Fe3O4 Nanoparticles in Targeted Drug/Gene Delivery Systems
Abstract
:1. Introduction
2. Synthesis of Fe3O4 NPs
2.1. Co-Precipitation
2.2. Thermal Decomposition
2.3. Solvothermal Synthesis
3. Functionalization of Fe3O4 NPs
3.1. Polymer Coating
3.2. Silica/Mesoporous Silica (SiO2/mSiO2) Coating
3.3. Graphene Oxide Coating
3.4. Carbon Coating
3.5. Other Coating Materials
4. Fe3O4 NPs for Targeted Drug/Gene Delivery Systems
4.1. Fe3O4 NP Drug Delivery of Chemotherapeutic Agents
4.1.1. Doxorubicin
4.1.2. 5-Fluorouracil
4.1.3. Ciprofloxacin
4.1.4. Gemcitabine
4.1.5. Dihydroartemisinin
4.2. Fe3O4 NP Gene Delivery for Gene Therapy
4.3. Fe3O4 NP Drug/Gene Co-Delivery for Chemo–Gene Combined Therapy
5. Conclusions and Future Prospects
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Chen, M.; Zhuo, Y.; Chai, Y.; Xu, W.; Yuan, R. In situ electrodeposited synthesis of electrochemiluminescent Ag nanoclusters as signal probe for ultrasensitive detection of Cyclin-D1 from cancer cells. Anal. Chem. 2017, 89, 6787–6793. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, Y.; Lu, X.; Wang, K.; Wang, Z.; Zhang, H. Polydopamine nanoparticles modulating stimuli-responsive PNIPAM hydrogels with cell/tissue adhesiveness. ACS Appl. Mater. Interfaces 2016, 8, 29088–29100. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zhang, K.; Cao, Y.; Chen, X.; Wang, K.; Liu, M.; Pei, R. Hydrophobic IR-780 dye encapsulated in cRGD-conjugated solid lipid nanoparticles for NIR imaging-guided photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 12217–12226. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, B.; Wang, Q.; Dong, Z.; Li, H.; Jin, Q.; Hong, H.; Zhang, J.; Wang, Y. An integrative folate-based metal complex nanotube as a potent antitumor nanomedicine as well as an efficient tumor-targeted drug carrier. Bioconjugate Chem. 2016, 27, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, X.; Wang, S.; Qian, J.; He, S. Biologically inspired polydopamine capped gold nanorods for drug delivery and light-mediated cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 24368–24384. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, D.; Dougherty, C.A.; Lu, W.; Wu, H.; He, X.; Cai, T.; Van Dort, M.E.; Ross, B.D.; Hong, H. In vivo targeting and positron emission tomography imaging of tumor with intrinsically radioactive metal-organic frameworks nanomaterials. ACS Nano 2017, 11, 4315–4327. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Mohanta, S.C.; Deka, K.; Deb, P.; Devi, P.S. Surface-engineered multifunctional Eu:Gd2O3 nanoplates for targeted and pH-responsive drug delivery and imaging applications. ACS Appl. Mater. Interfaces 2017, 9, 4126–4141. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.A.; Shim, M.S.; Heo, C.Y.; Kwon, Y.J. “Combo” nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Miao, Y.; Yu, B.; Ma, P.; Li, L.; Fan, H. Large-scale, facile transfer of oleic acid-stabilized iron oxide nanoparticles to the aqueous phase for biological applications. Langmuir 2017, 33, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Schrittwieser, S.; Pelaz, B.; Parak, W.J.; Lentijo-Mozo, S.; Soulantica, K.; Dieckhoff, J.; Ludwing, F.; Guenther, A.; Tschöpe, A.; Schotter, J. Homogeneous biosensing based on magnetic particle lables. Sensors 2016, 16, 828. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Doong, R. Catalytic nanoreactors of Au@Fe3O4 yolk-shell nanostructures with various Au sizes for efficient nitroarene reduction. J. Phys. Chem. C 2017, 121, 7844–7853. [Google Scholar] [CrossRef]
- Han, X.; Lee, H.K.; Lim, W.C.; Lee, Y.H.; Phan-Quang, G.C.; Phang, I.Y.; Ling, X.Y. Spinning liquid marble and its dual applications as microcentrifuge and miniature localized viscometer. ACS Appl. Mater. Interfaces 2016, 8, 23941–23946. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Filpponen, I.; Johansson, L.S.; Mohammadi, P.; Latikka, M.; Linder, M.B.; Ras, R.H.A.; Rojas, O.J. Complexes of magnetic nanoparticles with cellulose nanocrystals as regenerable, highly efficient, and selective platform for protein separation. Biomacromolecules 2017, 18, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Y.; Gao, S.; Jia, D.; Ma, J.; Liu, L. Sandwich-like CNT@Fe3O4@C coaxial nanocables with enhanced lithium-storage capability. ACS Appl. Mater. Interfaces 2017, 9, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zeng, T.; Wang, S.; Niu, H.; Cai, Y. Facile synthesis of magnetic covalent organic framework with three-dimensional bouquet-like structure for enhanced extraction of organic targets. ACS Appl. Mater. Interfaces 2017, 9, 2959–2965. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Ma, Y.; Yang, B.; Liang, C.; Chen, X.; Cai, C. The efficacy assessments of alkylating drugs induced by nano-Fe3O4/CA for curing breast and hepatic cancer. Spectrochim. Acta A 2017, 173, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Akbari, A.; Jamshidbeigi, M.; Falsafi, M. Functionalization of Fe3O4@SiO2 magnetic nanoparticles with nicotinamide and in vitro DNA interaction. J. Mol. Liq. 2016, 224, 227–233. [Google Scholar] [CrossRef]
