Effective Capture of Carbon Dioxide Using Hydrated Sodium Carbonate Powders
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of HSCPs
2.2. Characterization
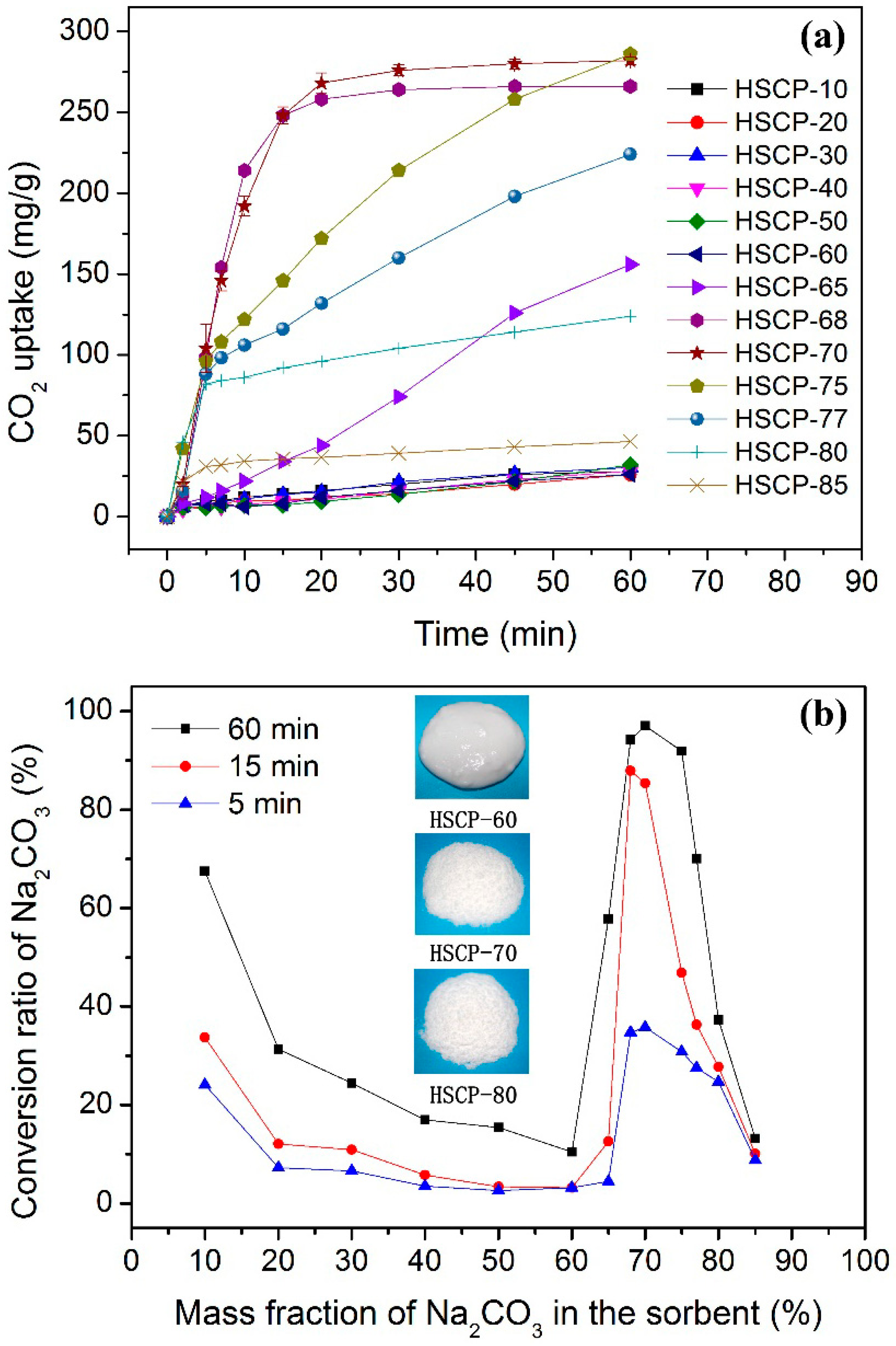
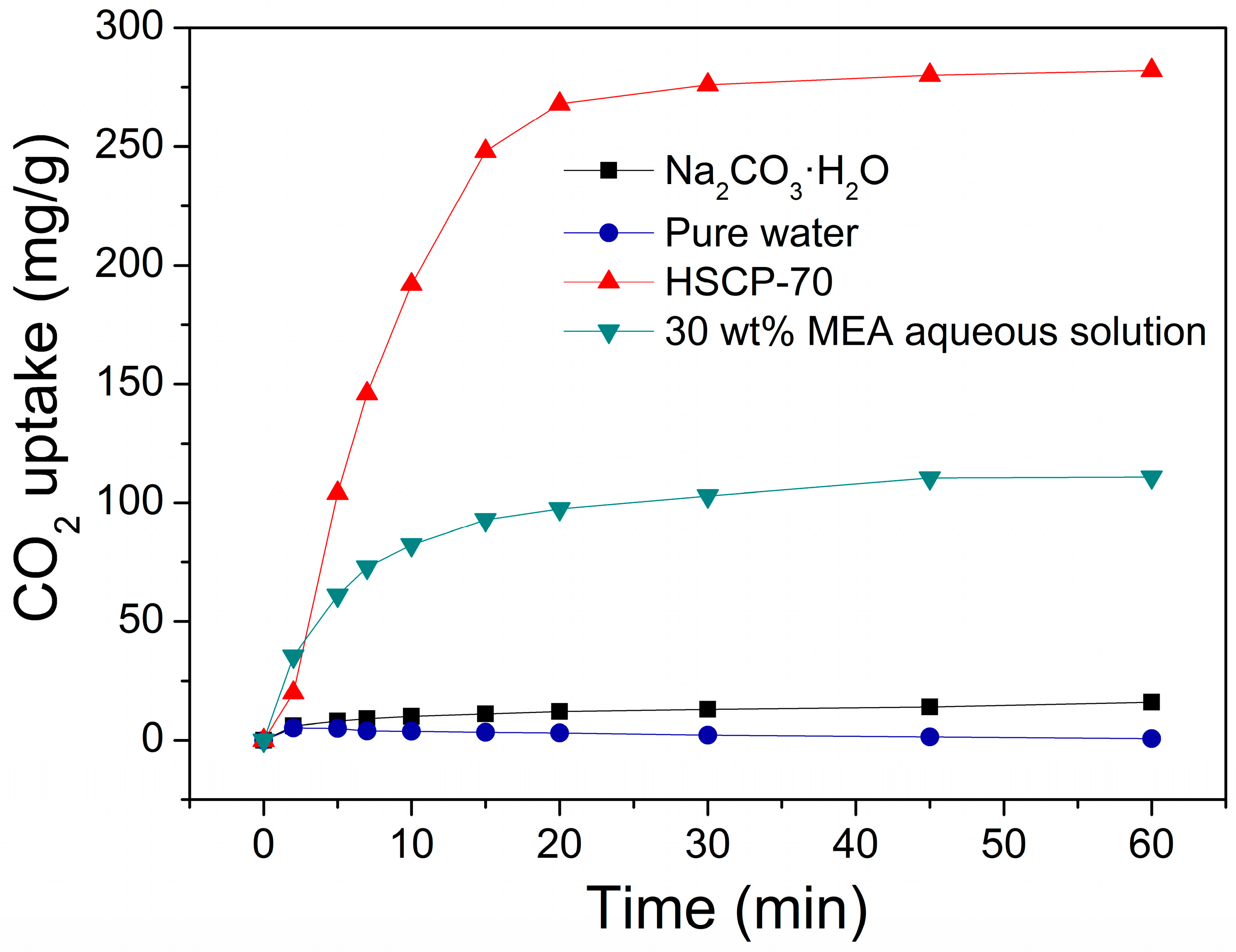
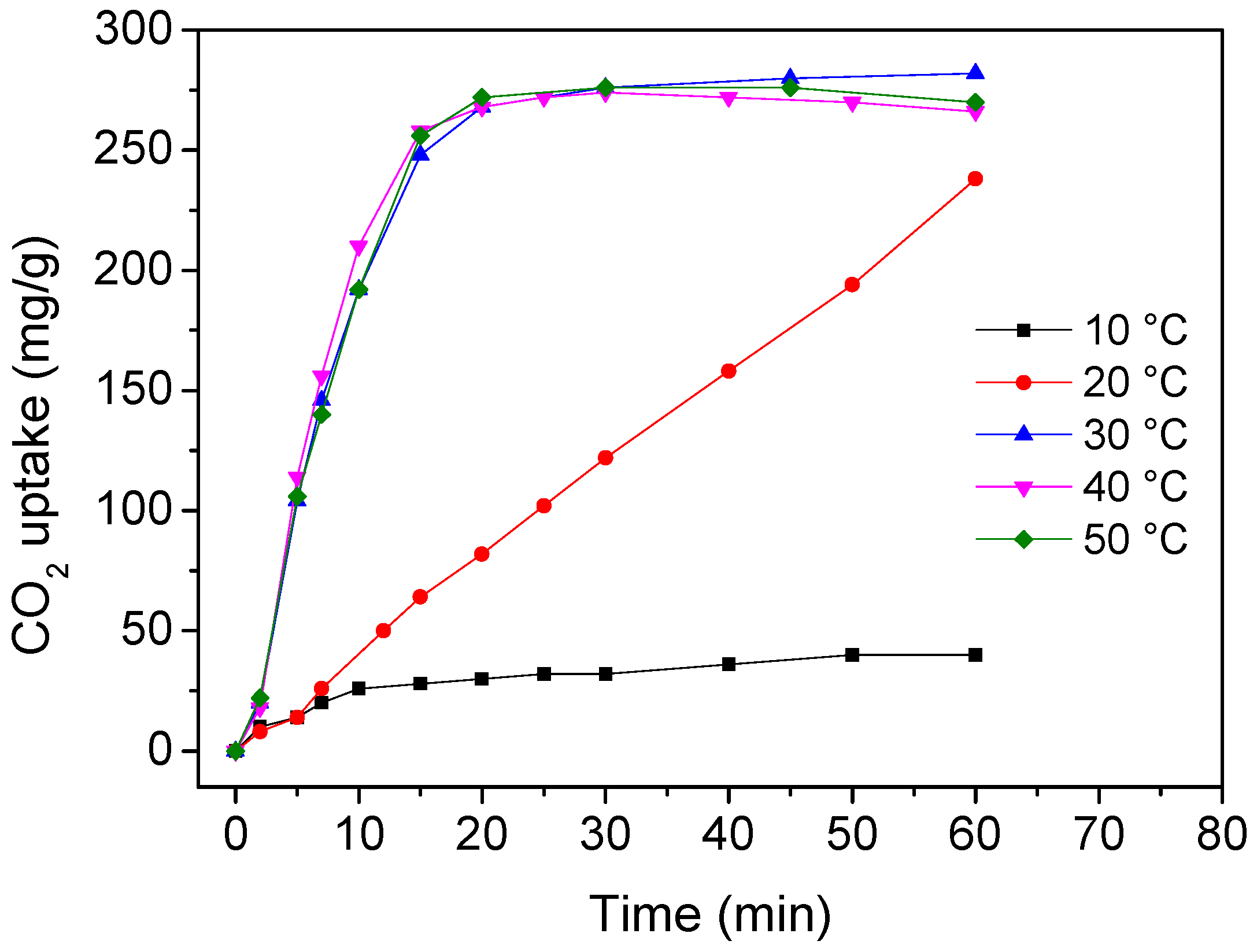
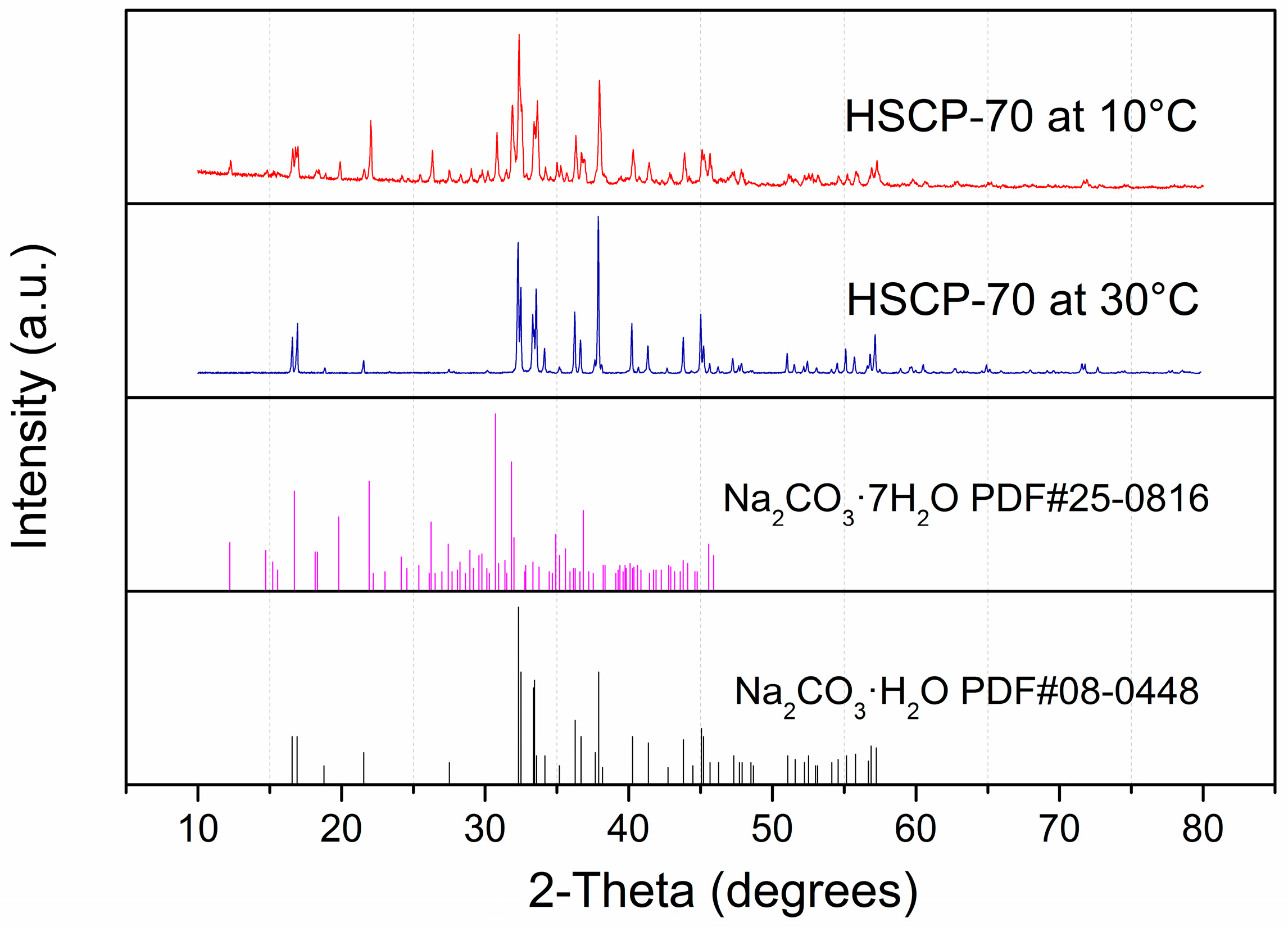
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bender, M.L.; Ho, D.T.; Hendricks, M.B.; Mika, R.; Battle, M.O.; Tans, P.P.; Conway, T.J.; Sturtevant, B.; Cassar, N. Atmospheric O2/N2 changes, 1993–2002: Implications for the partitioning of fossil fuel CO2 sequestration. Glob. Biogeochem. Cycles 2008, 19, 4057–4061. [Google Scholar]
- Rubin, E.S.; Chen, C.; Rao, A.B. Cost and performance of fossil fuel power plants with CO2 capture and storage. Energy Policy 2007, 35, 4444–4454. [Google Scholar] [CrossRef]
- Wolsky, A.M.; Daniels, E.J.; Jody, B.J. CO2 capture from the flue gas of conventional fossil-fuel-fired power plants. Environ. Prog. Sustain. Energy 1994, 13, 214–219. [Google Scholar] [CrossRef]
- Wang, W.; Ma, C.; Lin, P.; Sun, L.; Cooper, A.I. Gas storage in renewable bioclathrates. Energy Environ. Sci. 2012, 6, 105–107. [Google Scholar] [CrossRef]
- Cullinane, J.T.; Rochelle, G.T. Carbon dioxide absorption with aqueous potassium carbonate promoted by piperazine. Chem. Eng. Sci. 2004, 59, 3619–3630. [Google Scholar] [CrossRef]
- Macdowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef] [Green Version]
