Effect of Titanium Implants Coated with Radiation-Crosslinked Collagen on Stability and Osseointegration in Rat Tibia
Abstract
:1. Introduction
2. Results
2.1. In Vitro Results
2.1.1. Observations of Collagen Coated onto Ti Surface
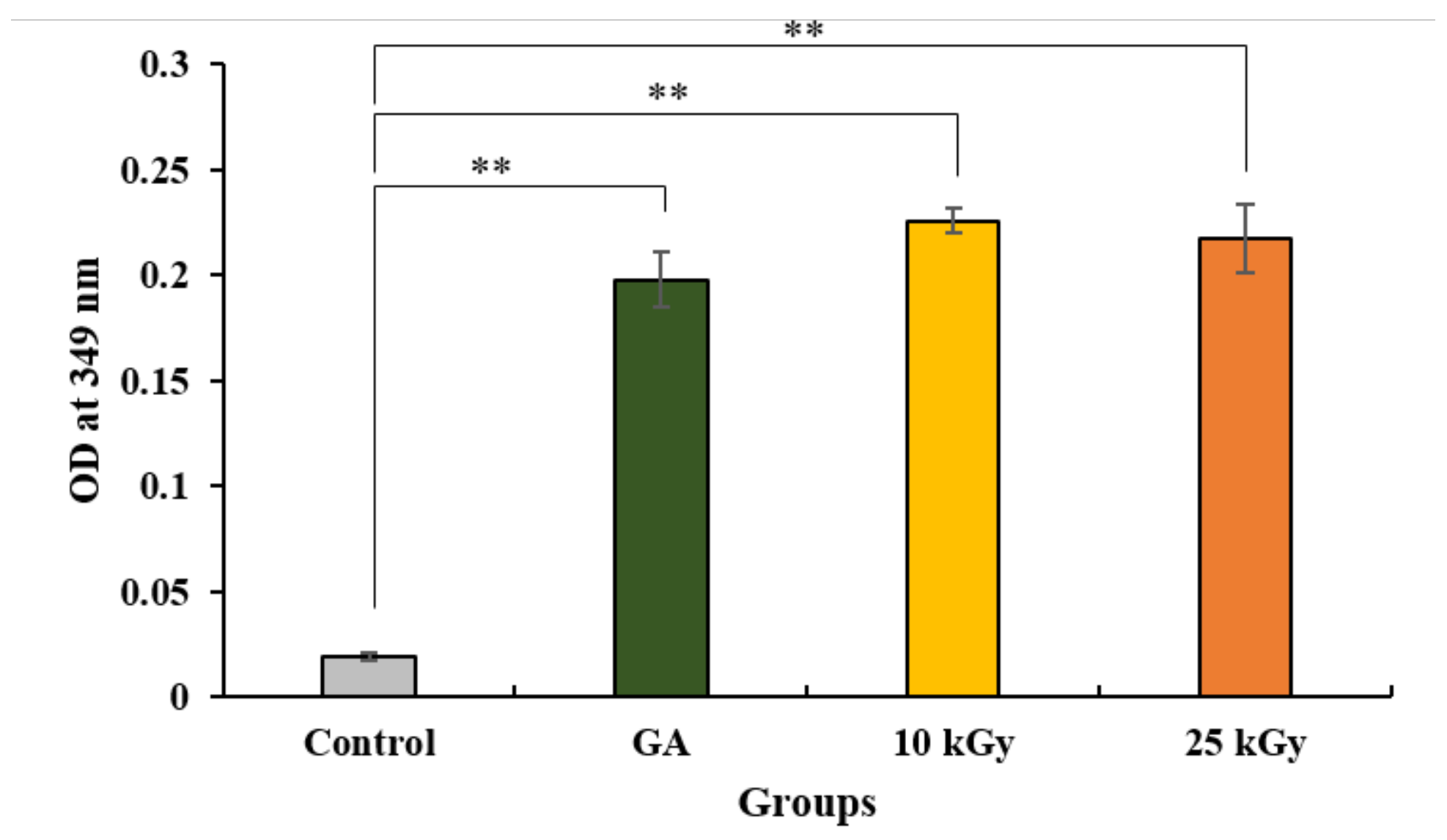
2.1.2. Quantification of Crosslinked Collagen Coated on Ti
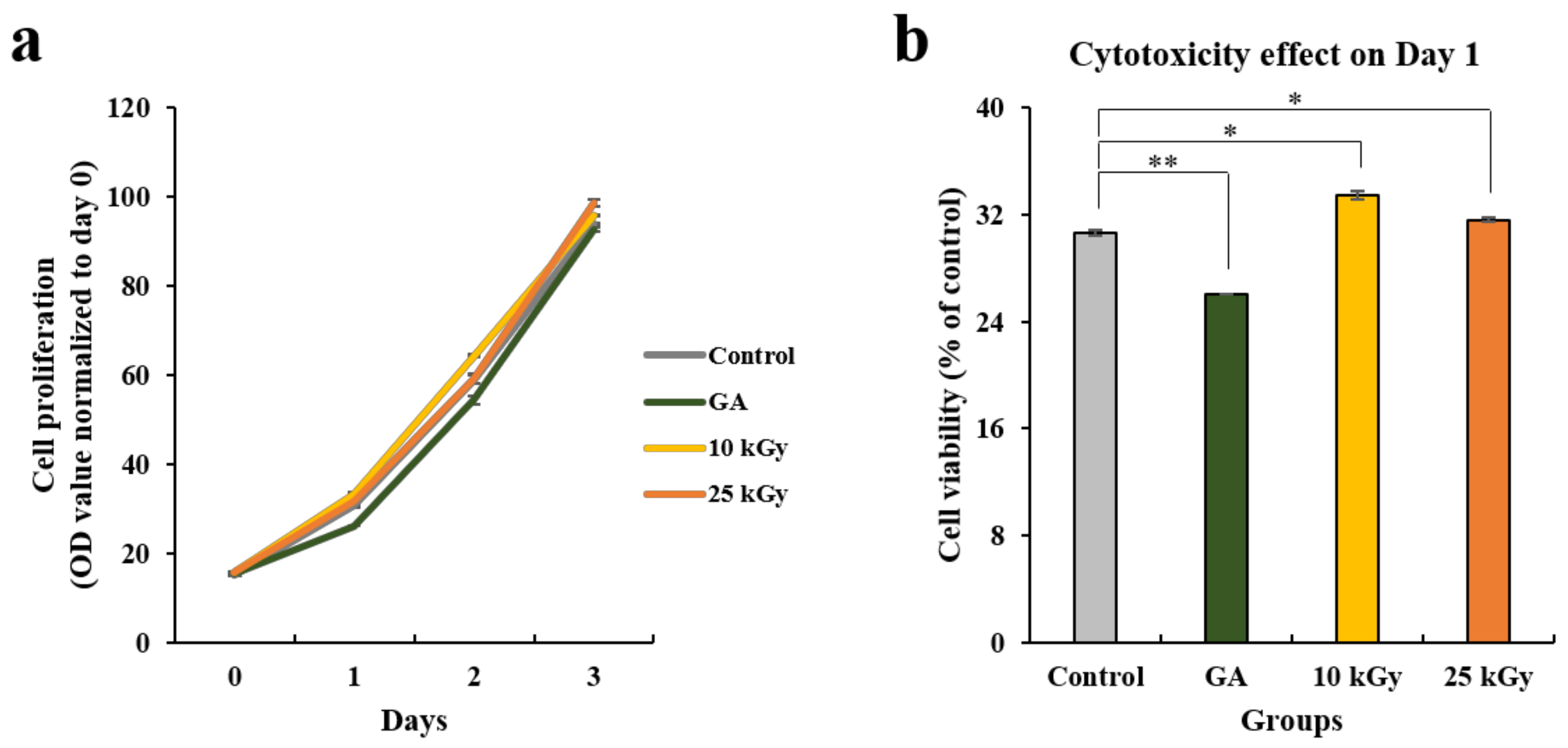
2.1.3. Cell Proliferation and Toxicity
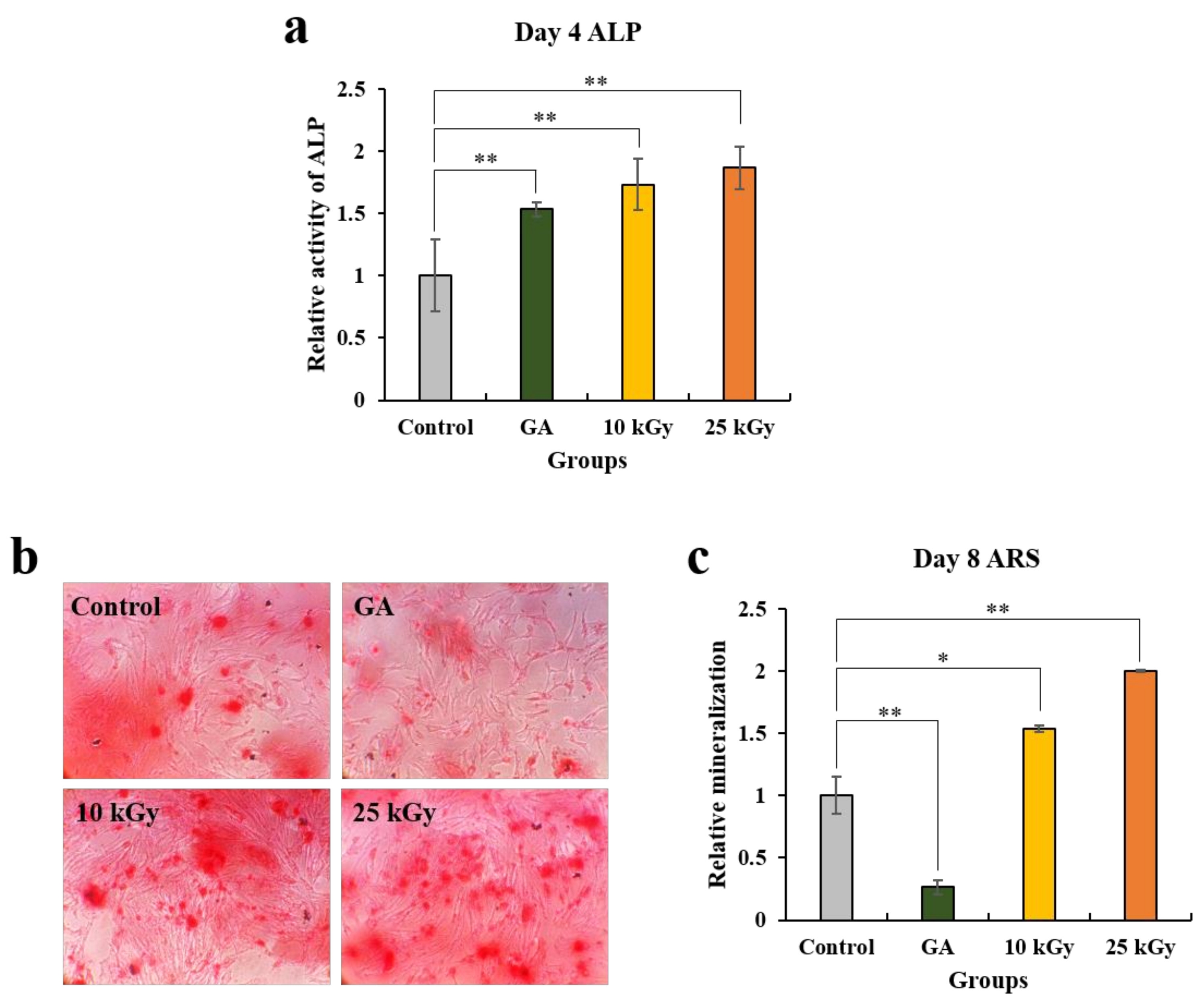
2.1.4. Analysis of Alkaline Phosphatase (ALP) Activity Assay and Alizarin Red S (ARS) Staining
2.1.5. Analysis of Real-time Polymerase Chain Reaction (PCR)
2.2. In Vivo Results
2.2.1. Clinical Findings
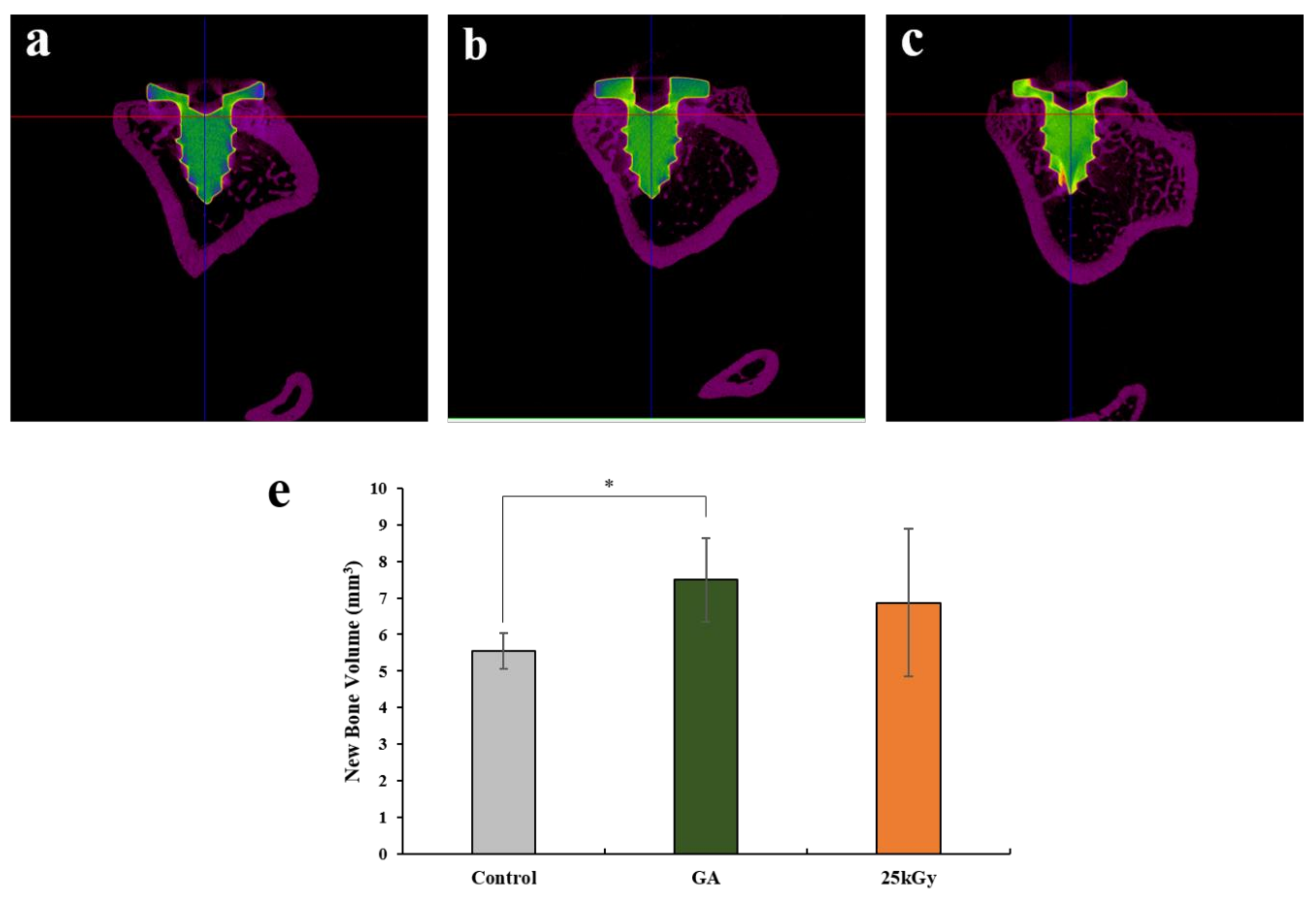
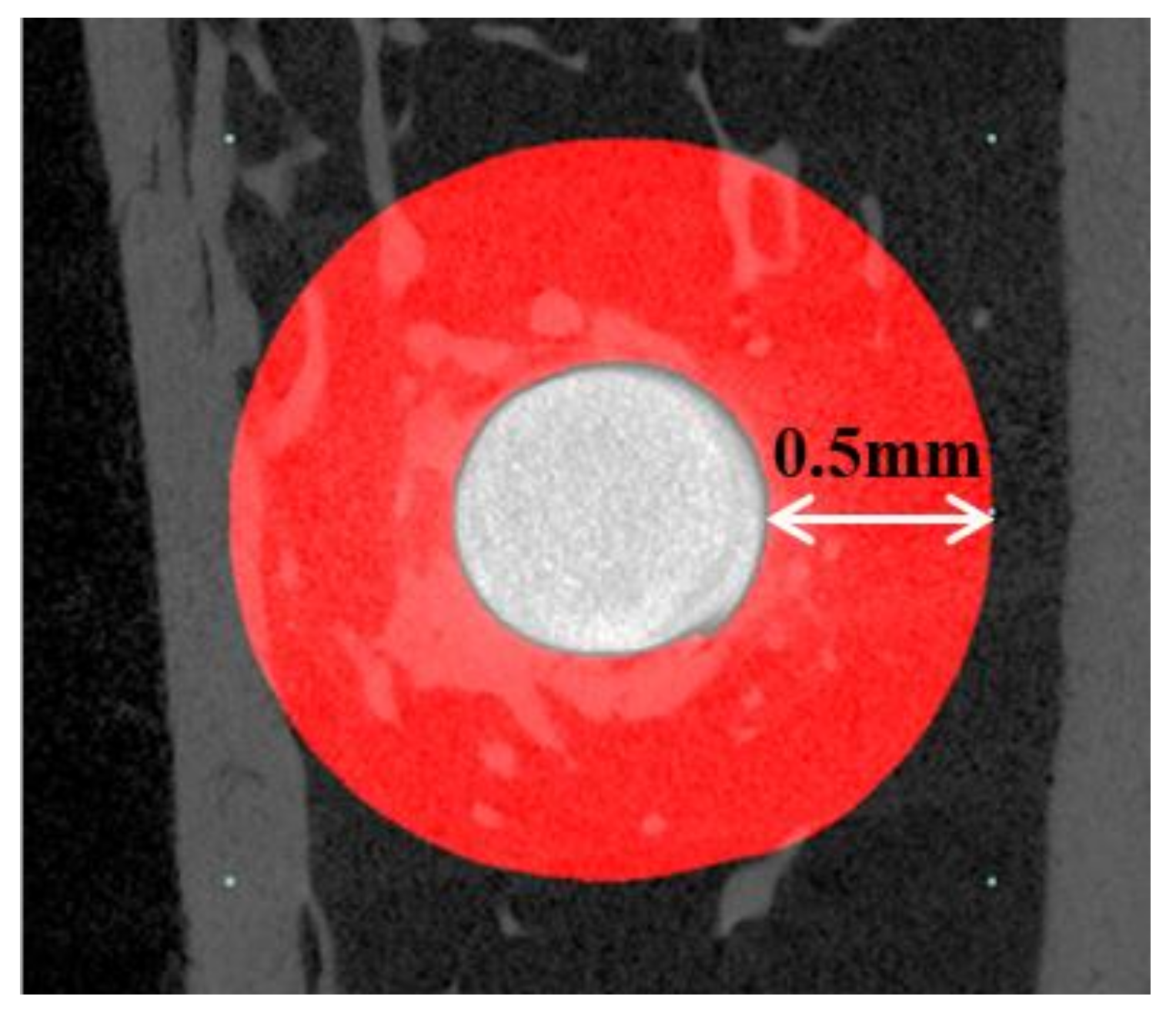
2.2.2. Micro-Computed Tomography (μCT) Findings
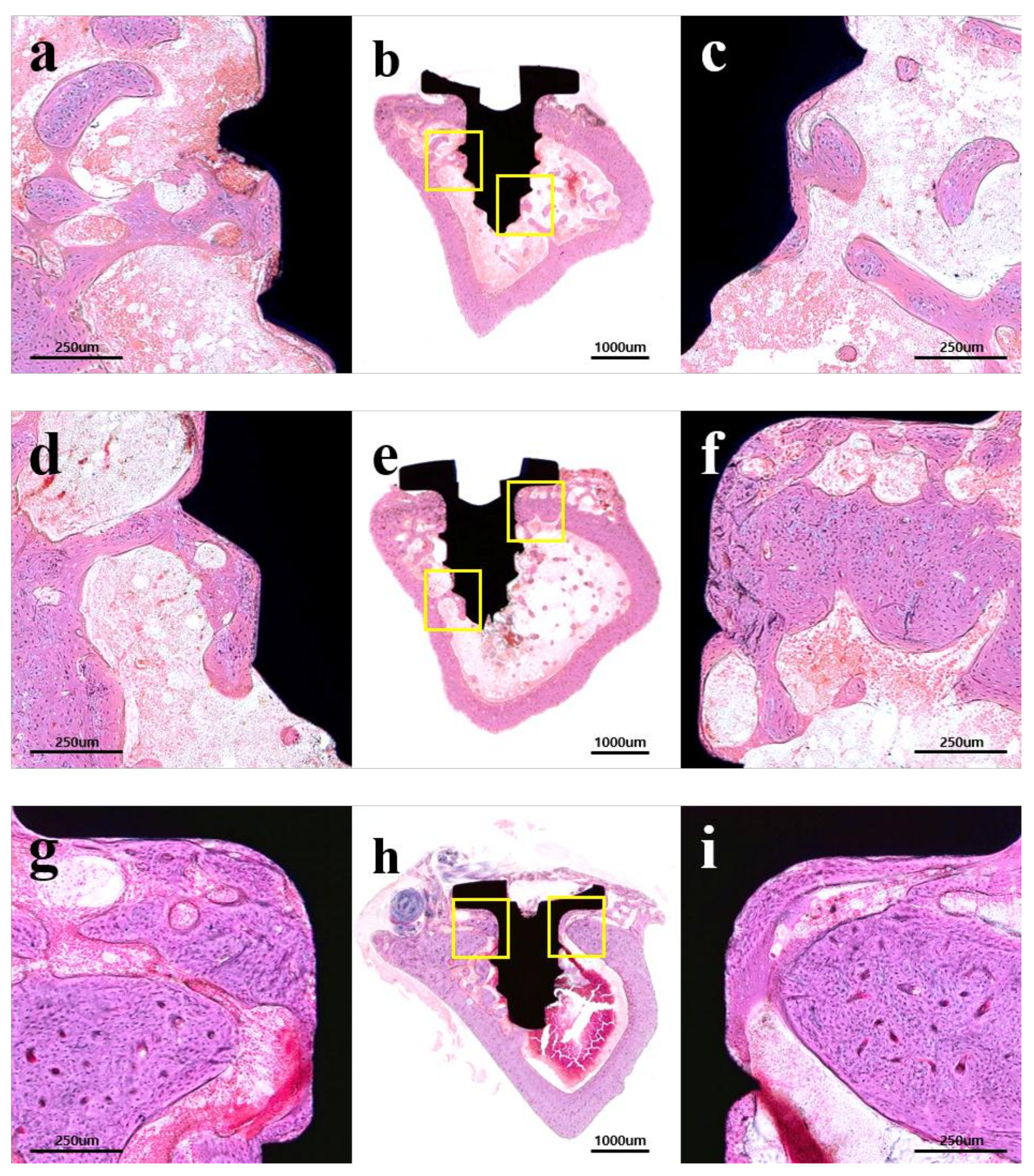
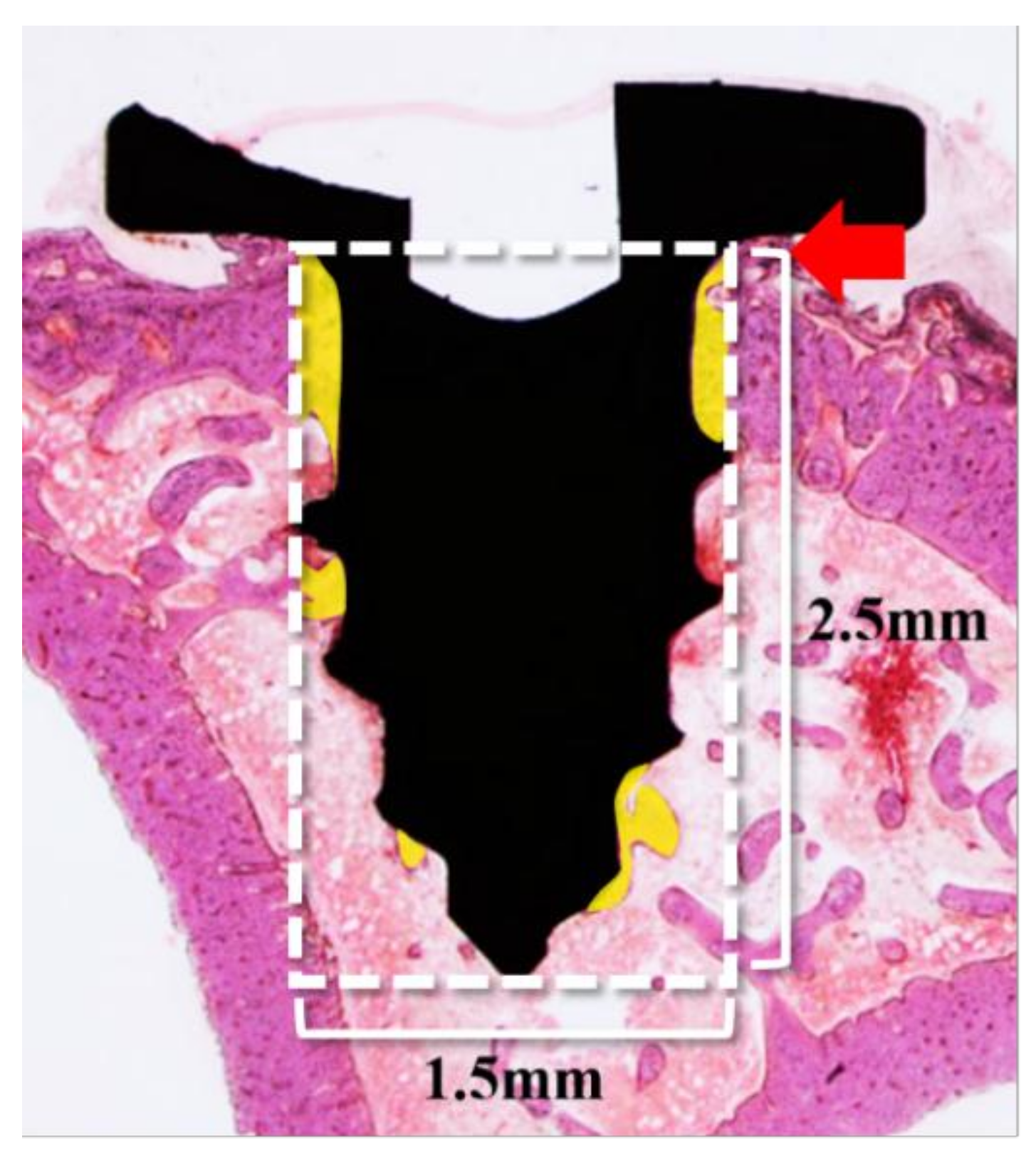
2.2.3. Histological Findings