- Xing, R.; Liu, G.; Zhu, J.; Hou, Y.; Chen, X. Functional magnetic nanoparticles for non-viral gene delivery and MR imaging. Pharm. Res. 2014, 31, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Saei, A.A.; Barzegari, A.; Majd, M.H.; Asgari, D.; Omidi, Y. Fe3O4 nanoparticles engineered for plasmid DNA delivery to Escherichia coli. J. Nanopart. Res. 2014, 16, 1–11. [Google Scholar] [CrossRef]
- Ma, M.; Yan, F.; Yao, M.; Wei, Z.; Zhou, D.; Yao, H.; Zheng, H.; Chen, H.; Shi, J. Template-free synthesis of hollow/porous organosilica-Fe3O4 hybrid nanocapsules toward magnetic resonance imaging-guided high-intensity focused ultrasound therapy. ACS Appl. Mater. Interfaces 2016, 8, 29986–29996. [Google Scholar] [CrossRef] [PubMed]
- Monaco, I.; Arena, F.; Biffi, S.; Locatelli, E.; Bortot, B.; La Cava, F.; Marini, G.M.; Severini, G.M.; Terreno, E.; Comes Franchini, M. Synthesis of lipophilic core-shell Fe3O4@SiO2@Au nanoparticles and polymeric entrapment into nanomicelles: A novel nanosystem for in vivo active targeting and magnetic resonance-photoacoustic dual imaging. Bioconjugate Chem. 2017, 28, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Seeni, R.Z.; Yu, X.; Chang, H.; Chen, P.; Liu, L.; Xu, C. Iron oxide nanoparticle-powered micro-optical coherence tomography for in situ imaging the penetration and swelling of polymeric microneedles in the skin. ACS Appl. Mater. Interfaces 2017, 9, 20340–20347. [Google Scholar] [CrossRef] [PubMed]
- Voronin, D.V.; Sindeeva, O.A.; Kurochkin, M.A.; Mayorova, O.; Fedosov, I.V.; Semyachkina-Glushkovskaya, O.; Gorin, D.A.; Tuchin, V.V.; Sukhorukov, G.B. In vitro and in vivo visualization and trapping of fluorescent magnetic microcapsules in a bloodstream. ACS Appl. Mater. Interfaces 2017, 9, 6885–6893. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Wang, Y.T.; Tseng, W.L. Amplified peroxidase-like activity in iron oxide nanoparticles using adenosine monophosphate: Application to urinary protein sensing. ACS Appl. Mater. Interfaces 2017, 9, 10069–10077. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Sau, S.; Kesharwani, P.; Iyer, A.K. Polyvalent folate-dendrimer-coated iron oxide theranostic nanoparticles for simultaneous magnetic resonance imaging and precise cancer cell targeting. Biomacromolecules 2017, 18, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Zhang, X.; Liu, Y.; Zhou, M.; Chen, X.; Zhang, X. Dual-targeted multifunctional nanoparticles for magnetic resonance imaging guided cancer diagnosis and therapy. ACS Appl. Mater. Interfaces 2017, 9, 9986–9995. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sahoo, S.K. Magnetic nanopartilces: A novel platform for cancer theranostics. Drug Discov. Today 2014, 19, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, W.; Luo, L.; Wang, Z.; Li, Q.; Kong, F.; Zhang, H.; Yang, J.; Zhu, C.; Du, Y.; et al. External magnetic field-enhanced chemo-photothermal combination tumor therapy via iron oxide nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 16581–16593. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ma, L.; Liu, L.; Wang, L.; Liu, Y.; Jia, Q.; Guo, Q.; Zhang, G.; Zhou, J. Polydopamine-encapsulated Fe3O4 with an adsorbed HSP70 inhibitor for improved photothermal inactivation of bacteria. ACS Appl. Mater. Interfaces 2016, 8, 24455–24462. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Ma, Y.; Yu, S.; Ji, C. Smart multifunctional magnetic nanoparticle-based drug delivery system for cancer thermo-chemotherapy and intracellular imaging. ACS Appl. Mater. Interfaces 2016, 8, 24502–24508. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhou, H.; Liu, J.; Liu, J.; Li, W.; Wang, Y.; Hu, F.; Huo, Q.; Li, J.; Liu, Y.; et al. Dual-mode imaging-guided synergistic chemo- and magnetohyperthermia therapy in a versatile nanoplatform to eliminate cancer stem cells. ACS Appl. Mater. Interfaces 2017, 9, 23497–23507. [Google Scholar] [CrossRef] [PubMed]
- Stephen, Z.R.; Dayringer, C.J.; Lim, J.J.; Revia, R.A.; Halbert, M.V.; Jeon, M.; Bakthavatsalam, A.; Ellenbogen, R.G.; Zhang, M. An approach to rapid synthesis and functionalization of iron oxide nanoparticles for high gene transfection. ACS Appl. Mater. Interfaces 2016, 8, 6320–6328. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, Q.; Al-Rehili, S.; Omar, H.; Almalik, A.; Alshamsan, A.; Zhang, J.; Khashab, N.M. Hybrid iron oxide-graphene oxide-polysaccharides microcapsule: A micro-matryoshka for on-demand drug release and antitumor therapy in vivo. ACS Appl. Mater. Interfaces 2016, 8, 6859–6868. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Dong, L.; Zhong, S.; Shi, C.; Sun, Y.; Chen, P. Sonochemical fabrication of folic acid functionalized multistimuli-responsive magnetic graphene oxide-based nanocapsules for targeted drug delivery. Chem. Eng. J. 2017, 326, 839–848. [Google Scholar] [CrossRef]
