Hydroxyapatite Microspheres as an Additive to Enhance Radiopacity, Biocompatibility, and Osteoconductivity of Poly(methyl methacrylate) Bone Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. HA Microspheres Synthesis
2.2. Morphology and Microstructure Characterization
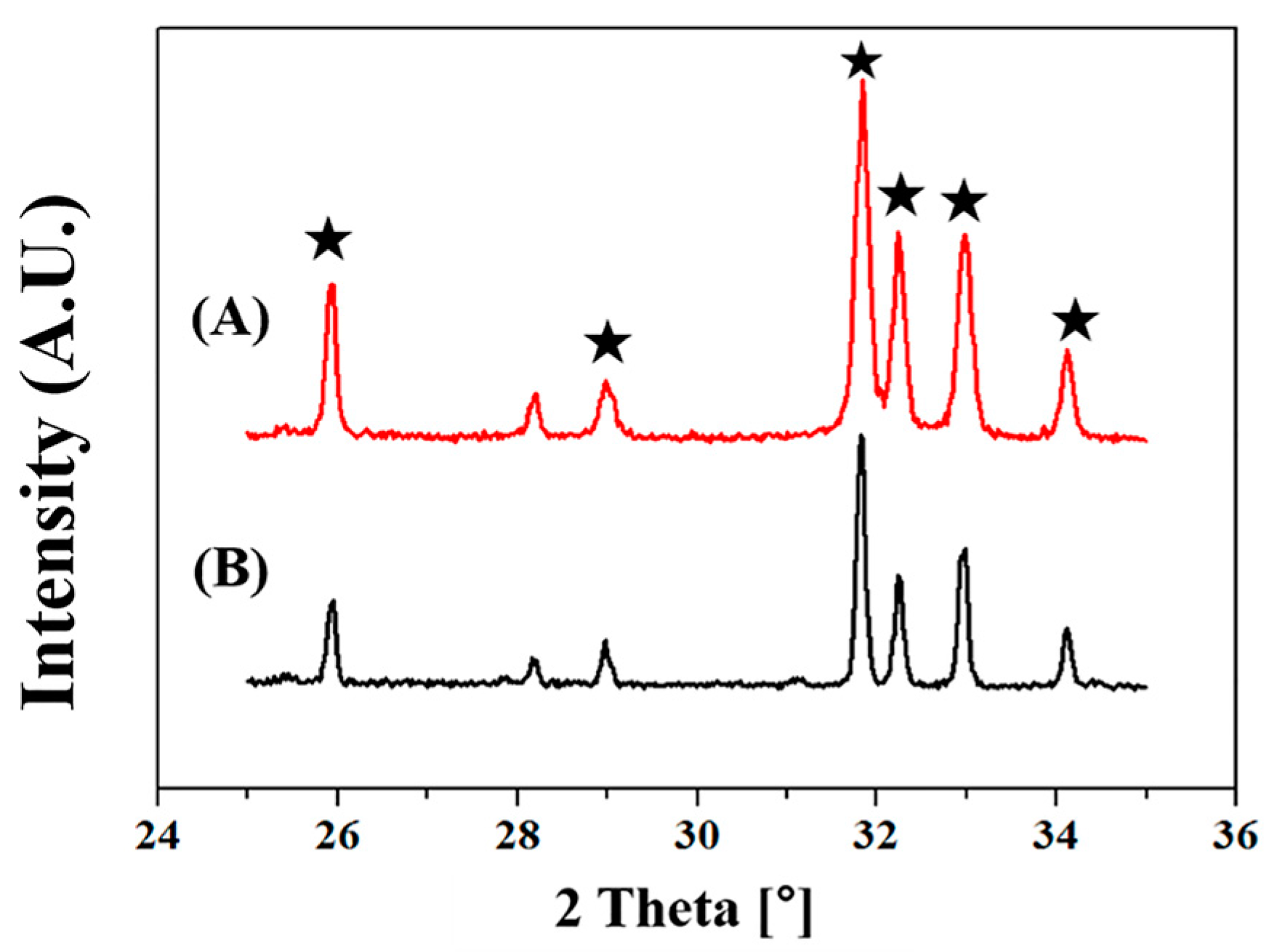
2.3. Crystalline Phases Analysis
2.4. PMMA/HA Microsphere Composites Preparation
2.5. Mechanical Properties Measurement
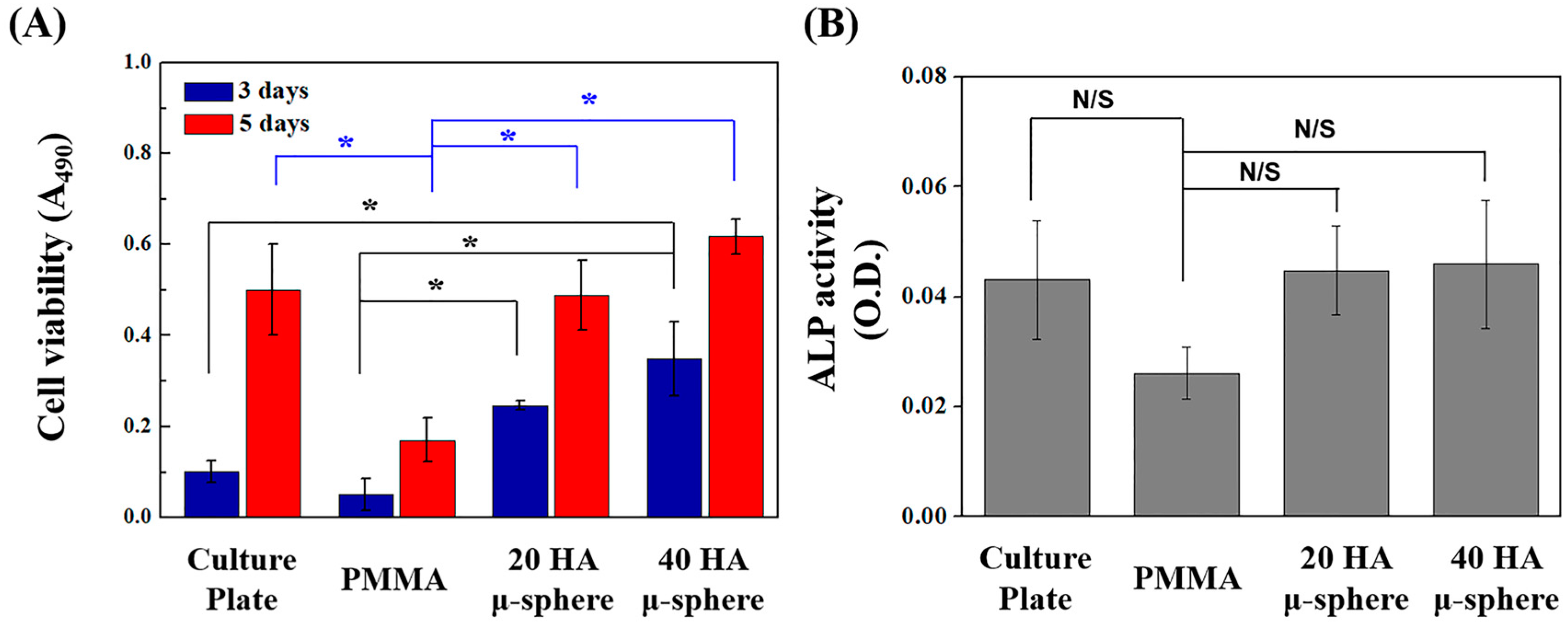
2.6. In Vitro Biocompatibility Evaluation
2.7. In Vitro Radiopaque Properties Evaluation
2.8. In Vivo Animal Tests
2.9. In Vivo Radiopaque Properties Evaluation
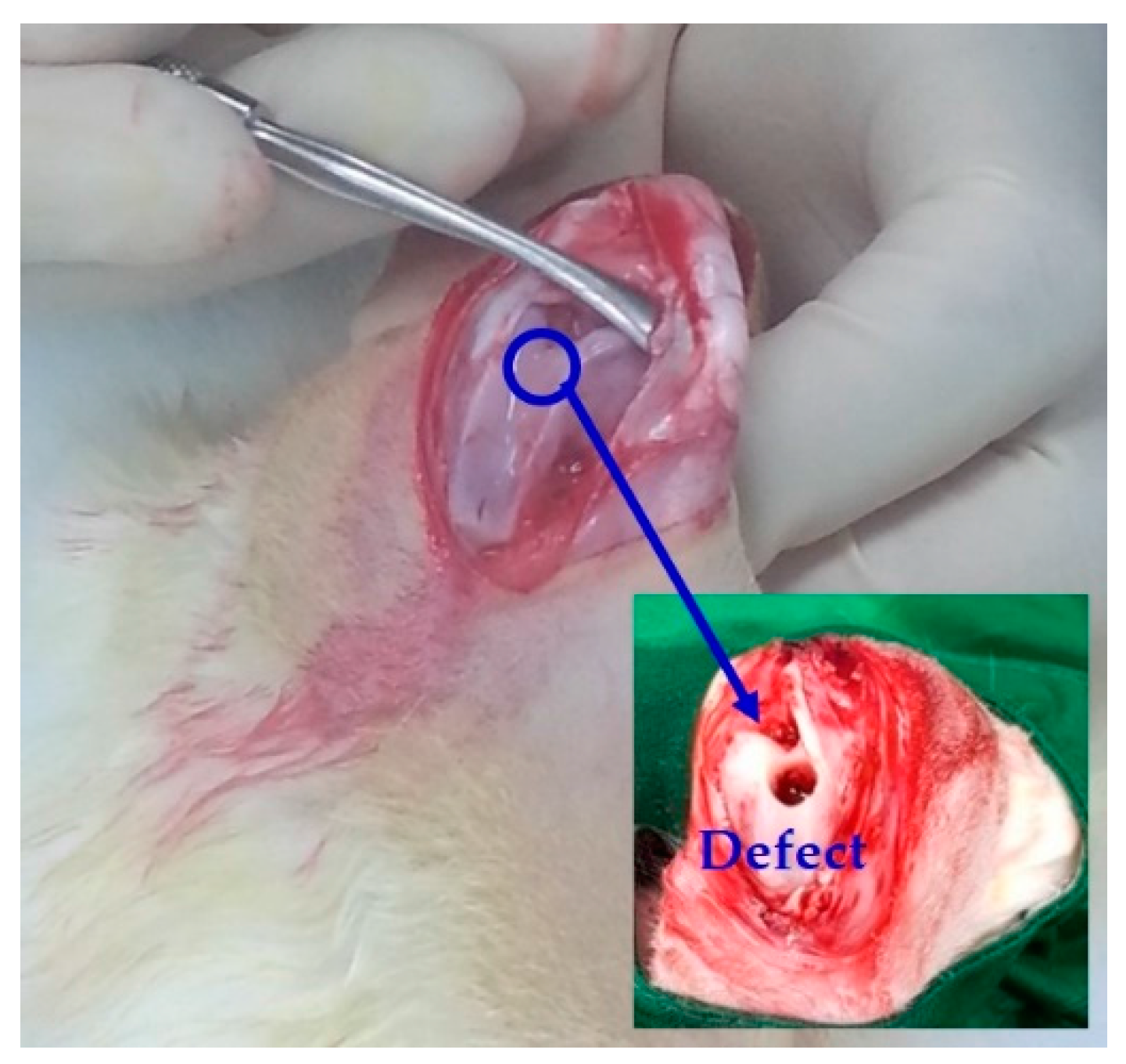
2.10. In Vivo Osteoconductivity Evaluation
2.11. Statistical Analysis
3. Results and Discussion
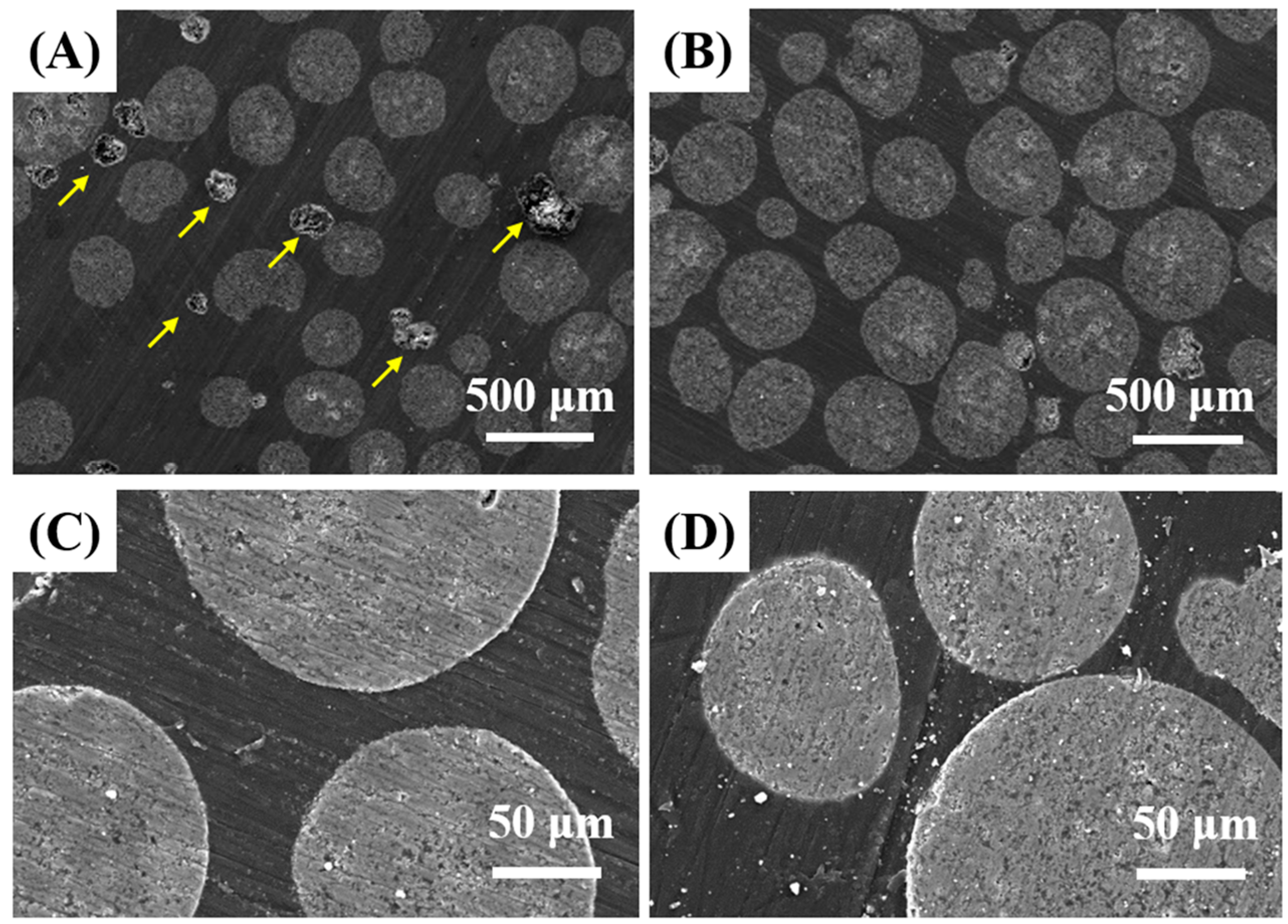
3.1. Morphology and Microstructure of HA Microspheres
3.2. Crystalline Phases of HA Microspheres
3.3. Mixing Behavior of HA Microspheres with PMMA Bone Cements
3.4. Microstructure of PMMA/HA Composites
3.5. Mechanical Properties of PMMA/HA Composites
3.6. In Vitro Biocompatibility of PMMA/HA Composites
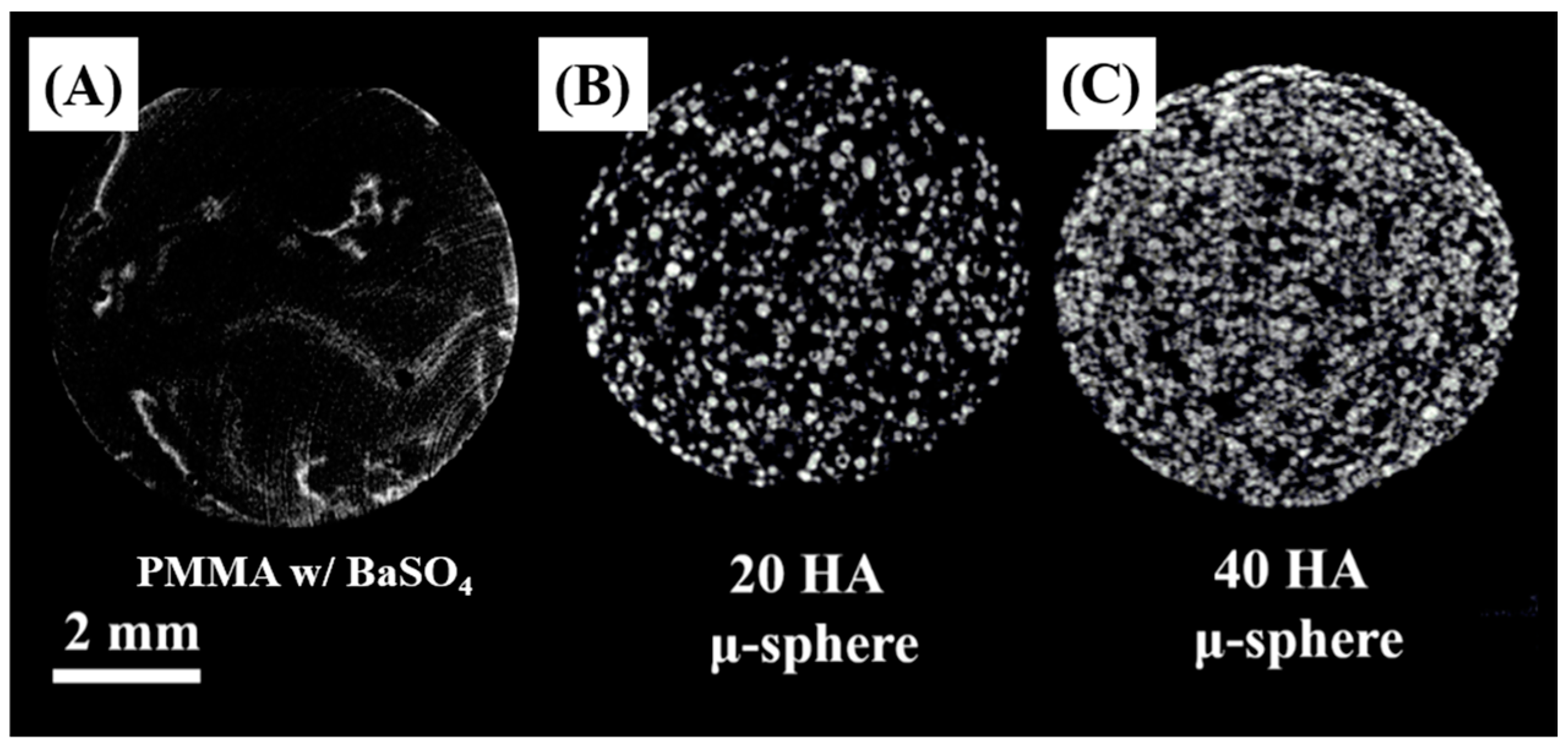
3.7. Radio-Opacifying Properties of PMMA/HA Composites
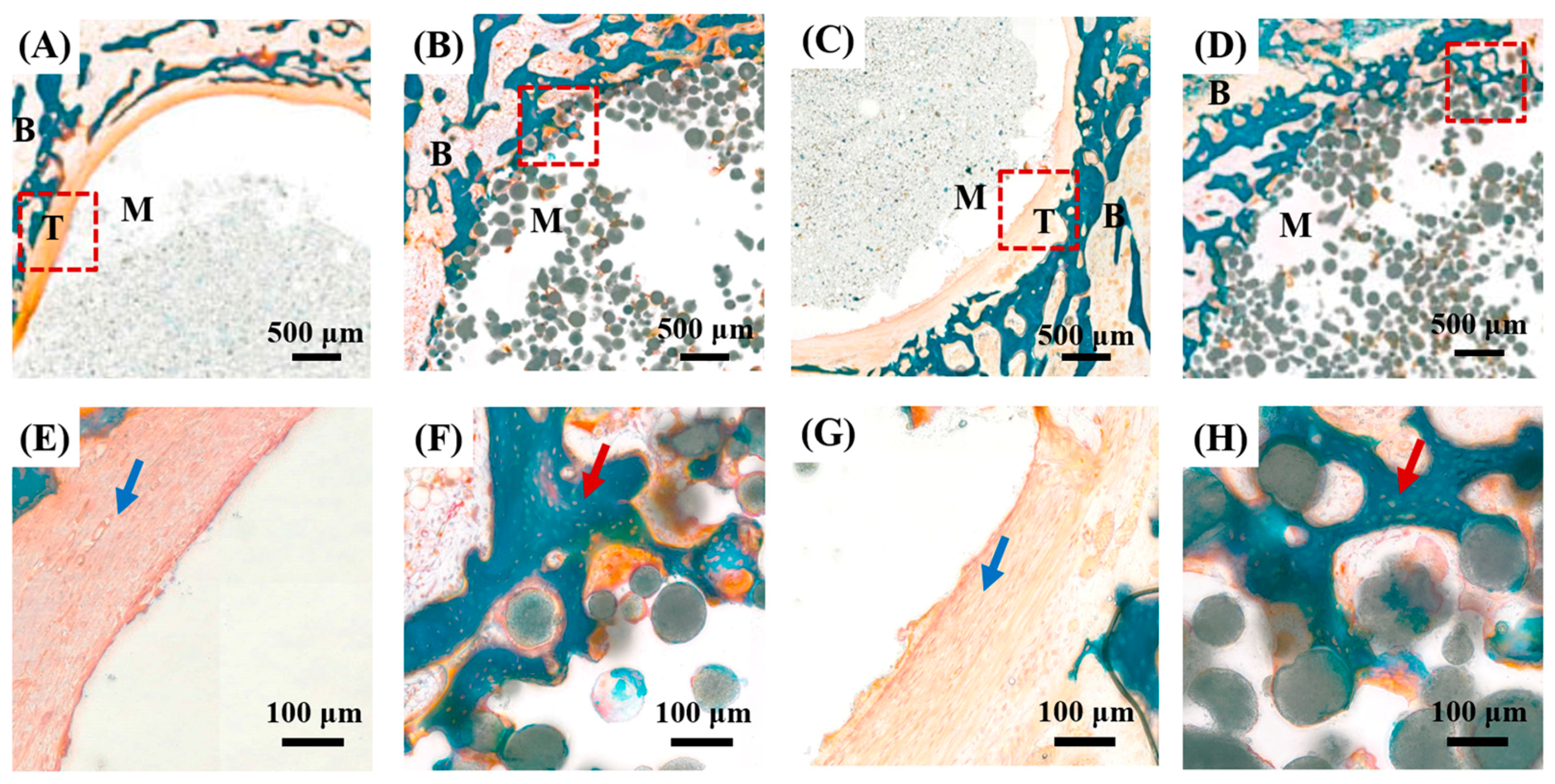
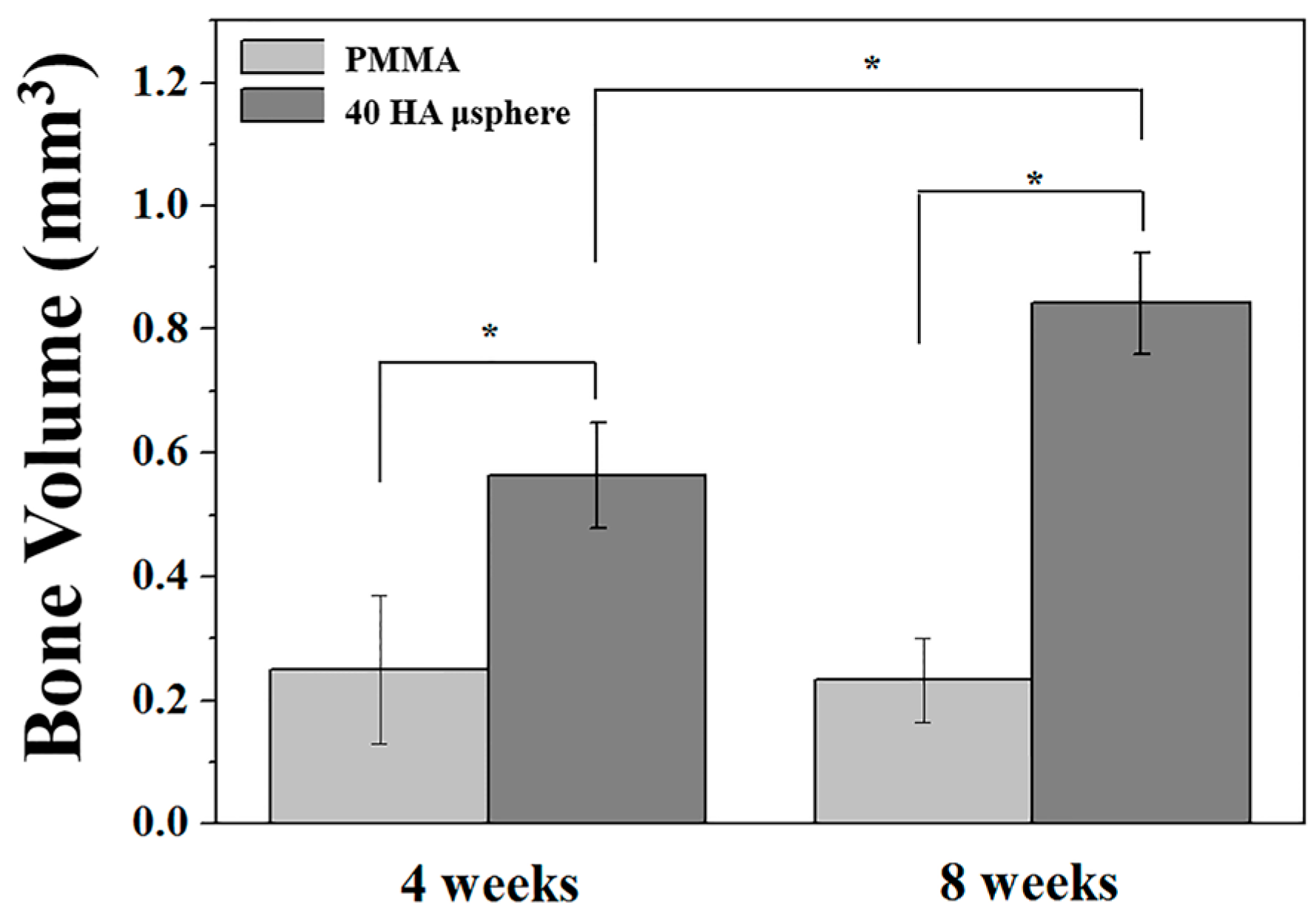