- Drage, T.C.; Snape, C.E.; Stevens, L.A.; Wood, J.; Wang, J.; Cooper, A.I.; Dawson, R.; Guo, X.; Satterley, C.; Irons, R. Materials challenges for the development of solid sorbents for post-combustion carbon capture. J. Mater. Chem. 2011, 22, 2815–2823. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Prakash, G.K.S.; Olah, G.A. ChemInform Abstract: Air as the Renewable Carbon Source of the Future: An Overview of CO2 Capture from the Atmosphere. Energy Environ. Sci. 2012, 44, 7833–7853. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Liu, J.; Sun, L. Coassembled Ionic Liquid/Laponite Hybrids as Effective CO2 Adsorbents. J. Energy Chem. 2017, 26, 1026–1029. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Min, X.; Meng, Y.; Sun, L. Designing Supported Ionic Liquids (ILs) within Inorganic Nanosheets for CO2 Capture Applications. ACS Appl. Mater. Interfaces 2016, 8, 5547–5555. [Google Scholar] [CrossRef] [PubMed]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.B.; Rubin, E.S. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef] [PubMed]
- Alie, C.; Backham, L.; Croiset, E.; Douglas, P.L. Simulation of CO2 capture using MEA scrubbing: A flowsheet decomposition method. Energy Convers. Manag. 2005, 46, 475–487. [Google Scholar] [CrossRef]
- And, I.J.U.; Idem, R.O. Studies of SO2− and O2−-Induced Degradation of Aqueous MEA during CO2 Capture from Power Plant Flue Gas Streams. Ind. Eng. Chem. Res. 2007, 46, 2558–2566. [Google Scholar]