- Song, M.M.; Xu, H.L.; Liang, J.X.; Xiang, H.H.; Liu, R.; Shen, Y.X. Lactoferrin modified graphene oxide iron oxide nanocomposite for glioma-targeted drug delivery. Mater. Sci. Eng. C 2017, 77, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, Y.; Zhao, N.; Xu, F.J. Well-defined peapod-like magnetic nanoparticles and their controlled modification for effective imaging guided gene therapy. ACS Appl. Mater. Interfaces 2016, 8, 11298–11308. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Fan, Y.; Huang, Y. Facile low temperature hydrothermal synthesis of magnetic mesoporous carbon nanocomposite for adsorption removal of ciprofloxacin antibiotics. Ind. Eng. Chem. Res. 2013, 52, 2604–2612. [Google Scholar] [CrossRef]
- Pramanik, A.; Jones, S.; Pedraza, F.; Vangara, A.; Sweet, C.; Williams, M.S.; Ruppa-Kasani, V.; Risher, S.E.; Sardar, D.; Ray, P.C. Fluorescent, magnetic multifunctional carbon dots for selective separation, identification, and eradication of drug-resistant superbugs. ACS Omega 2017, 2, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Cui, B.; Li, G.; Yang, J.; Peng, H.; Wang, Y.; Li, N.; Gao, R.; Chang, Z.; Wang, Y. Novel Fe3O4@ZnO@mSiO2 nanocarrier for targeted drug delivery and controllable release with microwave irradiation. J. Phys. Chem. C 2014, 11, 14929–14937. [Google Scholar] [CrossRef]
- Li, X.; Chen, L. Fluorescence probe based on an amino-functionalized fluorescent magnetic nanocomposite for detection of folic acid in serum. ACS Appl. Mater. Interfaces 2016, 8, 31832–31840. [Google Scholar] [CrossRef] [PubMed]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, K.; Zhang, R.; Yin, H.; Zhou, Y.; Ai, S. A colorimetric assay of DNA methyltransferase activity based on the keypad lock of duplex DNA modified meso-SiO2@Fe3O4. Anal. Chim. Acta 2016, 920, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Rezayan, A.H.; Mousavi, M.; Kheirjou, S.; Amoabediny, G.; Ardestani, M.S.; Mohammadnejad, J. Monodisperse magnetite (Fe3O4) nanoparticles modified with water soluble polymers for the diagnosis of breast cancer by MRI method. J. Magn. Magn. Mater. 2016, 420, 210–217. [Google Scholar] [CrossRef]
- Qiao, Y.; Shen, L.; Li, X.; Jin, C.; Sun, Z. Preparation of Fe3O4/SiO2 composite particles for cadmium (II) adsorption from aqueous solution. J. North Uni. China (Nat. Sci. Ed.) 2015, 36, 343–347, 353. [Google Scholar] [CrossRef]
- Ge, R.; Li, X.; Lin, M.; Wang, D.; Li, S.; Liu, S.; Tang, Q.; Liu, Y.; Jiang, J.; Liu, L.; et al. Fe3O4@polydopamine composite theranostic superparticles employing preassembled Fe3O4 nanoparticles as the core. ACS Appl. Mater. Interfaces 2016, 8, 22942–22952. [Google Scholar] [CrossRef] [PubMed]
- Pan, U.N.; Khandelia, R.; Sanpui, P.; Das, S.; Paul, A.; Chattopadhyay, A. Protein-based multifunctional nanocarriers for imaging, photothermal therapy, and anticancer drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 19495–19501. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Chiang, P.H.; Hsiao, W.C.; Chuang, C.C.; Chang, C.W. Redox-sensitive polymer/SPIO nanocomplexes for efficient magnetofection and MR imaging of human cancer cells. Langmuir 2015, 31, 6523–6531. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Lee, E.; Kim, J.; Seo, Y.; Lee, K.H.; Hong, J.W.; Gilad, A.A.; Park, H.; Choi, J. Effective delivery of immunosuppressive drug molecules by silica coated iron oxide nanoparticles. Colloid. Surface. B 2016, 142, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mao, K.; Zhang, B.; Zhao, Y. Superparamagnetic iron oxide nanoparticles conjugated with folic acid for dual target-specific drug delivery and MRI in cancer theranostics. Mater. Sci. Eng. C 2017, 70, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Troyer, L.D.; Lee, S.S.; Wu, J.; Kim, C.; Lafferty, B.J.; Catalano, J.G.; Fortner, J.D. Engineering nanoscale iron oxides for uranyl sorption and separation: Optimization of particle core size and bilayer surface coatings. ACS Appl. Mater. Interfaces 2017, 9, 13163–13172. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tian, H.; He, J. Adsorptive performance and catalytic activity of superparamagnetic Fe3O4@nSiO2@mSiO2 core-shell microspheres towards DDT. J. Colloid Interface Sci. 2014, 419, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Xin, T.; Ma, M.; Zhang, H.; Gu, J.; Wang, S.; Liu, M.; Zhang, Q. A facile approach for the synthesis of magnetic separable Fe3O4@TiO2, core-shell nanocomposites as highly recyclable photocatalysts. Appl. Surf. Sci. 2014, 288, 51–59. [Google Scholar] [CrossRef]
- Zhao, H.; Cui, H.J.; Fu, M.L. A general and facile method for improving carbon coat on magnetic nanoparticles with a thickness control. J. Colloid Interface Sci. 2016, 461, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. 2005, 117, 2842–2845. [Google Scholar] [CrossRef]