3.8. Osteoconductive Properties of PMMA/HA Composites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jager, M.; Wilke, A. Comprehensive biocompatibility testing of a new PMMA-HA bone cement versus conventional PMMA cement in vitro. J. Biomater. Sci.-Polym. E 2003, 14, 1283–1298. [Google Scholar] [CrossRef]
- Heini, P.F.; Walchli, B.; Berlemann, U. Percutaneous transpedicular vertebroplasty with PMMA: Operative technique and early results—A prospective study for the treatment of osteoporotic compression fractures. Eur. Spine J. 2000, 9, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Winking, M.; Stahl, J.P.; Oertel, M.; Schnettler, R.; Boker, D.K. Treatment of pain from osteoporotic vertebral collapse by percutaneous PMMA vertebroplasty. Acta Neurochir. 2004, 146, 469–476. [Google Scholar] [PubMed]
- Boger, A.; Bohner, M.; Heini, P.; Verrier, S.; Schneider, E. Properties of an injectable low modulus PMMA bone cement for osteoporotic bone. J. Biomed. Mater. Res. B 2008, 86b, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Boger, A.; Bisig, A.; Bohner, M.; Heini, P.; Schneider, E. Variation of the mechanical properties of PMMA to suit osteoporotic cancellous bone. J. Biomater. Sci.-Polym. E 2008, 19, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.E.; Evans, A.J.; Mathis, J.M.; Kallmes, D.F.; Cloft, H.J.; Dion, J.E. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: Technical aspects. Am. J. Neuroradiol. 1997, 18, 1897–1904. [Google Scholar] [PubMed]
