The Effect of Surface Treatments on the Degradation of Biomedical Mg Alloys—A Review Paper
Abstract
:1. Introduction
2. Mechanical Surface Treatments
2.1. Grinding and Polishing
2.2. Burnishing
2.3. Machining
3. Chemical Surface Treatments and Coatings
3.1. Acid Etching
3.2. Coatings
3.3. Ion Implantation
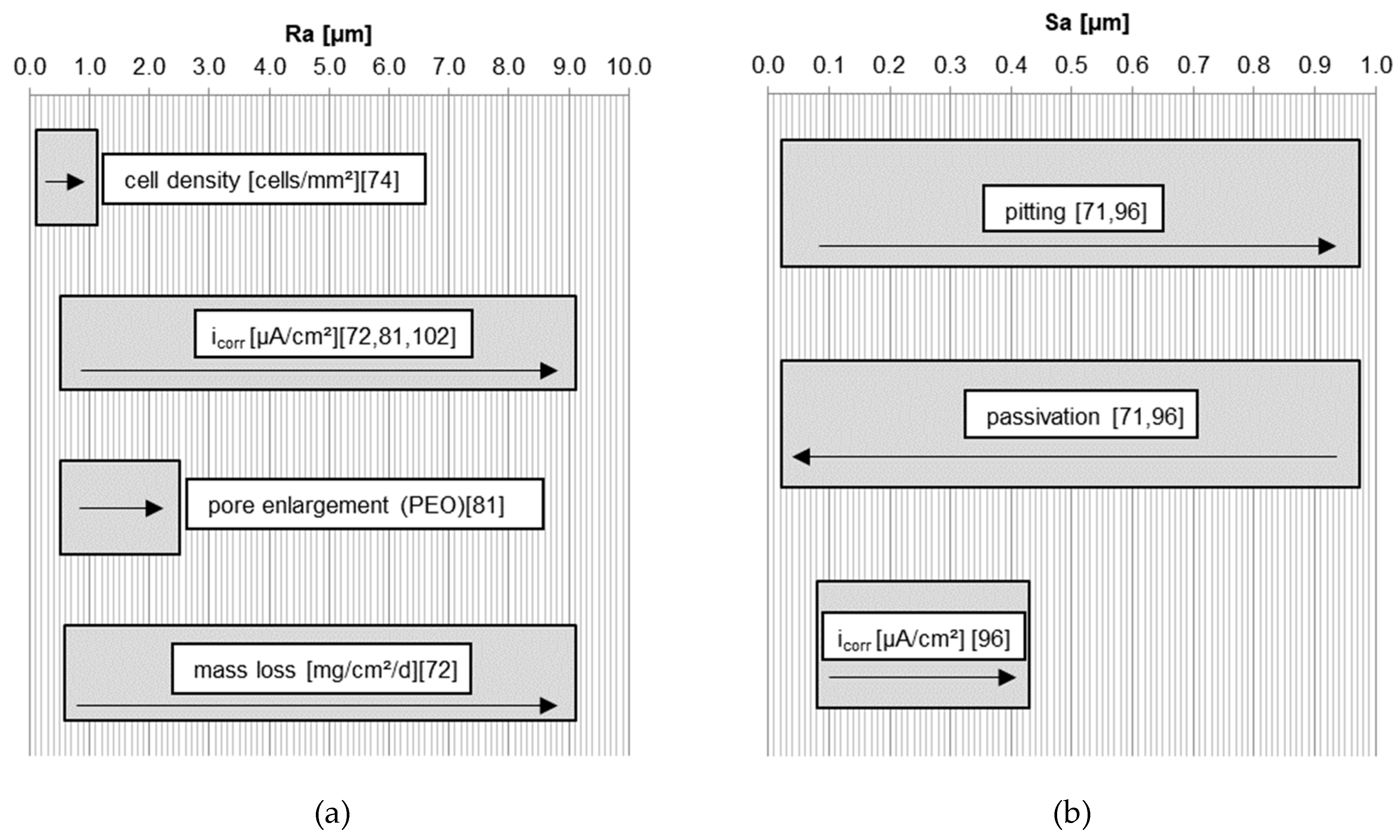
4. Summary of the Influence of Roughness on Degradation
4.1. Mechanical Surface Treatments
4.2. Chemical Surface Treatments and Coatings
5. Discussion
5.1. Suitable Roughness Values for Biodegradable Mg Implants
5.2. Suitable Treatments for Biodegradable Mg Implants
6. Conclusions
- Considering different roughness values arising from the same type of surface treatment, especially mechanical surface treatments, a trend of increased degradation rate can be seen with higher surface roughness.
- Roughness values arising from different surface treatments are non-comparable, and thus, cannot be compared against a degradation result.
- The roughness of a Mg implant is thought to have a greater influence on initial degradation, compared to long-term degradation. The duration for implant acceptance by the body is negligibly affected by the implant’s surface roughness.
- Implant surfaces with roughness values above Sa or Ra = 0.2 µm are unsuitable for initial cell adherence and cell viability. Higher roughness should be avoided, as increased degradation is expected, and consequently, greater local alkalization will occur.
- Ca/P coatings lead to a uniform surface morphology which results in a more uniform degradation over the surface, and decreases the degradation rate compared to uncoated material. Ca/P coated Mg alloys exhibited non-toxic and biocompatible properties.
- Differences in surface roughness and additions of K4P2O7 or KF into the electrolyte varied the pore size of PEO coatings, which, in turn, affected the degradation rate of implant materials. A smaller pore size of the PEO coating resulted in higher degradation.
- Acid etching provides a treatment over the entire surface, removing contamination and impurities by removing surface material. In particular, acetic acid and phosphoric acid etching improved the degradation behavior, i.e., by reducing the degradation rate. Etching allows the surface properties to be tailored in order to adjust the initial and long-term degradation.