- Li, B.; Fan, H.; Zhao, Q.; Wang, C. Synthesis, characterization and cytotoxicity of novel multifunctional Fe3O4@SiO2@GdVO4:Dy3+ core-shell nanocomposite as a drug carrier. Materials 2016, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Hao, Y.; Zhang, L.; Cui, X.; Liu, D.; Zhang, M.; Tang, Y.; Zheng, Y. A facile method for protein imprinting on directly carboxyl-functionalized magnetic nanoparticles using non-covalent template immobilization strategy. Chem. Eng. J. 2016, 284, 139–148. [Google Scholar] [CrossRef]
- Landarani-Isfahani, A.; Moghadam, M.; Mohammadi, S.; Royvaran, M.; Moshtael-Arani, N.; Rezaei, S.; Tangestaninejad, S.; Mirkhani, V.; Mohammadpoor-Baltork, I. Elegant pH-responsive nanovehicle for drug delivery based on triazine dendrimer modified magnetic nanoparticles. Langmuir 2017, 33, 8503–8515. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Rinaldi-Montes, N.; Alonso, J.; Amghouz, Z.; Garaio, E.; García, J.A.; Gorria, P.; Blanco, J.A.; Phan, M.H.; Srikanth, H. Boosted hyperthermia therapy by combined AC magnetic and photothermal exposures in Ag/Fe3O4 nanoflowers. ACS Appl. Mater. Interfaces 2016, 8, 25162–25169. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dong, B.; Xu, H.; Xu, S.; Zhang, X.; Lin, Y.; Xu, L.; Bai, X.; Zhang, S.; Song, H. Amphiphilic silane modified multifunctional nanoparticles for magnetically targeted photodynamic therapy. ACS Appl. Mater. Interfaces 2017, 9, 11451–11460. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, A.; Montis, C.; Berti, D.; Baglioni, P. Multifunctional magnetoliposomes for sequential controlled release. ACS Nano 2016, 10, 7749–7760. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Muthana, M.; Mukherjee, J.; Falconer, R.J.; Biggs, C.A.; Zhao, X. Magnetic-silk core-shell nanoparticles as potential carriers for targeted delivery of curcumin into human breast cancer cells. ACS Biomater. Sci. Eng. 2017, 3, 1027–1038. [Google Scholar] [CrossRef]
- Kaamyabi, S.; Habibi, D.; Amini, M.M. Preparation and characterization of the pH and thermosensitive magnetic molecular imprinted nanoparticle polymer for the cancer drug delivery. Bioorg. Med. Chem. Lett. 2016, 26, 2349–2354. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Lu, Y.J.; Chen, J.P. Magnetic graphene oxide as a carrier for targeted delivery of chemotherapy drugs in cancer therapy. J. Magn. Magn. Mater. 2017, 427, 34–40. [Google Scholar] [CrossRef]
- Yu, S.; Gao, X.; Baigude, H.; Hai, X.; Zhang, R.; Gao, X.; Shen, B.; Li, Z.; Tan, Z.; Su, H. Inorganic nanovehicle for potential targeted drug delivery to tumor cells, tumor optical imaging. ACS Appl. Mater. Interfaces 2015, 7, 5089–5096. [Google Scholar] [CrossRef] [PubMed]
- Prabha, G.; Raj, V. Formation and characterization of β-cyclodextrin (β-Cd)-polyethyleneglycol (PEG)-polyethyleneimine (PEI) coated Fe3O4 nanoparticles for loading and releasing 5-Fluorouracil drug. Biomed. Pharmacother. 2016, 80, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, M.; Shagholani, H.; Yunessnia lehi, A. Synthesis of a novel biocompatible nanocomposite of graphene oxide and magnetic nanoparticles for drug delivery. Int. J. Biol. Macromol. 2017, 98, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Prabha, G.; Raj, V. Preparation and characterization of polymer nanocomposites coated magnetic nanoparticles for drug delivery applications. J. Magn. Magn. Mater. 2016, 408, 26–34. [Google Scholar] [CrossRef]
- Shahabadi, N.; Falsafi, M.; Mansouri, K. Improving antiproliferative effect of the anticancer drug cytarabine on human promyelocytic leukemia cells by coating on Fe3O4@SiO2 nanoparticles. Colloid. Surface. B 2016, 141, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Parsian, M.; Unsoy, G.; Mutlu, P.; Yalcin, S.; Tezcaner, A.; Gunduz, U. Loading of Gemcitabine on chitosan magnetic nanoparticles increases the anti-cancer efficacy of the drug. Eur. J. Pharmacol. 2016, 784, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, H.; Tavakoli, T. Preparation of a new Fe3O4/starch-g-polyester nanocomposite hydrogel and a study on swelling and drug delivery properties. Carbohydr. Polym. 2016, 144, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Kariminia, S.; Shamsipur, A.; Shamsipur, M. Analytical characteristics and application of novel chitosan coated magnetic nanoparticles as an efficient drug delivery system for ciprofloxacin. Enhanced drug release kinetics by low-frequency ultrasounds. J. Pharm. Biomed. 2016, 129, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Du, R.; Zhang, L.; Zhang, G.; Zheng, X.; Qian, J.; Tian, X.; Zhou, J.; He, J.; Wang, Y.; et al. A pH-responsive yolk-like nanoplatform for tumor targeted dual-mode magnetic resonance imaging and chemotherapy. ACS Nano 2017, 11, 7049–7059. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Xiao, H.; Yu, C.; Liu, J.; Cheng, Z.; Song, H.; Zhang, X.; Li, C.; Wang, J.; Gu, Z.; et al. Enhanced cisplatin chemotherapy by iron oxide nanocarrier-mediated generation of highly toxic reactive oxygen species. Nano Lett. 2017, 17, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Cui, B.; Zhao, W.; Chen, P.; Peng, H.; Wang, Y. A novel microwave stimulus remote controlled anticancer drug release system based on Fe3O4@ZnO@mGd2O3:Eu@P(NIPAm-co-MAA) multifunctional nanocarriers. J. Mater. Chem. B 2015, 3, 6919–6927. [Google Scholar] [CrossRef]