- Kim, S.B.; Kim, Y.J.; Yoon, T.R.; Park, S.A.; Cho, I.H.; Kim, E.J.; Kim, I.A.; Shin, J.W. The characteristics of a hydroxyapatite-chitosan-PMMA bone cement. Biomaterials 2004, 25, 5715–5723. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Pal, S. Mechanical-properties of bone-cement—A review. J. Biomed. Mater. Res. 1984, 18, 435–462. [Google Scholar] [CrossRef] [PubMed]
- Molino, L.N.; Topoleski, L.D.T. Effect of baso4 on the fatigue crack propagation rate of PMMA bone cement. J. Biomed. Mater. Res. 1996, 31, 131–137. [Google Scholar] [CrossRef]
- Lopes, P.P.; Garcia, M.P.; Fernandes, M.H.; Fernandes, M.H.V. Acrylic formulations containing bioactive and biodegradable fillers to be used as bone cements: Properties and biocompatibility assessment. Mater. Sci. Eng. C-Mater. 2013, 33, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Sabokbar, A.; Pandey, R.; Quinn, J.M.W.; Athanasou, N.A. Osteoclastic differentiation by mononuclear phagocytes containing biomaterial particles. Arch. Orthop. Trauma Surg. 1998, 117, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Cunin, G.; Boissonnet, H.; Petite, H.; Blanchat, C.; Guillemin, G. Experimental