Author Contributions
Funding
Conflicts of Interest
References
- Kraus, T.; Fischerauer, S.; Treichler, S.; Martinelli, E.; Eichler, J.; Myrissa, A.; Zötsch, S.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. The influence of biodegradable magnesium implants on the growth plate. Acta Biomater. 2018, 66, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, L.; Wang, L.; Yuan, G.; Dai, K.; Pei, J.; Hao, Y. Dual modulation of bone formation and resorption with zoledronic acid-loaded biodegradable magnesium alloy implants improves osteoporotic fracture healing: An in vitro and in vivo study. Acta Biomater. 2018, 65, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.; Ullah, S.; Mueller, P. Advances and Challenges of Biodegradable Implant Materials with a Focus on Magnesium-Alloys and Bacterial Infections. Metals 2018, 8, 532. [Google Scholar] [CrossRef]
- Choo, J.T.; Lai, S.H.S.; Tang, C.Q.Y.; Thevendran, G. Magnesium-based bioabsorbable screw fixation for hallux valgus surgery—A suitable alternative to metallic implants. Foot Ankle Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Höhn, S.; Virtanen, S.; Boccaccini, A.R. Protein adsorption on magnesium and its alloys: A review. Appl. Surf. Sci. 2019, 464, 212–219. [Google Scholar] [CrossRef]
- Zhao, N.; Zhu, D. Collagen Self-Assembly on Orthopedic Magnesium Biomaterials Surface and Subsequent Bone Cell Attachment. PLoS ONE 2014, 9, e110420. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Dietzel, W.; Witte, F.; Hort, N.; Blawert, C. Progress and Challenge for Magnesium Alloys as Biomaterials. Adv. Eng. Mater. 2008, 10, B3–B14. [Google Scholar] [CrossRef]
- Vormann, J. Magnesium: nutrition and metabolism. Mol. Aspects Med. 2003, 24, 27–37. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Echeverry-Rendon, M.; Duque, V.; Quintero, D.; Robledo, S.M.; Harmsen, M.C.; Echeverria, F. Improved corrosion resistance of commercially pure magnesium after its modification by plasma electrolytic oxidation with organic additives. J. Biomater. Appl. 2018, 33, 725–740. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Li, L.; Gu, X.; Zhang, K.; Xia, J.; Fan, Y. Effect of stress on corrosion of high-purity magnesium in vitro and in vivo. Acta Biomater. 2018, 83, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.-N.; Li, S.-S.; Li, X.-M.; Fan, Y.-B. Magnesium based degradable biomaterials: A review. Front. Mater. Sci. 2014, 8, 200–218. [Google Scholar] [CrossRef]
- Castellani, C.; Lindtner, R.A.; Hausbrandt, P.; Tschegg, E.; Stanzl-Tschegg, S.E.; Zanoni, G.; Beck, S.; Weinberg, A.-M. Bone–implant interface strength and osseointegration: Biodegradable magnesium alloy versus standard titanium control. Acta Biomater. 2011, 7, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, N.; Betts, L.; Zhu, D. Bio-Adaption between Magnesium Alloy Stent and the Blood Vessel: A Review. J. Mater. Sci. Tech. 2016, 32, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Song, S. A Possible Biodegradable Magnesium Implant Material. Adv. Eng. Mater. 2007, 9, 298–302. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, Y.; Feyerabend, F.; Willumeit-Römer, R.; Kainer, K.U.; Hort, N. Microstructure and Mechanical Properties of Mg-Gd Alloys as Biodegradable Implant Materials. In TMS 2018 147th Annual Meeting & Exhibition Supplemental Proceedings; Materials Society, T.M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-72525-3. [Google Scholar]
- Li, P.; Zhou, N.; Qiu, H.; Maitz, M.F.; Wang, J.; Huang, N. In vitro and in vivo cytocompatibility evaluation of biodegradable magnesium-based stents: A review. Sci. Chin. Mater. 2018, 61, 501–515. [Google Scholar] [CrossRef]
- Kim, B.J.; Piao, Y.; Wufuer, M.; Son, W.-C.; Choi, T.H. Biocompatibility and Efficiency of Biodegradable Magnesium-Based Plates and Screws in the Facial Fracture Model of Beagles. J. Oral Maxillofac. Surg. 2018, 76, 1055. [Google Scholar] [CrossRef]
- Ferrando, W.A. Review of corrosion and corrosion control of magnesium alloys and composites. J. Mater. Eng. 1989, 11, 299–313. [Google Scholar] [CrossRef]
- Johnson, I.; Perchy, D.; Liu, H. In vitro evaluation of the surface effects on magnesium-yttrium alloy degradation and mesenchymal stem cell adhesion. J. Biomed. Mater. Res. Part A 2012, 100, 477–485. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, C.; Zhang, X.; Tang, G.; Tian, X.; Chu, P.K. Corrosion behavior of biomedical AZ91 magnesium alloy in simulated body fluids. J. Mater. Res. 2007, 22, 2004–2011. [Google Scholar] [CrossRef]
- Ghali, E. Corrosion and Protection of Magnesium Alloys. Mater. Sci. Forum 2000, 350–351, 261–272. [Google Scholar] [CrossRef]
- Makar, G.L.; Kruger, J. Corrosion of magnesium. Int. Mater. Rev. 1993, 38, 138–153. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; Stjohn, D.; Nairn, J.; Li, Y. The electrochemical corrosion of pure magnesium in 1 N NaCl. Corros. Sci. 1997, 39, 855–875. [Google Scholar] [CrossRef]
- Corrosion; ASM International; Korb, L.J. (Eds.) ASM handbook; [10. ed.], 7. print; ASM International: Materials Park, OH, USA, 2001; ISBN 978-0-87170-019-3. [Google Scholar]
- Hu, H.; Nie, X.; Ma, Y. Corrosion and Surface Treatment of Magnesium Alloys. In Magnesium Alloys—Properties in Solid and Liquid States; Czerwinski, F., Ed.; Intech Open: London, UK, 2014; ISBN 978-953-51-1728-5. [Google Scholar] [Green Version]
- Baboian, R.; Dean, S.; Hack, H.; Haynes, G.; Scully, J.; Sprowls, D. Corrosion Tests and Standards: Application and Interpretation; ASTM Manual Series: Philadelphia, PA, USA, 1995. [Google Scholar]