- Wang, R.; Li, D.F.; Zhou, C.; Liu, M.; Liang, D.T. Impact of DEA solutions with and without CO2 loading on porous polypropylene membranes intended for use as contactors. J. Membr. Sci. 2004, 229, 147–157. [Google Scholar] [CrossRef]
- Filburn, T.; And, J.J.H.; Weiss, R.A. Development of Supported Ethanolamines and Modified Ethanolamines for CO2 Capture. Ind. Eng. Chem. Res. 2005, 44, 1542–1546. [Google Scholar] [CrossRef]
- Hasib-Ur-Rahman, M.; Siaj, M.; Larachi, F. Ionic liquids for CO2 capture—Development and progress. Chem. Eng. Process. 2010, 49, 313–322. [Google Scholar] [CrossRef]
- Aparicio, S.; Atilhan, M. A computational study on choline benzoate and choline salicylate ionic liquids in the pure state and after CO2 adsorption. J. Phys. Chem. B 2012, 116, 9171–9185. [Google Scholar] [CrossRef] [PubMed]
- Aaron, D.; Tsouris, C. Separation of CO2 from Flue Gas: A Review. Sep. Sci. Technol. 2011, 40, 321–348. [Google Scholar] [CrossRef]
- Duke, M.C.; Ladewig, B.; Smart, S.; Rudolph, V.; Costa, J.C.D.D. Assessment of postcombustion carbon capture technologies for power generation. Front Chem. Sci. Eng. 2010, 4, 184–195. [Google Scholar] [CrossRef]
- Tuwati, A.; Fan, M.; Russell, A.G.; Wang, J.; Dacosta, H.F. New CO2 Sorbent Synthesized with Nanoporous TiO(OH)2 and K2CO3. Energy Fuels 2013, 27, 7628–7636. [Google Scholar] [CrossRef]
