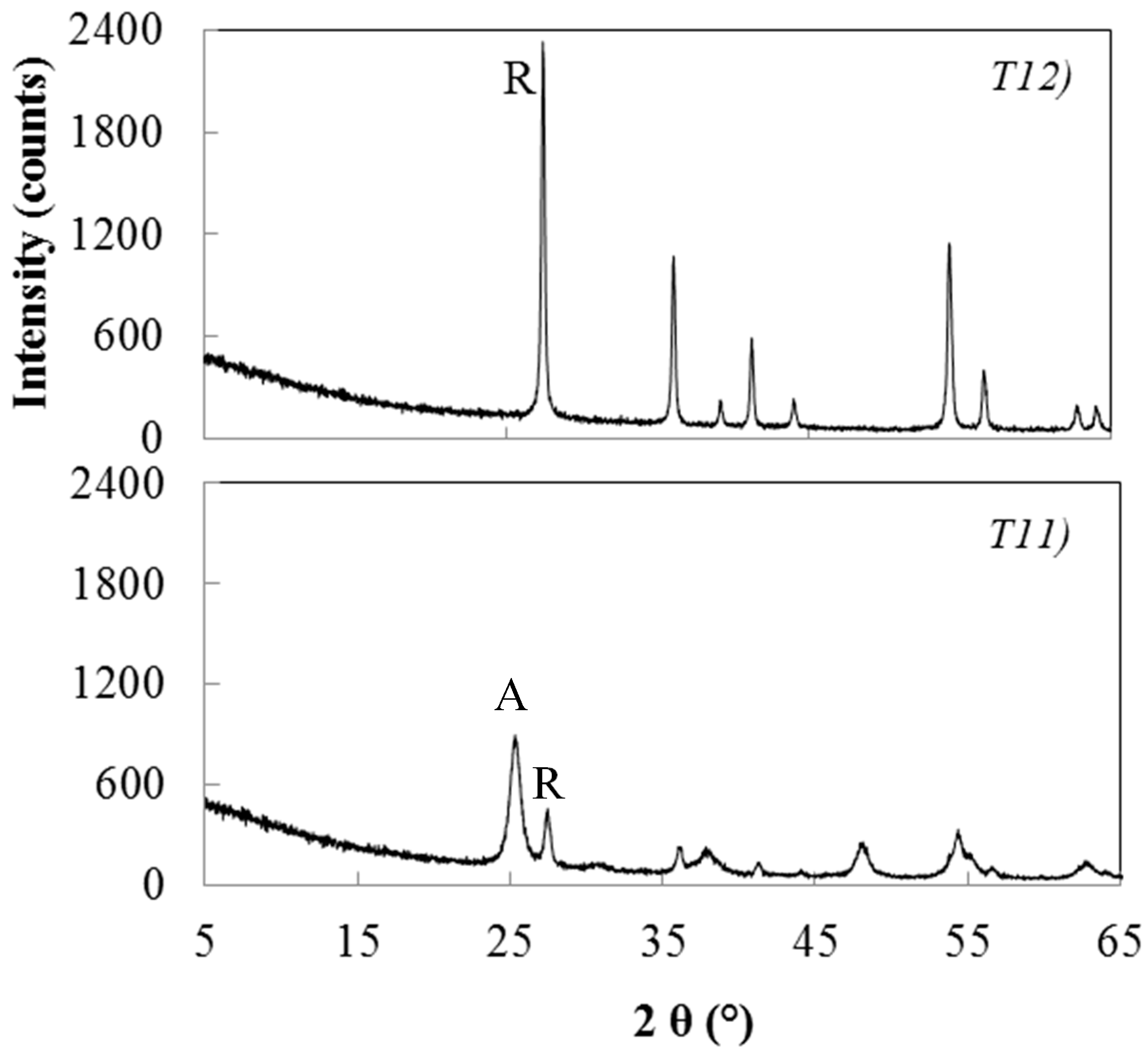
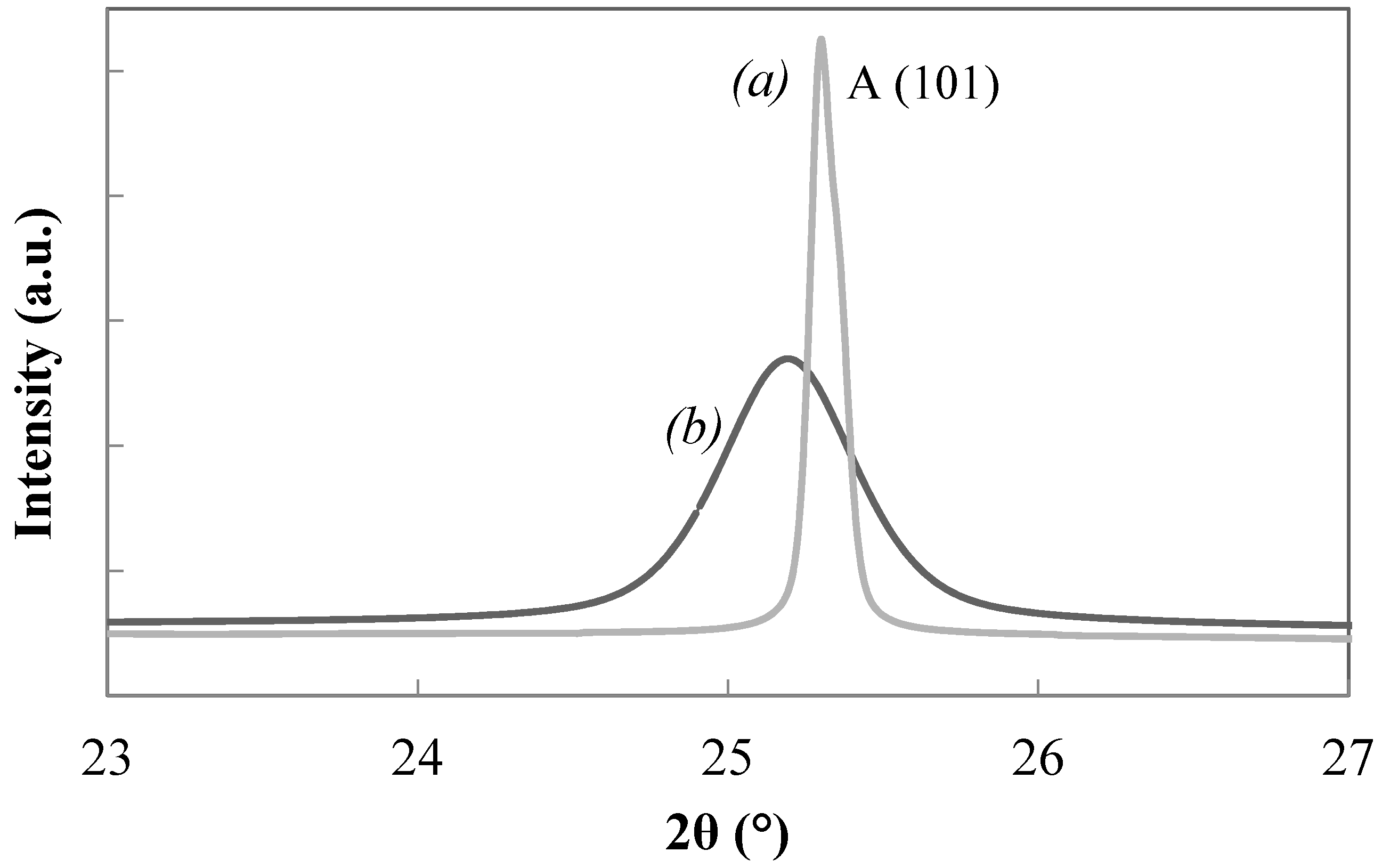
| T11 | 1.0/1 | 0.1/1 | 450/2 | 46.8 | 3.3 | 12.2 24.1 | 64.9 (A) 35.1 (R) |
| T12 | 1.0/1 | 0.1/1 | 600/2 | 10.5 | 5.2 | 48.1 | 100 (R) |
| T13 | 1.0/1 | 0.1/1 | 450/30 | 70.4 | 5.1 | 20.4 37.2 | 22.8 (A) 77.2 (R) |
| T14 | 1.0/1 | 0.1/3 | 450/2 | 30.3 | 3.3 | 17.0 43.1 | 73.3 (A) 26.7 (R) |
| T15 | 1.0/1 | 0.1/3 | 450/30 | - | - | 25.5 45.5 | 43.7 (A) 56.3 (R) |
| Sample | NH4OH Concentration (M)/Treatment Time (h) | HNO3 Concentration (M)/Treatment Time (h) | Calcination Temperature (°C)/Heating Rate (°C min−1) | Crystal Size by XRD (nm) | Polymorphic Phase A (Anatase) |
|---|---|---|---|---|---|
| E | 1.0/1 | 0/0 | 450/2 | 15.7 | A |
| F | 1.0/1 | 0/0 | 600/2 | 18.4 | A |
| G | 1.0/1 | 0.02/1 | 450/2 | 11.5 | A |
| H | 1.0/1 | 0.02/3 | 450/2 | 7.5 | A |
| I | 1.0/1 | 0.05/1 | 450/2 | 7.3 | A |
| J | 1.0/1 | 0.05/3 | 450/2 | 6.0 | A |
| L | 1.0/1 | 0.1/1 | 450/2 | 6.3 | A |
| M | 1.0/1 | 0.1/1 | 600/2 | 8.5 | A |
| N | 1.0/1 | 0.1/1 | 450/30 | 16.0 | A |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mascolo, M.C.; Ring, T.A. Recyclable Aggregates of Mesoporous Titania Synthesized by Thermal Treatment of Amorphous or Peptized Precursors. Materials 2018, 11, 381. https://doi.org/10.3390/ma11030381
Mascolo MC, Ring TA. Recyclable Aggregates of Mesoporous Titania Synthesized by Thermal Treatment of Amorphous or Peptized Precursors. Materials. 2018; 11(3):381. https://doi.org/10.3390/ma11030381
Chicago/Turabian StyleMascolo, Maria Cristina, and Terry Arthur Ring. 2018. "Recyclable Aggregates of Mesoporous Titania Synthesized by Thermal Treatment of Amorphous or Peptized Precursors" Materials 11, no. 3: 381. https://doi.org/10.3390/ma11030381