- Song, G.L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Iglesias, C.; Bodelón, O.G.; Montoya, R.; Clemente, C.; Garcia-Alonso, M.C.; Rubio, J.C.; Escudero, M.L. Fracture bone healing and biodegradation of AZ31 implant in rats. Biomed. Mater. 2015, 10, 025008. [Google Scholar] [CrossRef] [Green Version]
- Shalabi, M.M.; Gortemaker, A.; Hof, M.A.V.; Jansen, J.A.; Creugers, N.H.J. Implant Surface Roughness and Bone Healing: a Systematic Review. J. Dent. Res. 2006, 85, 496–500. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Zhang, P.; Li, Y.; Wang, J.; Lai, Y.; Qin, L. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: A general review. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1691–1701. [Google Scholar] [CrossRef]
- Kaesel, V.; Tai, P.-T.; Bach, F.-W.; Haferkamp, H.; Witte, F.; Windhagen, H. Approach to Control the Corrosion of Magnesium by Alloying. In Magnesium; Kainer, K.U., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 534–539. ISBN 978-3-527-60356-5. [Google Scholar]
- Yun, Y.; Dong, Z.; Lee, N.; Liu, Y.; Xue, D.; Guo, X.; Kuhlmann, J.; Doepke, A.; Halsall, H.B.; Heineman, W.; et al. Revolutionizing biodegradable metals. Mate. Today 2009, 12, 22–32. [Google Scholar] [CrossRef]
- Bland, L.G.; Gusieva, K.; Scully, J.R. Effect of Crystallographic Orientation on the Corrosion of Magnesium: Comparison of Film Forming and Bare Crystal Facets using Electrochemical Impedance and Raman Spectroscopy. Electrochim. Acta 2017, 227, 136–151. [Google Scholar] [CrossRef]
- Zhao, Y.-C.; Huang, G.-S.; Wang, G.-G.; Han, T.-Z.; Pan, F.-S. Influence of Grain Orientation on the Corrosion Behavior of Rolled AZ31 Magnesium Alloy. Acta Metall. Sin. 2015, 28, 1387–1393. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, D.; Zhao, M.-C.; Song, G.; Atrens, A. The effect of crystallographic orientation on the active corrosion of pure magnesium. Scr. Mater. 2008, 58, 421–424. [Google Scholar] [CrossRef]
- Song, G.-L.; Xu, Z. The surface, microstructure and corrosion of magnesium alloy AZ31 sheet. Electrochim. Acta 2010, 55, 4148–4161. [Google Scholar] [CrossRef]
- Pu, Z.; Song, G.-L.; Yang, S.; Outeiro, J.C.; Dillon, O.W.; Puleo, D.A.; Jawahir, I.S. Grain refined and basal textured surface produced by burnishing for improved corrosion performance of AZ31B Mg alloy. Corros. Sci. 2012, 57, 192–201. [Google Scholar] [CrossRef]
- Nwaogu, U.C.; Blawert, C.; Scharnagl, N.; Dietzel, W.; Kainer, K.U. Effects of organic acid pickling on the corrosion resistance of magnesium alloy AZ31 sheet. Corros. Sci. 2010, 52, 2143–2154. [Google Scholar] [CrossRef] [Green Version]
- Aung, N.N.; Zhou, W. Effect of grain size and twins on corrosion behaviour of AZ31B magnesium alloy. Corros. Sci. 2010, 52, 589–594. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Arrabal, R.; Viejo, F.; Matykina, E. Corrosion behaviour of magnesium/aluminium alloys in 3.5 wt.% NaCl. Corros. Sci. 2008, 50, 823–834. [Google Scholar] [CrossRef]
- Ambat, R.; Aung, N.N.; Zhou, W. Evaluation of microstructural effects on corrosion behaviour of AZ91D magnesium alloy. Corros. Sci. 2000, 42, 1433–1455. [Google Scholar] [CrossRef]
- Zheng, X.; Dong, J.; Xiang, Y.; Chang, J.; Wang, F.; Jin, L.; Wang, Y.; Ding, W. Formability, mechanical and corrosive properties of Mg-Nd-Zn-Zr magnesium alloy seamless tubes. Mater. Des. 2010, 31, 1417–1422. [Google Scholar] [CrossRef]
- Liu, Z.; Schade, R.; Luthringer, B.; Hort, N.; Rothe, H.; Müller, S.; Liefeith, K.; Willumeit-Römer, R.; Feyerabend, F. Influence of the Microstructure and Silver Content on Degradation, Cytocompatibility, and Antibacterial Properties of Magnesium-Silver Alloys In Vitro. Oxidat. Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Stellwagen, E.; Babul, J. Stabilization of the globular structure of ferricytochrome c by chloride in acidic solvents. Biochemistry 1975, 14, 5135–5140. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, M.; Persson, D. The influence of the microstructure on the atmospheric corrosion behaviour of magnesium alloys AZ91D and AM50. Corros. Sci. 2010, 52, 1077–1085. [Google Scholar] [CrossRef]
- Ben-Haroush, M.; Ben-Hamu, G.; Eliezer, D.; Wagner, L. The relation between microstructure and corrosion behavior of AZ80 Mg alloy following different extrusion temperatures. Corros. Sci. 2008, 50, 1766–1778. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 075005. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; You, C.; Chen, M. Effects of grain size on the corrosion resistance of pure magnesium by cooling rate-controlled solidification. Front. Mater. Sci. 2015, 9, 247–253. [Google Scholar] [CrossRef]
- Lu, Y.; Bradshaw, A.R.; Chiu, Y.L.; Jones, I.P. Effects of secondary phase and grain size on the corrosion of biodegradable Mg-Zn-Ca alloys. Mater. Sci. Eng. C 2015, 48, 480–486. [Google Scholar] [CrossRef]
- Kutniy, K.V.; Papirov, I.I.; Tikhonovsky, M.A.; Pikalov, A.I.; Sivtzov, S.V.; Pirozhenko, L.A.; Shokurov, V.S.; Shkuropatenko, V.A. Influence of grain size on mechanical and corrosion properties of magnesium alloy for medical implants. Materialwiss. Werkstofftech. 2009, 40, 242–246. [Google Scholar] [CrossRef]
- Ullmann, B.; Reifenrath, J.; Seitz, J.-M.; Bormann, D.; Meyer-Lindenberg, A. Influence of the grain size on the in vivo degradation behaviour of the magnesium alloy LAE442. Proc. Inst. Mech. Eng. H 2013, 227, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.-C.; Chen, J.; Dietzel, W.; Zettler, R.; dos Santos, J.F.; Lucia Nascimento, M.; Kainer, K.U. Corrosion of friction stir welded magnesium alloy AM50. Corros. Sci. 2009, 51, 1738–1746. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Li, Y.; Wang, F. Roles of β phase in the corrosion process of AZ91D magnesium alloy. Corros. Sci. 2006, 48, 1249–1264. [Google Scholar] [CrossRef]