vertebroplasty using osteoconductive granular material. Spine 2000, 25, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kang, Y.H.; Kim, J.K.; Park, J.B. Effect of bone mineral particles on the porosity of bone cement. Bio-Med. Mater. Eng. 1994, 4, 37–46. [Google Scholar]
- Hahn, B.D.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Choi, J.H.; Kim, J.W.; Ahn, C.W.; Kim, H.E.; Yoon, B.H.; et al. Osteoconductive hydroxyapatite coated PEEK for spinal fusion surgery. Appl. Surf. Sci. 2013, 283, 6–11. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Albuixech, L.; Fernández-Barragán, E.; Aparicio, C.; Gil, F.J.; San Román, J.; Vázquez, B.; Planell, J.A. Mechanical performance of acrylic bone cements containing different radiopacifying agents. Biomaterials 2002, 23, 1873–1882. [Google Scholar] [CrossRef]
- Sogal, A.; Hulbert, S. Mechanical properties of a composite bone cement: Polymethylmethacrylate and hydroxyapatite. Bioceramics 1992, 5, 213–224. [Google Scholar]
- Heikkila, J.T.; Aho, A.J.; Kangasniemi, I.; YliUrpo, A. Polymethylmethacrylate composites: Disturbed bone formation at the surface of bioactive glass and hydroxyapatite. Biomaterials 1996, 17, 1755–1760. [Google Scholar] [CrossRef]