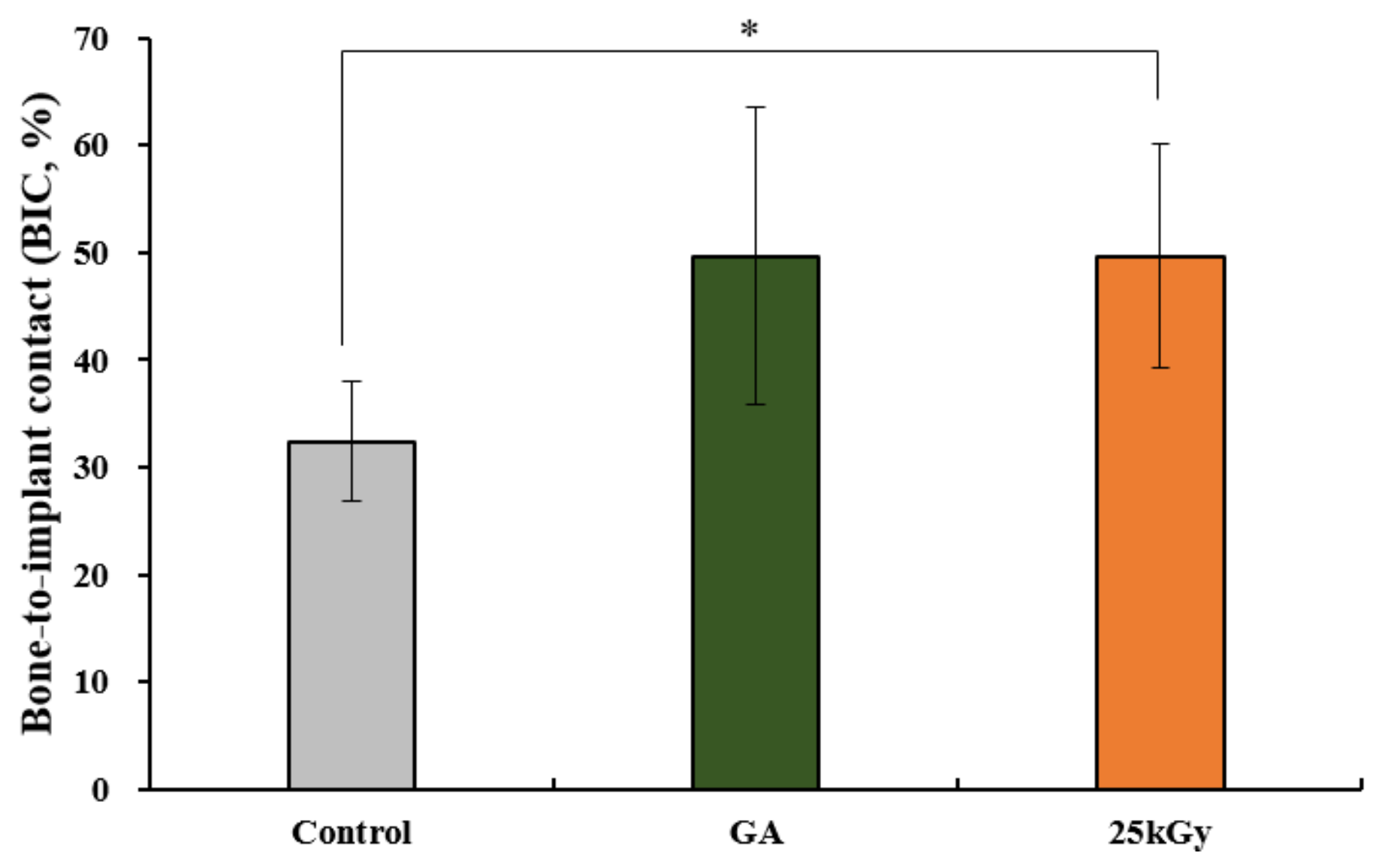
2.2.4. Histometric Findings
3. Discussion
4. Materials and Methods
4.1. Preparation of Crosslinked Collagen Coated Ti Implants
4.2. In Vitro Analysis
4.2.1. Scanning Electron Microscopy (SEM)
4.2.2. Trinitrobenzensulonic Acid (TNBS) Assay
4.2.3. Cell Culture
4.2.4. Cell Proliferation and Toxicity Assay
4.2.5. Alkaline Phosphatase (ALP) Activity Assay and Alizarin Red S (ARS) Staining
4.2.6. Real-Time Polymerase Chain Reaction (PCR) Analysis
4.3. In Vivo Analysis
4.3.1. Experimental Animals
4.3.2. Surgical Procedures
4.3.3. Micro-Computed Tomography (μCT) Analysis
4.3.4. Histology Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BIC | Bone-to-implant contact |
| Ti | Titanium |
| GA | Glutaraldehyde |
| ROI | Region of interest |
| SEM | Scanning electron microscopic |
| ALP | Alkaline phosphatase |
| ARS | Alizarin Red S |
| Real-time PCR | Real-time polymerase chain reaction |