- Nwaogu, U.C.; Blawert, C.; Scharnagl, N.; Dietzel, W.; Kainer, K.U. Influence of inorganic acid pickling on the corrosion resistance of magnesium alloy AZ31 sheet. Corros. Sci. 2009, 51, 2544–2556. [Google Scholar] [CrossRef] [Green Version]
- Gawlik, M.M.; Steiner, M.; Wiese, B.; González, J.; Feyerabend, F.; Dahms, M.; Ebel, T.; Willumeit-Römer, R. The Effects of HAc Etching on the Degradation Behavior of Mg-5Gd. J. Med. Mater. Tech. 2017, 1, 22–25. [Google Scholar]
- Snir, Y.; Ben-Hamu, G.; Eliezer, D.; Abramov, E. Effect of compression deformation on the microstructure and corrosion behavior of magnesium alloys. J. Alloys Compd. 2012, 528, 84–90. [Google Scholar] [CrossRef]
- Wang, B.J.; Xu, D.K.; Dong, J.H.; Ke, W. Effect of the crystallographic orientation and twinning on the corrosion resistance of an as-extruded Mg-3Al-1Zn (wt.%) bar. Scr. Mater. 2014, 88, 5–8. [Google Scholar] [CrossRef]
- Zou, G.; Peng, Q.; Wang, Y.; Liu, B. The effect of extension twinning on the electrochemical corrosion properties of Mg-Y alloys. J. Alloys Compd. 2015, 618, 44–48. [Google Scholar] [CrossRef]
- Liu, H. The effects of surface and biomolecules on magnesium degradation and mesenchymal stem cell adhesion. J. Biomed. Mater. Res. Part A 2011, 99, 249–260. [Google Scholar] [CrossRef]
- Kieke, M.; Feyerabend, F.; Lemaitre, J.; Behrens, P.; Willumeit-Römer, R. Degradation rates and products of pure magnesium exposed to different aqueous media under physiological conditions. BioNanoMaterials 2016, 17, 131–143. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P.K. Influence of Test Solutions on In Vitro Studies of Biomedical Magnesium Alloys. J. Electrochem. Soc. 2010, 157, C238. [Google Scholar] [CrossRef]
- Agha, N.A.; Feyerabend, F.; Mihailova, B.; Heidrich, S.; Bismayer, U.; Willumeit-Römer, R. Magnesium degradation influenced by buffering salts in concentrations typical of in vitro and in vivo models. Mater. Sci. Eng. C 2016, 58, 817–825. [Google Scholar] [CrossRef]
- Uddin, M.S.; Hall, C.; Murphy, P. Surface treatments for controlling corrosion rate of biodegradable Mg and Mg-based alloy implants. Sci. Tech. Adv. Mater. 2015, 16, 053501. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Cui, F.; Lee, I.S. Surface Modifications of Magnesium Alloys for Biomedical Applications. Ann. Biomed. Eng. 2011, 39, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Surface Modification of Magnesium and Its Alloys for Biomedical Applications; Sankara Narayanan, T.S.N.; Park, I.-S.; Lee, M.-H. (Eds.) Woodhead Publishing series in biomaterials; Elsevier/Woodhead Publishing: Cambridge, UK; Waltham, MA, USA, 2015; ISBN 978-1-78242-077-4. [Google Scholar]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Gray-Munro, J.E.; Seguin, C.; Strong, M. Influence of surface modification on the in vitro corrosion rate of magnesium alloy AZ31. J. Biomed. Mater. Res. Part A 2009, 91A, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.; Kannan, M.B.; He, Y.; Sandham, A. Effect of surface roughness on the in vitro degradation behaviour of a biodegradable magnesium-based alloy. Appl. Surf. Sci. 2013, 279, 343–348. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Blanquet, A.; Staiger, M.P.; Dias, G.J.; Woodfield, T.B.F. On the role of surface roughness in the corrosion of pure magnesium in vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1310–1318. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Hwang, D.-Y.; Lee, D.-H.; Yoo, B.; Shin, D.-H. Influence of potassium pyrophosphate in electrolyte on coated layer of AZ91 Mg alloy formed by plasma electrolytic oxidation. Trans. Nonferr. Met. Soc. China 2009, 19, 824–828. [Google Scholar] [CrossRef]
- Lorenz, C.; Brunner, J.G.; Kollmannsberger, P.; Jaafar, L.; Fabry, B.; Virtanen, S. Effect of surface pre-treatments on biocompatibility of magnesium. Acta Biomater. 2009, 5, 2783–2789. [Google Scholar] [CrossRef]
- Laycock, N.J.; Noh, J.S.; White, S.P.; Krouse, D.P. Computer simulation of pitting potential measurements. Corros. Sci. 2005, 47, 3140–3177. [Google Scholar] [CrossRef]
- Burstein, G.T.; Vines, S.P. Repetitive Nucleation of Corrosion Pits on Stainless Steel and the Effects of Surface Roughness. J. Electrochem. Soc. 2001, 148, B504. [Google Scholar] [CrossRef]
- Suter, T.; Müller, Y.; Schmutz, P.; von Trzebiatowski, O. Microelectrochemical Studies of Pit Initiation on High Purity and Ultra High Purity Aluminum. Adv. Eng. Mater. 2005, 7, 339–348. [Google Scholar] [CrossRef]
- Guo, H.F.; An, M.Z. Growth of ceramic coatings on AZ91D magnesium alloys by micro-arc oxidation in aluminate–fluoride solutions and evaluation of corrosion resistance. Appl. Surf. Sci. 2005, 246, 229–238. [Google Scholar] [CrossRef]
- Hwang, D.Y.; Kim, Y.M.; Shin, D.H. Corrosion Resistance of Plasma-Anodized AZ91 Mg Alloy in the Electrolyte with/without Potassium Fluoride. Mater. Trans. 2009, 50, 671–678. [Google Scholar] [CrossRef] [Green Version]
- Chiu, L.-H.; Chen, C.-C.; Yang, C.-F. Improvement of corrosion properties in an aluminum-sprayed AZ31 magnesium alloy by a post-hot pressing and anodizing treatment. Surf. Coat. Technol. 2005, 191, 181–187. [Google Scholar] [CrossRef]
- Yoo, B.; Shin, K.R.; Hwang, D.Y.; Lee, D.H.; Shin, D.H. Effect of surface roughness on leakage current and corrosion resistance of oxide layer on AZ91 Mg alloy prepared by plasma electrolytic oxidation. Appl. Surf. Sci. 2010, 256, 6667–6672. [Google Scholar] [CrossRef]
- Hwang, D.K.; Yoo, B.Y.; Cho, J.Y.; Lee, D.H.; Shin, D.H. Effect of surface roughness on corrosion resistance of oxide layer on AZ91 Mg alloy prepared by plasma electrolytic oxidation. In Proceedings of the 214th ECS Meeting, Honolulu, HI, USA, 12–17 October 2008. [Google Scholar]