- Peng, H.; Hu, C.; Hu, J.; Tian, X.; Wu, T. Fe3O4@mZnO nanoparticles as magnetic and microwave responsive drug carriers. Microporous Mesoporous Mater. 2016, 226, 140–145. [Google Scholar] [CrossRef]
- Zhao, W.; Cui, B.; Qiu, H.; Chen, P.; Wang, Y. Multifunctional Fe3O4@WO3@mSiO2-APTES nanocarrier for targeted drug delivery and controllable release with microwave irradiation triggered by WO3. Mater. Lett. 2016, 169, 185–188. [Google Scholar] [CrossRef]
- Wu, L.; Chen, L.; Liu, F.; Qi, X.; Ge, Y.; Shen, S. Remotely controlled drug release based on iron oxide nanoparticles for specific therapy of cancer. Colloid. Surface. B 2017, 152, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, J.; Chen, R.; Shi, R.; Xia, G.; Zhou, S.; Liu, Z.; Zhang, N.; Wang, H.; Guo, Z.; et al. Magnetically guided delivery of DHA and Fe ions for enhanced cancer therapy based on pH-responsive degradation of DHA-loaded Fe3O4@C@MIL-100(Fe) nanoparticles. Biomaterials 2016, 107, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, Y.Q.; Sun, K.K.; Zhang, R.; Fan, L.; Liang, K.K.; Mao, L.B. Controllable preparation and drug loading properties of core-shell microspheres Fe3O4@MOFs/GO. Mater. Lett. 2016, 162, 207–210. [Google Scholar] [CrossRef]
- Kievit, F.M.; Veiseh, O.; Fang, C.; Bhattarai, N.; Lee, D.; Ellenbogen, R.G.; Zhang, M. Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano 2010, 4, 4587–4594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, L.; Zhang, X.; Liu, H.; Yan, X.; Qiu, J.; Yeung, K.L. Synthesis and characterization of ZIF-8@SiO2@Fe3O4 core@double-shell microspheres with noble metal nanoparticles sandwiched between two shell layers. Mater. Lett. 2015, 148, 17–21. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, Y.; Zou, B.; Yu, Y.; Ruan, W.; Wang, Y. Template-etching route to construct uniform rattle-type Fe3O4@SiO2 hollow microspheres as drug carrier. Mater. Sci. Eng. C 2017, 75, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chatterjee, K. Comprehensive review on the use of graphene-based substrates for regenerative medicine and biomedical devices. ACS Appl. Mater. Interfaces 2016, 8, 26431–26457. [Google Scholar] [CrossRef] [PubMed]
- Dinda, S.; Kakran, M.; Zeng, J.; Sudhaharan, T.; Ahmed, S.; Das, D.; Selvan, S.T. Grafting of ZnS:Mn-doped nanocrystals and an anticancer drug onto graphene oxide for delivery and cell labeling. ChemPlusChem 2016, 81, 100–107. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhang, F.; Song, Y.; Song, S.; Zhang, R.; Hou, W. Synthesis of magnetite-graphene oxide-layered double hydroxide composites and applications for the removal of Pb(II) and 2,4-dichlorophenoxyacetic acid from aqueous solutions. ACS Appl. Mater. Interfaces 2015, 7, 7251–7263. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Deng, W.; Yang, L.; Tan, Y.; Xie, Q.; Yao, S. Copper-based metal-organic framework nanoparticles with peroxidase-like activity for sensitive colorimetric detection of staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 24440–24445. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Yan, B. A postsynthetic modified MOF hybrid as heterogeneous photocatalyst for α-phenethyl alcohol and reusable fluorescence sensor. Inorg. Chem. 2016, 55, 11831–11838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, Q.; Xia, T.; Zhang, J.; Yang, Y.; Cui, Y.; Chen, B.; Qian, G. Turn-on and ratiometric luminescent sensing of hydrogen sulfide based on metal-organic frameworks. ACS Appl. Mater. Interfaces 2016, 8, 32259–32265. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hill, M.R. Low-energy CO2 release from metal-organic frameworks triggered by external stimuli. Acc. Chem. Res. 2017, 50, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Chowdhuri, A.R.; Laha, D.; Chandra, S.; Karmakar, P.; Sahu, S.K. Synthesis of multifunctional upconversion NMOFs for targeted antitumor drug delivery and imaging in triple negative breast cancer cells. Chem. Eng. J. 2017, 319, 200–211. [Google Scholar] [CrossRef]