| H&E | Hematoxylin-eosin |
| MTT | 3-[45-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide |
| μCT | Micro-computed tomography |
| ANOVA | Analysis of variance |
| RT | Room temperature |
| CMC | Carboxymethyl cellulose |
| MC | Methylcellulose |
References
- Dard, M.; Sewing, A.; Meyer, J.; Verrier, S.; Roessler, S.; Scharnweber, D. Tools for tissue engineering of mineralized oral structures. Clin. Oral. Investig. 2000, 4, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.C.; Lang, N.P.; Giannobile, W.V. Evaluation of functional dynamics during sseointegration and regeneration associated with oral implants. Clin. Oral Implants Res. 2010, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.T.; Han, I.H.; Huh, J.B.; Kang, J.K.; Ryu, J.J. Review of the developmental trend of implant surface modification using organic biomaterials. J. Korean Acad. Prosthodont. 2011, 49, 254–262. [Google Scholar] [CrossRef]
- Stenzel, K.H.; Miyata, T.; Rubin, A.L. Collagen as a biomaterial. Annu. Rev. Biophys. Bioeng. 1974, 3, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Chvapil, M. Collagen sponge: Theory and practice of medical applications. J. Biomed. Mater. Res. 1997, 11, 721–741. [Google Scholar] [CrossRef]
- Mao, C.; Kisaalita, W.S. Characterization of 3-D Collagen Hydrogels for Functional Cell-based Biosensing. Biosens. Bioelectron. 2004, 19, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Kim, S.Y.; Chun, T.; Byun, H.J.; Lee, Y.M. Collagen Scaffolds Derived from a Marine Source and Their Biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef]
- Costa, D.G.; Ferraz, E.P.; Abuna, R.P.; de Oliveira, P.T.; Morra, M.; Beloti, M.M.; Rosa, A.L. The effect of collagen coating on titanium with nanotopography on in vitro osteogenesis. J. Biomed. Mater. Res. A 2017, 105, 2783–2788. [Google Scholar] [CrossRef]