- Burstein, G.T.; Pistorius, P.C. Surface Roughness and the Metastable Pitting of Stainless Steel in Chloride Solutions. Corros. Sci. 1995, 51, 380–385. [Google Scholar] [CrossRef]
- Parekh, R.B.; Shetty, O.; Tabassum, R. Surface Modifications for Endosseous Dental Implants. Int. J. Oral Implantol. Clin. Res. 2012, 3, 116–121. [Google Scholar] [CrossRef]
- Lacefield, W.R. Materials Characteristics of Uncoated/Ceramic-Coated Implant Materials. Adv. Dent. Res. 1999, 13, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Aoki, K.; Ohya, K. Effects of surface roughness of titanium implants on bone remodeling activity of femur in rabbits. Bone 1997, 21, 507–514. [Google Scholar] [CrossRef]
- Von der Höh, N.; von Rechenberg, B.; Bormann, D.; Lucas, A.; Meyer-Lindenberg, A. Influence of different surface machining treatments of resorbable magnesium alloy implants on degradation—EDX-analysis and histology results. Materialwiss. Werkstofftech. 2009, 40, 88–93. [Google Scholar] [CrossRef]
- Zhao, Y.; Jamesh, M.I.; Li, W.K.; Wu, G.; Wang, C.; Zheng, Y.; Yeung, K.W.K.; Chu, P.K. Enhanced antimicrobial properties, cytocompatibility, and corrosion resistance of plasma-modified biodegradable magnesium alloys. Acta Biomater. 2014, 10, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Lopez, B.S.; Hultenby, K.; Wennerberg, A.; Arvidson, K. Attachment and proliferation of human oral fibroblasts to titanium surfaces blasted with TiO2 particles. A scanning electron microscopic and histomorphometric analysis. Clin. Oral Implants Res. 1998, 9, 195–207. [Google Scholar] [CrossRef]
- Soskolne, W.A.; Cohen, S.; Shapira, L.; Sennerby, L.; Wennerberg, A. The effect of titanium surface roughness on the adhesion of monocytes and their secretion of TNF-alpha and PGE2. Clin. Oral Implants Res. 2002, 13, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Derhami, K.; Wolfaardt, J.F.; Wennerberg, A.; Scott, P.G. Quantifying the adherence of fibroblasts to titanium and its enhancement by substrate-attached material. J. Biomed. Mater. Res. 2000, 52, 315–322. [Google Scholar] [CrossRef]
- Boyan, B.D.; Lohmann, C.H.; Dean, D.D.; Sylvia, V.L.; Cochran, D.L.; Schwartz, Z. Mechanisms Involved in Osteoblast Response to Implant Surface Morphology. Ann. Rev. Mater. Res. 2001, 31, 357–371. [Google Scholar] [CrossRef]
- Höh, N.V.D.; Bormann, D.; Lucas, A.; Denkena, B.; Hackenbroich, C.; Meyer-Lindenberg, A. Influence of Different Surface Machining Treatments of Magnesium-based Resorbable Implants on the Degradation Behavior in Rabbits. Adv. Eng. Mater. 2009, 11, B47–B54. [Google Scholar] [CrossRef]
- Alvarez, R.B.; Martin, H.J.; Horstemeyer, M.F.; Chandler, M.Q.; Williams, N.; Wang, P.T.; Ruiz, A. Corrosion relationships as a function of time and surface roughness on a structural AE44 magnesium alloy. Corros. Sci. 2010, 52, 1635–1648. [Google Scholar] [CrossRef]
- Supplit, R.; Koch, T.; Schubert, U. Evaluation of the anti-corrosive effect of acid pickling and sol–gel coating on magnesium AZ31 alloy. Corros. Sci. 2007, 49, 3015–3023. [Google Scholar] [CrossRef]
- Walter, R.; Kannan, M.B. Influence of surface roughness on the corrosion behaviour of magnesium alloy. Mater. Des. 2011, 32, 2350–2354. [Google Scholar] [CrossRef]
- Sharma, A.K. Text Book of Correlation and Regression; Discovery Publishing House: New Delhi, Delhi, India, 2005; ISBN 978-81-7141-935-7. [Google Scholar]
- Characterisation of areal surface texture; Leach, R. (Ed.) Springer: Berlin, Germany, 2013; ISBN 978-3-642-36458-7. [Google Scholar]
- Jin, S.; Amira, S.; Ghali, E. Electrochemical Impedance Spectroscopy Evaluation of the Corrosion Behavior of Die Cast and Thixocast AXJ530 Magnesium Alloy in Chloride Solution. Adv. Eng. Mater. 2007, 9, 75–83. [Google Scholar] [CrossRef]
- Song, G.L.; St John, D.H.; Abbott, T. Corrosion behaviour of a pressure die cast magnesium alloy. Int. J. Cast Met. Res. 2005, 18, 174–180. [Google Scholar] [CrossRef]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.J.; de With, G.; Sommerdijk, N.A.J.M. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mhaede, M.; Pastorek, F.; Hadzima, B. Influence of shot peening on corrosion properties of biocompatible magnesium alloy AZ31 coated by dicalcium phosphate dihydrate (DCPD). Mater. Sci. Eng. C 2014, 39, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Pompa, L.; Rahman, Z.U.; Munoz, E.; Haider, W. Surface characterization and cytotoxicity response of biodegradable magnesium alloys. Mater. Sci. Eng. C 2015, 49, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Abdul-Kadir, M.R. Synthesis and in vitro degradation evaluation of the nano-HA/MgF2 and DCPD/MgF2 composite coating on biodegradable Mg-Ca-Zn alloy. Surf. Coat. Technol. 2013, 222, 79–89. [Google Scholar] [CrossRef]
- Jamesh, M.; Wu, G.; Zhao, Y.; Chu, P.K. Effects of silicon plasma ion implantation on electrochemical corrosion behavior of biodegradable Mg-Y-RE Alloy. Corros. Sci. 2013, 69, 158–163. [Google Scholar] [CrossRef]
- Almen, J.O. Shot Blasting to Increase Fatigue Resistance; SAE International: Warrendale, PA, USA, 1943. [Google Scholar]
- Guagliano, M. Relating Almen intensity to residual stresses induced by shot peening: A numerical approach. J. Mater. Process.Tech. 2001, 110, 277–286. [Google Scholar] [CrossRef]
- Denkena, B.; Lucas, A. Biocompatible Magnesium Alloys as Absorbable Implant Materials—Adjusted Surface and Subsurface Properties by Machining Processes. CIRP Ann.-Manuf. Technol. 2007, 56, 113–116. [Google Scholar] [CrossRef]
- American Society of Mechanical Engineers Surface Texture Symbols; ASME: New York, 1996; ISBN 978-0-7918-2319-4.