- Ohtsuki, C.; Miyazaki, T.; Kyomoto, M.; Tanihara, M.; Osaka, A. Development of bioactive PMMA-based cement by modification with alkoxysilane and calcium salt. J. Mater. Sci.-Mater. Med. 2001, 12, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Ormsby, R.; McNally, T.; Mitchell, C.; Dunne, N. Influence of multiwall carbon nanotube functionality and loading on mechanical properties of PMMA/MWCNT bone cements. J. Mater. Sci.-Mater. Med. 2010, 21, 2287–2292. [Google Scholar] [CrossRef] [PubMed]
- Harper, E.J. Bioactive bone cements. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jang, T.S.; Song, J.; Kim, H.E.; Jung, H.D. The production of porous hydroxyapatite scaffolds with graded porosity by sequential freeze-casting. Materials 2017, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 81–98. [Google Scholar] [CrossRef]
- Dalby, M.J.; Di Silvio, L.; Harper, E.J.; Bonfield, W. Increasing hydroxyapatite incorporation into poly(methylmethacrylate) cement increases osteoblast adhesion and response. Biomaterials 2002, 23, 569–576. [Google Scholar] [CrossRef]
- Arcos, D.; Lopez-Noriega, A.; Ruiz-Hernandez, E.; Terasaki, O.; Vallet-Regi, M. Ordered mesoporous microspheres for bone grafting and drug delivery. Chem. Mater. 2009, 21, 1000–1009. [Google Scholar] [CrossRef]