- Nagai, M.; Hayakawa, T.; Fukatsu, A.; Yamamoto, M.; Fukumoto, M.; Nagahama, F.; Kato, T. In vitro study of collagen coating of titanium implants for initial cell attachment. Dent. Mater. 2002, 21, 250–260. [Google Scholar] [CrossRef]
- Sverzut, A.T.; Crippa, G.E.; Morra, M.; de Oliveira, P.T.; Beloti, M.M.; Rosa, A.L. Effects of type I collagen coating on titanium osseointegration: Histomorphometric, cellular and molecular analyses. Biomed. Mater. 2012, 7, 035007. [Google Scholar] [CrossRef] [PubMed]
- De Assis, A.F.; Beloti, M.M.; Crippa, G.E.; de Oliveira, P.T.; Morra, M.; Rosa, A.L. Development of the osteoblastic phenotype in human alveolar bone-derived cells grown on a collagen type I-coated titanium surface. Clin. Oral Implants Res. 2009, 20, 240–246. [Google Scholar] [CrossRef]
- Yamanouchi, K.; Satomura, K.; Gotoh, Y.; Kitaoka, E.; Tobiume, S.; Kume, K.; Nagayama, M. Bone formation by transplanted human osteoblasts cultured within collagen sponge with dexamethasone in vitro. J. Bone Miner. Res. 2001, 16, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Casser-Bette, M.; Murray, A.B.; Closs, E.I.; Erfle, V.; Schmidt, J. Bone formation by osteoblast-like cells in a three-dimensional cell culture. Calcif. Tissue Int. 1990, 46, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Morra, M.; Cassinelli, C.; Meda, L.; Fini, M.; Giavaresi, G.; Giardino, R. Surface analysis and effects on interfacial bone microhardness of collagen-coated titanium implants: A rabbit model. Int. J. Oral Maxillofac. Implants 2005, 20, 23–30. [Google Scholar]
- Bernhardt, R.; van den Dolder, J.L.; Bierbaum, S.; Beutner, R.; Scharnweber, D.; Jansen, J.; Worch, H. Osteoconductive modifications of Ti-implants in a goat defect model: Characterization of bone growth with SR muCT and histology. Biomaterials 2005, 26, 3009–3019. [Google Scholar] [CrossRef]