- Guo, Y.B.; Salahshoor, M. Process mechanics and surface integrity by high-speed dry milling of biodegradable magnesium–calcium implant alloys. CIRP Ann.-Manuf. Technol. 2010, 59, 151–154. [Google Scholar] [CrossRef]
- Denkena, B.; Lucas, A.; Thorey, F.; Waizy, H.; Angrisani, N.; Meyer-Lindenberg, A. Biocompatible Magnesium Alloys as Degradable Implant Materials—Machining Induced Surface and Subsurface Properties and Implant Performance. In Special Issues on Magnesium Alloys; Monteiro, W.A., Ed.; Intech Open: London, UK, 2011; ISBN 978-953-307-391-0. [Google Scholar]
- Dorozhkin, S.V. Calcium orthophosphate-based biocomposites and hybrid biomaterials. J. Mater. Sci. 2009, 44, 2343–2387. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V. Calcium Orthophosphate Cements and Concretes. Materials 2009, 2, 221–291. [Google Scholar] [CrossRef] [Green Version]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophanides, T., Ed.; Intech Open: London, UK, 2012; ISBN 978-953-51-0537-4. [Google Scholar]
- El Kady, A.M.; Mohamed, K.R.; El-Bassyouni, G.T. Fabrication, characterization and bioactivity evaluation of calcium pyrophosphate/polymeric biocomposites. Ceram. Int. 2009, 35, 2933–2942. [Google Scholar] [CrossRef]
- Introduction to Biomaterials; Shi, D. (Ed.) Tsinghua University Press; World Scientific: Beijing, China; Singapore; Hackensack, NJ, USA, 2006; ISBN 978-7-302-10807-8. [Google Scholar]
- Duan, H.; Yan, C.; Wang, F. Growth process of plasma electrolytic oxidation films formed on magnesium alloy AZ91D in silicate solution. Electrochim. Acta 2007, 52, 5002–5009. [Google Scholar] [CrossRef]
- Novaes, A.B.; de Souza, S.L.S.; de Barros, R.R.M.; Pereira, K.K.Y.; Iezzi, G.; Piatelli, A. Influence of Implant Surfaces on Osseointegration. Braz. Dent. J. 2010, 21, 471–481. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Suggested guidelines for the topographic evaluation of implant surfaces. Int. J. Oral Maxillofac. Implant. 2000, 15, 331–344. [Google Scholar]
- Sanchez, A.H.M.; Luthringer, B.J.C.; Feyerabend, F.; Willumeit, R. Mg and Mg alloys: How comparable are in vitro and in vivo corrosion rates? A review. Acta Biomater. 2015, 13, 16–31. [Google Scholar] [CrossRef] [Green Version]


| Alloy | Sample | Experiment | Solution | Time | Grinding/ Polishing | Initial Roughness | Results | Ref |
|---|---|---|---|---|---|---|---|---|
| Mg | Disk | pH | MEM 1 | 2 h | Polishing: 6 µm + lubricant | Ra = 0.10 µm | pH = 8.01 | [74] |
| Cell viability | MEM 1 +FBS 2 | 24 h | * CD 3 = 10 cells/mm2 | |||||
| Foil | Mass loss | DMEM 4 + 10% FBS 2 | up to 80 d | 1200 grit | - | Max DR 5 = 0.09 mg cm−2 d−1 | [61] | |
| DI 6 Water | Max DR 5 = 0.28 mg/cmWütr²/d | |||||||
| Cell adhesion | DMEM 4 + 10% FBS 2 | 24 h | 1200 grit | - | 14.8% cell adhesion | |||
| Disk | Collagen quantification, cell attachment | - | 2 h | 180 grit | Ra = 1.89 µm | Trend of higher collagen adsorption with higher Ra, lowest CD 3 for highest Ra. | [6] | |
| 800 grit | Ra = 0.29 µm | |||||||
| 1200 grit | Ra = 0.15 µm | |||||||
| AE44 | Plate | Immersion | 3.5 wt.% NaCl | 4 and 12 h, 1.5 and 2.5 d | 1400 grit | - | intergranular degradation started earlier after polishing | [94] |
| AZ31 | - | PDP 7 | 0.9 wt.% NaCl | - | P1000 emery Paper | Ra = 0.33 µm | icorr = 3.64 µAcm−2 | [102] |
| EIS 8 | Rp = 934 Ωcm2 | |||||||
| Sheet | Hydrogen | 5 wt.% NaCl | 24 h | 1200 grit | Ra = 0.07 µm | 1.11 mg/dcm² | [38] | |
| Disk | PDP 7 (1 cm2) | PBS 9 | - | 1200 grit | Sa = 48.58 ± 23.45 nm | icorr = 34.5 ± 3.5 µA cm−2 | [103] | |
| CR 10 = 0.76 ± 0.06 mm/y | ||||||||
| Cytotoxicity | α-MEM 11 | 21 d | 1200 grit | Sa = 48.58 ± 23.45 nm | * Cell survival: 92 % | |||
| Disk | Immersion, Hydrogen, PDP 7 | 5 wt.% NaCl | 30, 200 h Immersion, 7 h Hydrogen | 4000 grit | Ra = 0.2 µm | Burnishing lead to a better corrosion behavior | [39] | |
| Dry Burnishing | ||||||||
| Cryogenic Burnishing | ||||||||
| Disk | Collagen quantification, cell attachment | - | 2 h | 180 grit | Ra = 1.89 µm | Trend of higher collagen adsorption with higher Ra, lowest CD 3 for highest Ra. | [6] | |
| 800 grit | Ra = 0.29 µm | |||||||
| 1200 grit | Ra = 0.15 µm | |||||||
| AZ91 | - | EIS 8 (0.785 cm2) | SBF 12 | 12 h | 120 grit | Sa = 0.022 µm | passivation layers on smoother surfaces last longer | [71] |
| Polishing: 3 µm | Sa = 0.973 µm | |||||||
| - | PDP 7(0.75 cm2) | 0.5 wt.% NaCl | - | 320 grit | Sa = 0.430 µm | icorr = 6.92 µA cm−2 | [96] | |
| 600 grit | Sa = 0.248 µm | icorr = 4.79 µA cm−2 | ||||||
| 1200 grit | Sa = 0.145 µm | icorr = 3.73 µA cm−2 | ||||||
| Polishing: 3 µm | Sa= 0.08 µm | icorr = 2.19 µA cm−2 | ||||||
| Disk | PDP 7 (1 cm2) | PBS 9 | - | 1200 grit | Sa = 29.76 ± 12.69 nm | icorr = 36.6 ± 3.2 µA cm−2 | [103] | |
| CR 10 = 0.78 ± 0.07 mm/y | ||||||||
| Cytotoxicity | α-MEM 11 | 21 d | 1200 grit | Sa = 29.76 ± 12.69 nm | * Cell survival: 87 % | |||
| M-4Y | Disk | Mass loss | DMEM 4 + 10% FBS 2 | 9.04 d | 1200 grit | Ra = 65 ± 31 nm | * 89.7 % | [21] |
| DI 6 Water | * 0.33 % | |||||||
| pH | DMEM 4 + 10% FBS 2 | 24 h | 1200 grit | Ra = 65 ± 31 nm | * pH = 8.32 | |||
| DI 6 Water | * pH = 9.00 | |||||||
| Cell adhesion | DMEM 4 + 10% FBS 2 | 1200 grit | Ra = 65 ± 31 nm | * 22.4 % | ||||
| ZK60A | Disk | PDP (1 cm2) | PBS 9 | - | 1200 grit | Sa = 78.30 ± 21.63 nm | icorr = 32.3 ± 2.6 µA cm−2 | [103] |
| CR 10 = 0.68 ± 0.01 mm/y | ||||||||
| Cytotoxicity | α-MEM 11 | 21 d | 1200 grit | Sa = 78.30 ± 21.63 nm | * Cell survival: 32 % | |||
| Mg-0.5Ca-6Zn | Rectangular prism | PDP 7 (1 cm2) | Kokubo | - | 2000 grit | Rq = 210 nm | icorr = 365 µA cm−2 | [104] |
| CR 10 = 8.34 mm/y | ||||||||
| Hydrogen | 10 d | 2000 grit | Rq = 210 nm | 4.92 mL/cm²/d | ||||