- Kuehn, K.-D.; Ege, W.; Gopp, U. Acrylic bone cements: Composition and properties. Orthop. Clin. N. Am. 2005, 36, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Chou, L.; Marek, B.; Wagner, W.R. Effects of hydroxylapatite coating crystallinity on biosolubility, cell attachment efficiency and proliferation in vitro. Biomaterials 1999, 20, 977–985. [Google Scholar] [CrossRef]
- Hanein, D.; Sabanay, H.; Addadi, L.; Geiger, B. Selective interactions of cells with crystal surfaces. Implications for the mechanism of cell adhesion. J. Cell Sci. 1993, 104, 275–288. [Google Scholar] [PubMed]
- Maxian, S.H.; Stefano, T.D.; Melican, M.C.; Tiku, M.L.; Zawadsky, J.P. Bone cell behavior on Matrigel®-coated Ca/P coatings of varying crystallinities. J. Biomed. Mater. Res. 1998, 40, 171–179. [Google Scholar] [CrossRef]
- Morgan, J.; Holtman, K.R.; Keller, J.C.; Stanford, C.M. In vitro mineralization and implant calcium phosphate-hydroxyapatite crystallinity. Implant Dent. 1996, 5, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.J.; Ye, F.; Yang, R.N.; Lu, X.F.; Shi, Y.J.; Li, L.; Fan, H.S.; Bu, H. Osteoinduction of hydroxyapatite/beta-tricalcium phosphate bioceramics in mice with a fractured fibula. Acta Biomater. 2010, 6, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Vallo, C.I.; Montemartini, P.E.; Fanovich, M.A.; Lopez, J.M.P.; Cuadrado, T.R. Polymethylmethacrylate-based bone cement modified with hydroxyapatite. J. Biomed. Mater. Res. 1999, 48, 150–158. [Google Scholar] [CrossRef]