- Li, W.; Qi, X.; Zhao, C.Y.; Xu, X.F.; Tang, A.N.; Kong, D.M. A rapid and facile detection for specific small-sized amino acids based on target-triggered destruction of metal organic frameworks. ACS Appl. Mater. Interfaces 2017, 9, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Tang, G.; Wang, Z.; Li, Q.; Gao, J.; Wu, S. Magnetic porous carbon nanocomposites derived from metal-organic frameworks as a sensing platform for DNA fluorescent detection. Anal. Chim. Acta 2016, 940, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Huang, G.W.; Li, Y.Q.; Xiao, H.M.; Feng, Q.P.; Hu, N.; Fu, S.Y. Enhanced microwave absorption performance of coated carbon nanotubes by optimizing the Fe3O4 nanocoating structure. ACS Appl. Mater. Interfaces 2017, 9, 2973–2983. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Mei, B.; Wu, H.; Wei, H.; Fang, X.; Xu, Y. Microwave electromagnetic and absorption properties of N-doped ordered mesoporous carbon decorated with ferrite nanoparticles. J. Phys. Chem. C 2017, 121, 3846–3853. [Google Scholar] [CrossRef]
- Long, D.; Liu, T.; Tan, L.; Shi, H.; Liang, P.; Tang, S.; Wu, Q.; Yu, J.; Dou, J.; Meng, X. Multisynergistic platform for tumor therapy by mild microwave irradiation-activated chemotherapy and enhanced ablation. ACS Nano 2016, 10, 9516–9528. [Google Scholar] [CrossRef] [PubMed]
- Leonard, K.C.; Nam, K.M.; Lee, H.C.; Kang, S.H.; Park, H.S.; Bard, A.J. ZnWO4/WO3 composite for improving photoelectrochemical water oxidation. J. Phys. Chem. C 2013, 117, 15901–15910. [Google Scholar] [CrossRef]
- Poß, M.; Tower, R.J.; Napp, J.; Appold, L.C.; Lammers, T.; Alves, F.; Glüer, C.C.; Boretius, S.; Feldmann, C. Multimodal [GdO]+[ICG]− nanoparticles for optical, photoacoustic, and magnetic resonance imaging. Chem. Mater. 2017, 29, 3547–3554. [Google Scholar] [CrossRef]
- Cao, C.; Xue, M.; Zhu, X.; Yang, P.; Feng, W.; Li, F. Energy transfer highway in Nd3+-sensitized nanoparticles for efficient near-infrared bioimaging. ACS Appl. Mater. Interfaces 2017, 9, 18540–18548. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.P.; Jadhav, N.V.; Sharma, S.; Pandey, B.N.; Srivastava, S.K.; Ningthoujam, R.S. Hybrid nanomaterials YVO4:Eu/Fe3O4 for optical imaging and hyperthermia in cancer cells. J. Mater. Chem. C 2015, 3, 1965–1975. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Cheng, G.; Zhang, J.; Hong, G.; Ni, J. Synthesis and characterization of Fe3O4@YPO4:Eu3+ multifunctional microspheres. Mater. Lett. 2015, 152, 224–227. [Google Scholar] [CrossRef]
- Fan, H.; Li, B.; Feng, Y.; Qiu, D.; Song, Y. Multifunctional Fe3O4@SiO2@GdVO4:Eu3+ core-shell nanocompositen for a potential drug carrier. Ceram. Int. 2016, 42, 13326–13330. [Google Scholar] [CrossRef]
- Mujokoro, B.; Adabi, M.; Sadroddiny, E.; Adabi, M.; Khosravani, M. Nano-structures mediated co-delivery of therapeutic agents for glioblastoma treatment: A review. Mater. Sci. Eng. C 2016, 69, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R. Coupling the magnetic and heat dissipative properties of Fe3O4 particles to enable applications in catalysis, drug delivery, tissue destruction and remote biological interfacing. RSC Adv. 2016, 6, 4262–4270. [Google Scholar] [CrossRef]
- Du, B.; Cao, X.; Zhao, F.; Su, X.; Wang, Y.; Yan, X.; Jia, S.; Zhou, J.; Yao, H. Multimodal imaging-guided, dual-targeted photothermal therapy for cancer. J. Mater. Chem. B 2016, 4, 2038–2050. [Google Scholar] [CrossRef]
- Su, X.; Chan, C.; Shi, J.; Tsang, M.K.; Pan, Y.; Cheng, C.; Gerile, O.; Yang, M. A graphene quantum dot@Fe3O4@SiO2 based nanoprobe for drug delivery sensing and dual-modal fluorescence and MRI imaging in cancer cells. Biosens. Bioelectron. 2017, 92, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.J.; Lankelma, J.; Kok, R.M.; Noordhuis, P.; van Groeningen, C.J.; van der Wilt, C.L.; Meyer, S.; Pinedo, H.M. Prolonged retention of high concentration of 5-fluorouracil in human and murine tumors as compared with plasma. Cancer Chemother. Pharm. 1993, 31, 269–276. [Google Scholar] [CrossRef]
- Boncel, S.; Herman, A.P.; Budniok, S.; Jędrysiak, R.G.; Jakóbik-Kolon, A.; Skepper, J.N.; Müller, K.H. In vitro targeting and selective killing of T47D breast cancer cells by purpurin and 5-Fluorouracil anchored to magnetic CNTs: Nitrene-based functionalization versus uptake, cytotoxicity, and intracellular fate. ACS Biomater. Sci. Eng. 2016, 2, 1273–1285. [Google Scholar] [CrossRef]
- Picó, Y.; Andreu, V. Fluoroquinolones in soil-risks and challenges. Anal. Bioanal. Chem. 2007, 387, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, J.; Bomba, H.N.; Zhu, Y.; Gu, Z. Mechanical force-triggered drug delivery. Chem. Rev. 2016, 116, 12536–12563. [Google Scholar] [CrossRef] [PubMed]
- Toschi, L.; Finocchiaro, G.; Bartolini, S.; Gioia, V.; Cappuzzo, F. Role of gemcitabine in cancer therapy. Future Oncol. 2005, 1, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Joubert, F.; Martin, L.; Perrier, S.; Pasparakis, G. Development of a gemcitabine-polymer conjugate with prolonged cytotoxicity against a pancreatic cancer cell line. ACS Macro Lett. 2017, 6, 535–540. [Google Scholar] [CrossRef]