| WE43 | Plate | SBF 12 | - | Polishing: 1 µm | - | icorr = 642 ± 125 µA cm−2 | [105] | |
| Mg-1.0Ca | Rectangular prism | Mass loss | SBF 12 | 3 d | 1200 grit | Sa = 4.67 nm | * 9.63 mg | [88] |
| Cell viability | Extract DMEM 4 + 10% FBS 2 | 3 d + 4 h | 1200 grit | Sa = 4.67 nm | * 100% | |||
| EIS 8 (10 × 10 mm2) | SBF 12 | - | 1200 grit | Sa = 4.67 nm | icorr = 2.3 × 102 µA cm−2 | |||
| Mg-0.5Sr | Mass loss | SBF 12 | 3 d | 1200 grit | Sa = 2.16 nm | * 14.3 mg | ||
| Cell viability | Extract DMEM 4 + 10% FBS 2 | 3 d + 4 h | 1200 grit | Sa = 2.16 nm | * 100 % | |||
| EIS 8 (10 × 10 mm2) | SBF 12 | - | 1200 grit | Sa = 2.16 nm | icorr = 1.0 × 103 µA cm−2 |
| Alloy | Sample | Experiment | Solution | Time | Machining | Initial Roughness | Results | Ref |
|---|---|---|---|---|---|---|---|---|
| Mg | - | PDP 1 | HBSS 2 + HEPES 3 | 6 h | SFF 4 | Ra =0.59 ± 0.04 µm | icorr = 94.52 µAcm−2 | [72] |
| Ra = 2.68 ± 0.74 µm | icorr ~ 189.04 µAcm−2 | |||||||
| Ra = 9.12 ± 0.44 µm | icorr ~ 567.12 µAcm−2 | |||||||
| Mass loss | Ra =0.59 ± 0.04 µm | 2.74 mg cm−2 d−1 | ||||||
| Ra = 2.68 ± 0.74 µm | 28.43 mg cm−2 d−1 | |||||||
| Ra = 9.12 ± 0.44 µm | 130.12 mg cm−2 d−1 | |||||||
| Foil | Mass loss | DMEM 5 + 10% FBS 6 + P/S 7 | 80 d | Rolling | - | Max DR 8 = 1.2 mg cm−2 d−1 | [61] | |
| DI 9 Water | Max DR 8 = 0.14 mg cm−2 d−1 | |||||||
| Cell adhesion | DMEM 5 + 10% FBS 6 + P/S 7 | 24 h | Rolling | - | 13.6 % cell adhesion | |||
| AZ31 | Sheet | Hydrogen | 5 wt.% NaCl | 1.5 h | Milling | Ra = 2.02 µm | 54.23 mg/dcm² | [38] |
| 0.25 h | HT 10 + SB60 11 | 563.49 mg/dcm² | ||||||
| - | PDP 1 | 0.9 wt.% NaCl | - | SP 12 0.042 mmN | Ra = 1.58 µm | icorr = 416.17 µAcm−2 | [102] | |
| SP 12 0.140 mmN | Ra = 1.72 µm | icorr = 882.77 µAcm−2 | ||||||
| SP 12 0.262 mmN | Ra = 1.95 µm | icorr = 1136.5 µAcm−2 | ||||||
| Sheet | Hydrogen | 5 wt.% NaCl | 6.55 h | Rolling | - | * CR 13 = 7.17 mg cm−2 d−1 | [95] | |
| Plate | PDP 1 | 3.5 wt.% | - | SB40 14 | - | icorr = 2.1 µA cm−2 | [80] | |
| M-4Y | Disk | 217 h | EDM 15 | Ra = 196 ±47 nm | * 75.2 % | [21] | ||
| DI 9 Water | * 45.9 % | |||||||
| 24 h | EDM 15 | Ra = 196 ±47 nm | * pH = 8.48 | |||||
| DI 9 Water | * pH = 8.98 | |||||||
| Cell adhesion | DMEM 5 + 10% FBS 6 + P/S 7 | EDM 15 | Ra = 196 ±47 nm | * 7.82 % | ||||
| Mg-3.0Ca | Cylinder | Mass loss From hydrogen generation | 0.9 wt.% NaCl | 93 h | Turning: ap = 0.5 mm, vc = 10 m/min, f = 0.1 mm | * Rz = 4.48 µm | * 0.89 g/cm² | [108] |
| Turning: ap = 0.5 mm, vc = 100 m/min, f = 0.1 mm | * Rz = 3.75 µm | * 1.35 g/cm² | ||||||
| Turning: ap = 0.5 mm, vc = 100 m/min, f = 0.05 mm | * Rz = 2.17 µm | * 1.29 g/cm² | ||||||
| 240 h | Deep Rolling 16: Fr = 50 N | * Rz = 1.26 µm | * 0.07 g/cm² | |||||
| Deep Rolling 16: Fr = 200 N | * Rz = 0.91 µm | * 0.02 g/cm² | ||||||
| Deep Rolling 16: Fr = 500 N | * Rz = 1.26 µm | * 0.02 g/cm² | ||||||
| Cylinder | Hydrogen evolution | 0.9 wt.% NaCl | 29 h | Turning: ap = 200 µm, vc = 100 m/min, f = 0.1 mm | * Rz = 3.98 µm | * 20.2 mL/cm² | [111] | |
| Deep Rolling 16: Fr = 50 N | * Rz = 0.63 µm | * 1.27 mL/cm² | ||||||
| Deep Rolling 16: Fr = 200 N | * Rz = 0.47 µm | * 0.76 mL/cm² | ||||||
| µ-CT | 0.9 wt.% NaCl | 29 h | Turning: ap = 200 µm, vc = 100 m/min, f = 0.1 mm | * Rz = 3.98 µm | * PV 17 = 19.6 mL | |||
| Deep Rolling 16: Fr = 50 N | * Rz = 0.63 µm | * PV 17 = 1.44 mL | ||||||
| Deep Rolling 16: Fr = 200 N | * Rz = 0.47 µm | * PV 17 = 1.05mL | ||||||
| Mg-0.8Ca | Cylinder | Hydrogen evolution | 0.9 wt.% NaCl | 29 h | Turning: ap = 200 µm, vc = 100 m/min, f = 0.1 mm | * Rz = 4.00 µm | * 6.18 mL/cm² | [111] |
| Deep Rolling 16: Fr = 50 N | * Rz = 0.44 µm | * 5.42 mL/cm² | ||||||
| Deep Rolling 16: Fr = 200 N | * Rz = 0.76 µm | * 6.22 mL/cm² | ||||||
| µ-CT | 0.9 wt.% NaCl | 29 h | Turning: ap = 200 µm, vc = 100 m/min, f = 0.1 mm | * Rz = 4.00 µm | * PV 17 = 16.3 mL | |||
| Deep Rolling 16: Fr = 50 N | * Rz = 0.44 µm | * PV 17 = 12.1 mL | ||||||
| Deep Rolling 16: Fr = 200 N | * Rz = 0.76 µm | * PV 17 = 6.71 mL | ||||||
| Rabbit, µ-CT | - | 3 and 6 months | Turning | Ra = 3.65 µm | Turning lead to the lowest gas evolution and decomposition | [93] | ||
| Sand milling | Ra = 32.7 µm | |||||||
| Threading | - | |||||||
| Mg-5Gd | Disk | Mass loss | DMEM 5 + 10% FBS 6 + P/S 7 | 30 d | Milling | Sa =1.6 µm | CR 13 = 0.50 µm/d | [57] |
| Alloy | Sample | Experiment | Solution | Time | Acid Etching | Initial Roughness | Results | Ref |
|---|---|---|---|---|---|---|---|---|
| AZ31 | Sheet | SST 1 | 5 wt.% NaCl | 48 h | 50 g/L H2SO4 2, 15s | * Ra = 0.98 µm | CR 3 = 2.20 ± 0.18 mm/y | [56] |
| 80 g/L HNO3 4, 120 s | * Ra = 0.23 µm | CR 3 = 0.51 ± 0.10 mm/y | ||||||
| 80 g/L H3PO4 5, 60s | * Ra = 0.49 µm | CR 3 = 0.74 ± 0.31 mm/y | ||||||
| SST 1 | 5 wt.% NaCl | 48 h | 300 g/L CH3COOH 6, 120s | * Ra = 0.61 µm | CR 3 = 0.34 ± 0.08 mm/y | [40] | ||
| 80 g/L C2H2O4 7, 30s | * Ra = 0.48 µm | CR 3 = 0.59 ± 0.11 mm/y | ||||||
| 80 g/L C6H8O7 8, 60s | * Ra = 0.34 µm | CR 3 = 0.72 ± 0.07 mm/y | ||||||
| Hydrogen | 5 wt.% NaCl | ~48 h | 20% CH3COOH 6, 30 s | - | * CR 3 = 0.70 mg cm−2 d−1 | [95] | ||
| ~30 h | 50% H3PO4 5, 30 s | * CR 3 = 1.58 mg cm−2 d−1 | ||||||
| ~11 h | 3.3% HNO3 4, 20 s | * CR 3 = 4.59 mg cm−2 d−1 | ||||||
| ~23 h | 12% HF 9, 1200 s | * CR 3 = 1.68 mg cm−2 d−1 | ||||||
| Hydrogen | 5 wt.% NaCl | 24 h | HT 10 + 10 % H2SO4 2, 20 s | Ra = 2.50 µm | 0.97 mg/dcm² | [38] | ||
| Foil | Immersion | SBF 11 | 14 d | 90% H3PO4 5, 30 s | - | * CR 3 = 8.27 mg/d | [70] | |
| Mg-5Gd | Disk | Mass loss | DMEM 12 + 10% FBS 13 + P/S 14 | 30 d | 150 g/L CH3COOH 6, 150 s | Sa = 6.3 µm | CR 3 = 0.31 µm/d | [57] |
| 250 g/L CH3COOH 6, 150s | Sa = 5.6 µm | CR 3 = 0.30 µm/d | ||||||