- Liu, C.; Green, S.M.; Watkins, N.D.; Gregg, P.J.; McCaskie, A.W. Some failure modes of four clinical bone cements. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2001, 215, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Ridzwan, M.I.Z.; Shuib, S.; Hassan, A.Y.; Shokri, A.A.; Mohammad Ibrahim, M.N. Problem of stress shielding and improvement to the hip implant designs: A review. J. Med. Sci. 2007, 7, 460–467. [Google Scholar]
- Gruen, T.A.; Mcneice, G.M.; Amstutz, H.C. “Modes of failure” of cemented stem-type femoral components: A radiographic analysis of loosening. Clin. Orthop. Relat. Res. 1979, 141, 17–27. [Google Scholar] [CrossRef]
- Stauffer, R.N. Ten-year follow-up study of total hip replacement. JBJS 1982, 64, 983–990. [Google Scholar] [CrossRef]
- Bayraktar, H.H.; Morgan, E.F.; Niebur, G.L.; Morris, G.E.; Wong, E.K.; Keaveny, T.M. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J. Biomech. 2004, 37, 27–35. [Google Scholar] [CrossRef]
- Keller, T.S. Predicting the compressive mechanical-behavior of bone. J. Biomech. 1994, 27, 1159–1168. [Google Scholar] [CrossRef]