- Khatri, R.A.; Chuang, S.S.; Soong, Y.; Gray, M. Thermal and chemical stability of regenerable solid amine sorbent for CO2 capture. Energy Fuels 2006, 20, 1514–1520. [Google Scholar] [CrossRef]
- Chen, C.; Yang, S.T.; Ahn, W.S.; Ryoo, R. Amine-impregnated silica monolith with a hierarchical pore structure: Enhancement of CO2 capture capacity. Chem. Commun. 2009, 24, 3627–3629. [Google Scholar] [CrossRef] [PubMed]
- Bollini, P.; Didas, S.A.; Jones, C.W. Amine-oxide hybrid materials for acid gas separations. J. Mater. Chem. 2011, 21, 15100–15120. [Google Scholar] [CrossRef]
- Brunelli, N.A.; Didas, S.A.; Venkatasubbaiah, K.; Jones, C.W. Tuning cooperativity by controlling the linker length of silica-supported amines in catalysis and CO2 capture. J. Am. Chem. Soc. 2012, 134, 13950–13953. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Kang, D.Y.; Copeland, J.R.; Brunelli, N.A.; Didas, S.A.; Bollini, P.; Sievers, C.; Kamegawa, T.; Yamashita, H.; Jones, C.W. Dramatic enhancement of CO2 uptake by poly(ethyleneimine) using zirconosilicate supports. J. Am. Chem. Soc. 2012, 134, 10757–10760. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, X.; Sun, L.; Liu, X. Adsorption separation of carbon dioxide, methane and nitrogen on monoethanol amine modified β-zeolite. J. Nat. Gas Chem. 2009, 18, 167–172. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, X.; Zhao, C. Multiple-Cycles Behavior of K2CO3/Al2O3 for CO2 Capture in a Fluidized-Bed Reactor. Energy Fuels 2010, 24, 1009–1012. [Google Scholar] [CrossRef]