- Thanh Nga, T.T.; Ménage, C.; Bégué, J.P.; Bonnet-Delpon, D.; Gantier, J.C.; Pradines, B.; Doury, J.C.; Thac, T.D. Synthesis and antimalarial activities of fluoroalkyl derivatives of dihydroartemisinin. J. Med. Chem. 1998, 41, 4101–4108. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L.; Chou, H.L.; Liao, Z.X.; Huang, S.J.; Ke, J.H.; Liu, Y.S.; Chiu, C.C.; Wang, L.F. Chondroitin sulfate-polyethylenimine copolymer-coated superparamagnetic iron oxide nanoparticles as an efficient magneto-gene carrier for microRNA-encoding plasmid DNA delivery. Nanoscale 2015, 7, 8554–8565. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Jiang, Q.; He, Y.; Nie, Y.; Yue, D.; Gu, Z. Insight into the efficient transfection activity of a designed low aggregated magnetic polyethyleneimine/DNA complex in serum-containing medium and the application in vivo. Biomater. Sci. 2015, 3, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Pourianazar, N.T.; Gunduz, U. CpG oligodeoxynucleotide-loaded PAMAM dendrimer-coated magnetic nanoparticles promote apoptosis in breast cancer cells. Biomed. Pharmacother. 2016, 78, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.J.; Stutts, L.; Ryu, K.A.; Tom, J.K.; Esser-Kahn, A.P. Directing the immune system with chemical compounds. ACS Chem. Biol. 2014, 9, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhao, J.; Unkeless, J.C.; Feng, Z.H.; Xiong, H. TLR signaling by tumor and immune cells: A double-edged sword. Oncogene 2008, 27, 218–224. [Google Scholar] [CrossRef] [PubMed]
- So, E.Y.; Ouchi, T. The application of Toll like receptors for cancer therapy. Int. J. Biol. Sci. 2010, 6, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Chester, K.A.; Hawkins, R.E. Clinical issues in antibody design. Trends Biotechnol. 1995, 13, 294–300. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, Z.; Sun, L.; Fang, Y.; Xu, X.; Zhou, G. miR-186 regulates chemo-sensitivity to paclitaxel via targeting MAPT in non-small cell lung cancer (NSCLC). Mol. Biosyst. 2016, 12, 3417–3424. [Google Scholar] [CrossRef] [PubMed]
- Azhdarzadeh, M.; Atyabi, F.; Saei, A.A.; Varnamkhasti, B.S.; Omidi, Y.; Fateh, M.; Ghavami, M.; Shanehsazzadeh, S.; Dinarvand, R. Theranostic MUC-1 aptamer targeted gold coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging and photothermal therapy of colon cancer. Colloids Surfaces B 2016, 143, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Yang, C.; Wang, Q.; Zeng, S.; Hu, R.; Lin, G.; Tian, J.; Hu, S.; Lan, R.F.; Yoon, H.S.; et al. A light-driven therapy of pancreatic adenocarcinoma using gold nanorods-based nanocarriers for co-delivery of doxorubicin and siRNA. Theranostics 2015, 5, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yin, Q.; Su, J.; Sun, H.; Meng, Q.; Chen, Y.; Chen, L.; Huang, Y.; Gu, W.; Xu, M.; et al. Inhibition of metastasis and growth of breast cancer by pH-sensitive poly(β-amino ester) nanoparticles co-delivering two siRNA and paclitaxel. Biomaterials 2015, 48, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, J.; Li, B.; Meng, L.; Tian, Z. Recent advances in mechanism-based chemotherapy drug-siRNA pairs in co-delivery systems for cancer: A review. Colloids Surfaces B 2017, 157, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shen, X.; Geng, Y.; Chen, Z.; Li, L.; Li, S.; Yang, H.; Wu, C.; Zeng, H.; Liu, Y. Folate-functionalized magnetic-mesoporous silica nanoparticles for drug/gene codelivery to potentiate the antitumor efficacy. ACS Appl. Mater. Interfaces 2016, 8, 13748–13758. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.Z.; Wang, W.; Zheng, D.W.; Wang, X.; Yu, W.Y.; Li, S.Y.; Zhuo, R.X.; Zhao, Y.F.; Feng, J.; Zhang, X.Z. Multifunctional nanotherapeutics with all-in-one nanoentrapment of drug/gene/inorganic nanoparticle. ACS Appl. Mater. Interfaces 2016, 8, 6784–6789. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhu, J.; Wang, X.; Cheng, H.; Chen, G.; Zhao, Y.; Zeng, X.; Feng, J.; Zhang, X.; Zhuo, R. A boronate-linked linear-hyperbranched polymeric nanovehicle for pH-dependent tumor-targeted drug delivery. Biomaterials 2014, 35, 5240–5249. [Google Scholar] [CrossRef] [PubMed]






| Cell Lines | Loaded Drugs | Coating Materials | Releasing Factors | Refs. | |
|---|---|---|---|---|---|
| Co-precipitation | Doxorubicin (DOX) | Carboxymethyl chitosan (CS) | pH− | MCF-7, S180 (in vivo/in vitro) | [28] |
| DOX | Sodium alyinate (SA), chitosan (CS), graphene oxide (GO), hyaluronic acid (HA) | pH−, near-infrared (NIR) | HeLa (in vivo/in vitro) | [33] | |
| DOX | Lactoferrin, GO | pH− | C6 (in vitro) | [35] | |
| DOX | Poly(N-isopropylacrylamide) (PNIPAAM), 3-(trimethoxysilyl) propyl methacrylate (TMSPMC) | pH−, thermosensitive | – | [62] | |
| DOX, irinotecan | CS, GO, methoxypolyethylene glycol succinimidyl carbonate ester (mPEG-NHS) | pH− | U87 (in vitro) | [63] | |
| 5-Fluorouracil (5-Fu) | Zr(HPO4)2·H2O, folic acid (FA), CS, R6G | pH− | A549, HEK293, HeLa (in vitro) | [64] | |