- Baek, J.; Jung, H.-D.; Jang, T.-S.; Kim, S.W.; Kang, M.-H.; Kim, H.-E.; Koh, Y.-H. Synthesis and evaluation of bone morphogenetic protein (BMP)-loaded hydroxyapatite microspheres for enhanced bone regeneration. Ceram. Int. 2016, 42, 7748–7756. [Google Scholar] [CrossRef]
- He, Q.; Chen, H.; Huang, L.; Dong, J.; Guo, D.; Mao, M.; Kong, L.; Li, Y.; Wu, Z.; Lei, W. Porous surface modified bioactive bone cement for enhanced bone bonding. PLoS ONE 2012, 7, e42525. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, I.-G.; Park, C.-I.; Lee, H.; Kim, H.-E.; Lee, S.-M. Hydroxyapatite Microspheres as an Additive to Enhance Radiopacity, Biocompatibility, and Osteoconductivity of Poly(methyl methacrylate) Bone Cement. Materials 2018, 11, 258. https://doi.org/10.3390/ma11020258
Kang I-G, Park C-I, Lee H, Kim H-E, Lee S-M. Hydroxyapatite Microspheres as an Additive to Enhance Radiopacity, Biocompatibility, and Osteoconductivity of Poly(methyl methacrylate) Bone Cement. Materials. 2018; 11(2):258. https://doi.org/10.3390/ma11020258
Chicago/Turabian StyleKang, In-Gu, Cheon-Il Park, Hyun Lee, Hyoun-Ee Kim, and Sung-Mi Lee. 2018. "Hydroxyapatite Microspheres as an Additive to Enhance Radiopacity, Biocompatibility, and Osteoconductivity of Poly(methyl methacrylate) Bone Cement" Materials 11, no. 2: 258. https://doi.org/10.3390/ma11020258