- Seo, Y.; Jo, S.H.; Ryu, C.K.; Yi, C.K. Effects of water vapor pretreatment time and reaction temperature on CO2 capture characteristics of a sodium-based solid sorbent in a bubbling fluidized-bed reactor. Chemosphere 2007, 69, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Harrison, D.; Gupta, R.; Green, D.; McMichael, W. Carbon dioxide capture using dry sodium-based sorbents. Energy Fuels 2004, 18, 569–575. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, J.; Liu, J.; Guo, Z.; Umar, A.; Sun, L. Na+ and K+-Exchanged Zirconium Phosphate (ZrP) as High-Temperature CO2 Adsorbents. Sci. Adv. Mater. 2013, 5, 469–474. [Google Scholar] [CrossRef]
- Borhani, T.N.G.; Azarpour, A.; Akbari, V.; Alwi, S.R.W.; Manan, Z.A. CO2 Capture with Potassium Carbonate Solutions: A State-of-the-Art Review. Int. J. Greenh. Gas Control 2015, 41, 142–162. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, J.C. Dry Potassium-Based Sorbents for CO2 Capture. Catal. Surv. Asia 2007, 11, 171–185. [Google Scholar] [CrossRef]
- Charitos, A.; Rodríguez, N.; Hawthorne, C.; Alonso, M.; Zieba, M.; Arias, B.; Kopanakis, G.; Scheffknecht, G.; Abanades, J.C. Experimental Validation of the Calcium Looping CO2 Capture Process with Two Circulating Fluidized Bed Carbonator Reactors. Ind. Eng. Chem. Res. 2011, 50, 9685–9695. [Google Scholar] [CrossRef] [Green Version]