| 5-Fu | β-cyclodextrin, polyethylenimine (PEI), polyethyline glycol (PEG), | Shell thickness, pH−, temperature | L929, MCF-7 (in vitro) | [65] | |
| 5-Fu | GO, CS, polyvinyl alcohol (PVA) | pH− | – | [66] | |
| Curcumin | FA, polyamidoamine (PAMAM) | FA receptor | SKOV3, HeLa (in vitro) | [25] | |
| Curcumin | Silk fibroin | Silk fibroin concentration, pH− | MDA-MB-231 (in vitro) | [61] | |
| Curcumin | CS, PEG, polyvinylpyrrolidone (PVP) | Shell thickness, pH− | Caco-2, HCT-116 (in vitro) | [67] | |
| C6 | FA, GO, Oleic acid (OA) | Light- and reductive-triggered | HeLa, A549 (in vitro) | [34] | |
| C6, e6 | OA, silane | Light irradiation photodynamic | MCF-7 (in vivo/in vitro) | [59] | |
| Methoterxate (MTX) | Gold layer, Lipoic acid-PEG | NIR | KB, MRC-5, 4T1 (in vivo/in vitro) | [26] | |
| Nimustine, semustine, chlormethine | CA | Interact with DNA or prevent DNA relaxation | MHCC97-H, MCF-7 (in vitro) | [16] | |
| nicotinamide | SiO2 | DNA binding interaction | - | [17] | |
| Cytarabine | SiO2 | DNA binding interaction | HL-60, KG-1, Raji (in vitro) | [68] | |
| N-[N-(3,5-Difluor-ophenacetyl-l-al-anyl)]-S-phenylg-lycinet-butylester (DAPT) | Polypyrrole (PPy), HA | pH− | 4T, MDA-MB-231, MCF-7 (in vitro) | [31] | |
| Gemcitabine | CS | pH− | SKBR, MCF-7 (in vitro) | [69] | |
| Heteropolyacids (HPAs) | Starch-g-poly(EP) hydrogel biopolymer | Hydrolysis of polymer chains | – | [70] | |
| Ciprofloxacin | CS | Low-frequency ultrasound | – | [71] | |
| Thermal Decomposition | DOX | Gold nanorods and nanoclusters, bovine serum albumin (BSA) | NIR, magnetic triggered | HeLa (in vitro) | [46] |
| DOX | Graphene quantum dot, SiO2, FA | pH−, fluorescence resonance energy transfer (FRET) | HeLa (in vitro) | [51] | |
| DOX | PEG, PEI, FA | pH− | MCF-7 (in vivo/in vitro) | [49] | |
| Cisplatin | PEI, Gd2O3, FA, PEG | pH−, reactive oxygen species (ROS)-mediated toxicity | HeLa, NHLF (in vivo/in vitro) | [72] | |
| Cisplatin, DOX, artesunate | PEG, PEI, rhodamine B | pH−, ROS-mediated toxicity | A2780, ACP (in vivo/in vitro) | [73] | |
| Mycophenolic acid (MPA) | SiO2 | Release MPA by hydrolysis in cells | Peripheral blood mononuclear cells (PBMCs) (in vitro) | [48] | |
| VP16 | ZnO, mSiO2 | Microwave-triggered, pH−, temperature | – | [39] | |
| VP16 | ZnO, Gd2O3:Eu, P(NIPAm-co-MAA) | Microwave, pH− | – | [74] | |
| VP16 | mZnO | Microwave | – | [75] | |
| VP16 | WO3, mSiO2, (3-aminopropyl) trimethoxysilane (APTES) | Microwave, pH−, temperature | – | [76] | |
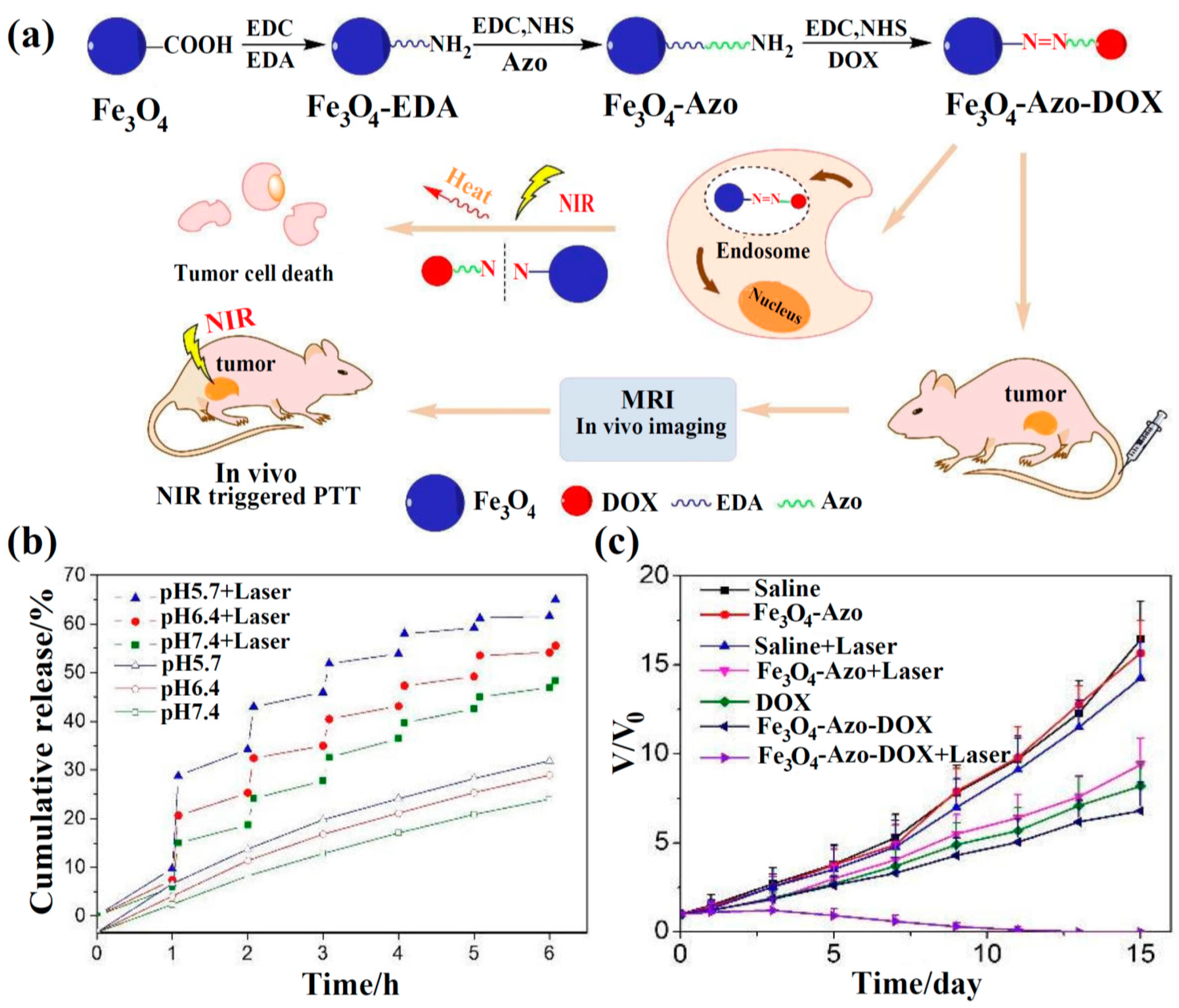
| Solvothermal Synthesis | DOX | Azo | pH−, NIR | MCF-7, S180 (in vivo/in vitro) | [77] |
| 5-Fu | PNIPAAM, mSiO2, CS, R6G | Thermoresponsive drug release | 7901 (in vitro) | [30] | |
| Dihydroartemisinin | C and MIL-100 (Fe) | pH, ROS-mediated cytotoxicity | A549, HeLa (in vivo/in vitro) | [78] | |
| HSP70 | Polydopamine | NIR | HCT116 (in vitro) | [29] | |
| Ibuprofen | Metal-organic frameworks, GO | Drug release controlled by layers | – | [79] |
| Preparation Method | Coating Materials | Loaded Gene | Gene Connection | Cell Lines | Refs. |
|---|---|---|---|---|---|
| Co-precipitation | CS, PEG, catechol, PEI | pRFP DNA | Electrostatic interactions between PEI and plasmid DNA | SF767 human glioblastoma multiforme (GBM) (in vitro) | [32] |
| Chlorotoxin, CS, PEG, PEI | Green fluorescent protein (GFP) encoding DNA | Electrostatic interactions between PEI and DNA | C6 (in vivo) | [80] | |
| CA-silane, PEI | p-encoding green fluorescent protein (pEGFP), pGL3, pCMV-Luc | Electrostatic interactions of PEI with DNA and carboxylic acid | HepG2 (in vitro/in vivo) | [117] | |
| PAMAM dendrimer | CpG oligodeoxynucleotide | Electrostatic interactions between PAMAM and DNA | MDA-MB231, SKBR3 (in vitro) | [118] | |
| Thermal Decomposition | Dopamine, PEG-NH2 | DNA, Pcambia, PGEM-T | Electrostatic interactions between amino groups and plasmid DNA | Escherichia coli cells (in vitro) | [19] |
| PEG, liposomes, gold | Chol-DNA | Au coating provided anchorage points for DNA to be attached | – | [60] | |
| PEI | DNA constructed by pGL3-basic to pcDNA3 vector | Electrostatic interactions between PEI and plasmid DNA | ALTS1C1, PC3, HEK293T (in vitro) | [47] | |
| Solvothermal Synthesis | Ethanolamin-functionalized poly(glycidyl methacrylate), SiO2, APTES | EGFP encoding plasmid DNA | Electrostatic interactions between linked polymer and plasmid DNA | HepG2, C6, HEK293 (in vitro/in vivo) | [36] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Li, B.; Qiao, Y. Fe3O4 Nanoparticles in Targeted Drug/Gene Delivery Systems. Materials 2018, 11, 324. https://doi.org/10.3390/ma11020324
Shen L, Li B, Qiao Y. Fe3O4 Nanoparticles in Targeted Drug/Gene Delivery Systems. Materials. 2018; 11(2):324. https://doi.org/10.3390/ma11020324
Chicago/Turabian StyleShen, Lazhen, Bei Li, and Yongsheng Qiao. 2018. "Fe3O4 Nanoparticles in Targeted Drug/Gene Delivery Systems" Materials 11, no. 2: 324. https://doi.org/10.3390/ma11020324