| 300 g/L CH3COOH 6, 90 s | Sa = 2.3 µm | CR 3 = 0.30 µm/d |
| Alloy | Sample | Experiment | Solution | Time | Coatings | Initial Roughness | Results | Ref |
|---|---|---|---|---|---|---|---|---|
| Mg | Disk | pH | MEM 1 | 2 h | Polished + NaOH | Ra = 0.23 µm | pH = 7.88 | [74] |
| Polished + M-SBF 2 | Ra = 1.12 µm | pH = 8.96 | ||||||
| Cell viability | MEM 1 + FBS 3 | 24 h | Polished + NaOH | Ra = 0.23 µm | * CD 4 = 177 cells/mm2 | |||
| Polishing + M-SBF 2 | Ra = 1.12 µm | * CD 4 = 838 cells/mm2 | ||||||
| AZ31 | Foil | Immersion | SBF 4 | 2 weeks | 90% H3PO4 5, 30 s + Ca/P | - | * CR 6 = 7.27 mg/d | [70] |
| 90% H3PO4 5, 30 s + PLA 7 | * CR 6 = 6.17 mg/d | |||||||
| 90% H3PO4 5, 30 s + poly (DTH 8 carbonate) | * CR 6 = 3.83 mg/d | |||||||
| - | PDP 9 | 0.9 wt.% NaCl | - | P1000 Ground + DCPD 10 | Ra = 4.29 µm | icorr = 1.57 µA cm−2 | [102] | |
| SP 11 0.042 mmN + DCPD 10 | Ra = 2.89 µm | icorr = 20.03 µA cm−2 | ||||||
| SP 11 0.140 mmN + DCPD 10 | Ra = 3.25 µm | icorr = 21.96 µA cm−2 | ||||||
| SP 11 0.262 mmN + DCPD 10 | Ra = 5.07 µm | icorr = 38.12 µA cm−2 | ||||||
| disk | PDP 9 (1 cm2) | PBS 12 | - | 1200 grit + anodizing | Sa = 49.0 ± 10.2 nm | icorr = 2.72 ± 0.8 µA cm−2 | [103] | |
| CR 6 = 0.06 ± 0.01 mm/y | ||||||||
| Cytotoxicity | MEM 1 alpha modification Media | 21 d | 1200 grit + anodizing | Sa = 49.0 ± 10.2 nm | * Cell survival: 67 % | |||
| Plate | PDP 9 | 3.5 wt.% | - | SB 13 + Al ASC 14 | Ra = 11.6 µm | icorr = 2.4 × 102 µA cm−2 | [80] | |
| SB 13 + Al ASC 14 + PHP 15 (800 MPa) | Ra = 4.89 µm | - | ||||||
| SB 13 + Al ASC 14 + PHP 15 (1600 MPa) | - | icorr = 0.8 µA cm−2 | ||||||
| SB 13 + Al ASC 14 + PHP 15 (2000 MPa) | Ra = 1.12 µm | - | ||||||
| SB 13 + Al ASC 14 + PHP 15 + 7 wt.% oxalic acid anodizing | - | icorr = 3.7 × 10−2 µA cm−2 | ||||||
| AZ91 | Plate | PDP 9 | 3.5 wt.% NaCl | - | PEO 16 without K4P2O7 | - | icorr = 19.6 µA cm−2 | [73] |
| PEO 16 + 0.03 mol/L K4P2O7 | icorr = 1.22 × 10−2 µA cm−2 | |||||||
| PEO 16 + 0.06 mol/L K4P2O7 | icorr = 2.27 µA cm−2 | |||||||
| PEO 16 + 0.15 mol/L K4P2O7 | icorr = 4.77 µA cm−2 | |||||||
| Plate | PDP 9 | 3.5 wt.% NaCl | - | Polishing 0.5 µm Al2O3 + PEO 16 | Ra = 0.5 µm | icorr = 7.26 × 10−3 µA cm−2 | [81] | |
| 1000 grit + PEO 16 | Ra = 1.0 µm | icorr = 5.17 × 10−2 µA cm−2 | ||||||
| 100 grit + PEO 16 | Ra = 2.5 µm | icorr = 0.38 µA cm−2 | ||||||
| Plate | 1000 grit + PEO 16 without KF 17 | - | Rp = 8.28 × 103 mΩm² | [79] | ||||
| 1000 grit + PEO + KF 17 | Rp = 4.67 × 103 mΩm² | |||||||
| disk | PDP 9 (1 cm2) | PBS 12 | - | 1200 grit + anodized | Sa = 204.8 ± 62.7 nm | icorr = 2.50 ± 0.5 µA cm−2 | [103] | |
| CR 6 = 0.05 ± 0.01 mm/y | ||||||||
| Cytotoxicity | MEM 1 alpha modification Media | 21 d | 1200 grit + anodized | Sa = 204.8 ± 62.7 nm | * Cell survival: 102 % | |||
| ZK60A | disk | PDP 9 (1 cm2) | PBS 12 | - | 1200 grit + anodizing | Sa = 75.88 ± 34.49 nm | icorr = 1.86 ± 0.2 µA cm−2 | [103] |
| CR 6 = 0.04 ± 0.01 mm/y | ||||||||
| Cytotoxicity | MEM alpha modification Media | 21 d | 1200 grit + anodizing | Sa = 75.88 ± 34.49 nm | * Cell survival: 30 % | |||
| Mg-0.5Ca-6Zn | Rectangular prism | PDP 9 (1 cm2) | Kokubo | - | 2000 grit + 40% HF 18 | Rq = 280 nm | icorr = 6.20 µA cm−2 | [104] |
| CR 6 = 0.14 mm/y | ||||||||
| 2000 grit + DCPD 10/MgF2 | Rq = 395 nm | icorr = 5.72 µA cm−2 | ||||||
| CR 6 = 0.13 mm/y | ||||||||
| 2000 grit + HA/MgF2 | Rq = 468 nm | icorr = 5.23 µA cm−2 | ||||||
| CR 6 = 0.11 mm/y | ||||||||
| Hydrogen | Kokubo | 240 h | 2000 grit + 40% HF 18 | Rq = 280 nm | 1.31 mL/cm²/d | |||
| 2000 grit + DCPD 10/MgF2 | Rq = 395 nm | 1.12 mL/cm²/d | ||||||
| 2000 grit + nano-(HA 19/MgF2) | Rq = 468 nm | 0.85 mL/cm²/d |
| Alloy | Sample | Experiment | Solution | Time | Implantation | Initial roughness | Results | Ref |
|---|---|---|---|---|---|---|---|---|
| WE43 | Plate | PDP 1 | SBF 2 | - | Polishing: 1 µm + Si ion plasma | - | icorr = 27 ± 32 µA cm−2 | [105] |
| Mg-1.0Ca | Rectangular prism | Mass loss | SBF 2 | 3 d | 1200 grit + Zr | Sa = 5.34 nm | * 8.03 mg | [88] |
| 1200 grit + ZrO | Sa = 9.42 nm | * 6.77 mg | ||||||
| Cell viability | Extract assay (DMEM 3) | 24 h + 72 h + 4 h | 1200 grit + Zr | Sa = 5.34 nm | * 101 % | |||
| 1200 grit + ZrO | Sa = 9.42 nm | * 103 % | ||||||
| EIS 4 (10 × 10 mm2) | SBF 2 | - | 1200 grit + Zr | Sa = 5.34 nm | icorr = 1.2 × 102 µA cm−2 | |||
| 1200 grit + ZrO | Sa = 9.42 nm | icorr = 2.6 × 101 µA cm−2 | ||||||
| Mg-0.5Sr | Rectangular prism | Mass loss | SBF 2 | 3 d | 1200 grit + Zr | Sa = 4.61 nm | * 13.7 mg | [88] |
| 1200 grit + ZrO | Sa = 7.29 nm | * 8.52 mg | ||||||
| Cell viability | Extract assay (DMEM 3) | 24 h + 72 h + 4 h | 1200 grit + Zr | Sa = 4.61 nm | * 110 % | |||
| 1200 grit + ZrO | Sa = 7.29 nm | * 126 % | ||||||
| EIS 4 (10 × 10 mm2) | SBF 2 | - | 1200 grit + Zr | Sa = 4.61 nm | icorr = 2.5 × 102 µA cm−2 | |||
| 1200 grit + ZrO | Sa = 7.29 nm | icorr = 1.7 × 102 µA cm−2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawlik, M.M.; Wiese, B.; Desharnais, V.; Ebel, T.; Willumeit-Römer, R. The Effect of Surface Treatments on the Degradation of Biomedical Mg Alloys—A Review Paper. Materials 2018, 11, 2561. https://doi.org/10.3390/ma11122561
Gawlik MM, Wiese B, Desharnais V, Ebel T, Willumeit-Römer R. The Effect of Surface Treatments on the Degradation of Biomedical Mg Alloys—A Review Paper. Materials. 2018; 11(12):2561. https://doi.org/10.3390/ma11122561
Chicago/Turabian StyleGawlik, Marcjanna Maria, Björn Wiese, Valérie Desharnais, Thomas Ebel, and Regine Willumeit-Römer. 2018. "The Effect of Surface Treatments on the Degradation of Biomedical Mg Alloys—A Review Paper" Materials 11, no. 12: 2561. https://doi.org/10.3390/ma11122561