- Borhani, T.N.G.; Akbari, V.; Hamid, M.K.A.; Manan, Z.A. Rate-based simulation and comparison of various promoters for CO2 capture in industrial DEA-promoted potassium carbonate absorption unit. J. Ind. Eng. Chem. 2015, 22, 306–316. [Google Scholar] [CrossRef]
- Dawson, R.; Stevens, L.A.; Williams, O.S.A.; Wang, W.; Carter, B.O.; Sutton, S.; Drage, T.C.; Blanc, F.; Adams, D.J.; Cooper, A.I. ‘Dry bases’: carbon dioxide capture using alkaline dry water. Energy Environ. Sci. 2014, 7, 1786–1791. [Google Scholar] [CrossRef]
- Lee, S.C.; Choi, B.Y.; Lee, S.J.; Jung, S.Y.; Chong, K.R.; Kim, J.C. CO2 Absorption and Regeneration using Na and K Based Sorbents. Stud. Surf. Sci. Catal. 2004, 153, 527–530. [Google Scholar]
- Choi, B.S. Sorption of Carbon Dioxide onto Sodium Carbonate. Sep. Sci. Technol. 2006, 41, 515–525. [Google Scholar]
- Park, S.W.; Sung, D.H.; Choi, B.S.; Lee, J.W.; Kumazawa, H. Carbonation kinetics of potassium carbonate by carbon dioxide. J. Ind. Eng. Chem. 2006, 12, 522–530. [Google Scholar]
- Dutcher, B.; Fan, M.; Leonard, B. Use of multifunctional nanoporous TiO(OH)2 for catalytic NaHCO3 decomposition-eventually for Na2CO3/NaHCO3 based CO2 separation technology. Sep. Purif. Technol. 2011, 80, 364–374. [Google Scholar] [CrossRef]
- Kondakindi, R.R.; Mccumber, G.; Aleksic, S.; Whittenberger, W.; Abraham, M.A. Na2CO3 -based sorbents coated on metal foil: CO2 capture performance. Int. J. Greenh. Gas Control 2013, 15, 65–69. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Choi, B.Y.; Ryu, C.K.; Ahn, Y.S.; Lee, T.J.; Kim, J.C. The effect of water on the activation and the CO2 capture capacities of alkali metal-based sorbents. Korean J. Chem. Eng. 2006, 23, 374–379. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, X.; Anthony, E.J.; Jiang, X.; Duan, L.; Wu, Y.; Dong, W.; Zhao, C. Capturing CO2 in flue gas from fossil fuel-fired power plants using dry regenerable alkali metal-based sorbent. Prog. Energy Combust. Sci. 2013, 39, 515–534. [Google Scholar] [CrossRef]
- Nakayama, F. Hydrolysis of sodium carbonate. J. Chem. Educ. 1970, 47, 67. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Wang, W.; Li, L.; Wang, Z.; Wang, S.; Ding, H.; Zhang, Z.; Sun, L.; Wang, W. Effective Capture of Carbon Dioxide Using Hydrated Sodium Carbonate Powders. Materials 2018, 11, 183. https://doi.org/10.3390/ma11020183
Cai Y, Wang W, Li L, Wang Z, Wang S, Ding H, Zhang Z, Sun L, Wang W. Effective Capture of Carbon Dioxide Using Hydrated Sodium Carbonate Powders. Materials. 2018; 11(2):183. https://doi.org/10.3390/ma11020183
Chicago/Turabian StyleCai, Yuanhao, Weilin Wang, Liang Li, Zhaofeng Wang, Suying Wang, Hao Ding, Zhengguo Zhang, Luyi Sun, and Weixing Wang. 2018. "Effective Capture of Carbon Dioxide Using Hydrated Sodium Carbonate Powders" Materials 11, no. 2: 183. https://doi.org/10.3390/ma11020183