- Chen, G.; Ushida, T.; Tateishi, T. Poly (DL-lactic-co-glycolic acid) sponge hybridized with collagen microsponges and deposited apatite particulates. J. Biomed. Mater. Res. 2001, 57, 8–14. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Luo, X.; Guo, Z.; He, P.; Chen, T.; Li, L.; Ding, S.; Li, H. Study on structure, mechanical property and cell cytocompatibility of electrospun collagen nanofibers crosslinked by common agents. Int. J. Biol. Macromol. 2018, 113, 476–486. [Google Scholar] [CrossRef]
- Zeeman, R.; Dijkstra, P.J.; van Wachem, P.B.; van Luyn, M.J.; Hendriks, M.; Cahalan, P.T.; Feijen, J. Successive epoxy and carbodiimide cross-linking of dermal sheep collagen. Biomaterials 1999, 20, 921–931. [Google Scholar] [CrossRef]
- Van Wachem, P.B.; Zeeman, R.; Dijkstra, P.J.; Feijen, J.; Hendriks, M.; Cahalan, P.T.; Van Luyn, M.J.A. Characterization and biocompatibility of epoxy-crosslinked dermal sheep collagens. Biomed. Mater. Symp. 1999, 47, 270–277. [Google Scholar] [CrossRef]
- Kim, Z.H.; Lee, Y.; Kim, S.M.; Kim, H.; Yun, C.K.; Choi, Y.S. A composite dermal filler comprising cross-linked hyaluronic acid and human collagen for tissue reconstruction. J. Microbiol. Biotechnol. 2015, 25, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Du, T.; Tang, X.; Liu, C.; Li, R.; Xu, C.; Tian, F.; Du, Z.; Wu, J. Comparison of the properties of collagen–chitosan scaffolds after γ-ray irradiation and carbodiimide cross-linking. J. Biomater. Sci. Polym. Ed. 2016, 27, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Vyavahare, N.R.; Jones, P.L.; Hirsch, D.; Schoen, F.J.; Levy, R.J. Prevention of glutaraldehyde-fixed bioprosthetic heart valve calcification by alcohol pretreatment: Further mechanistic studies. J. Heart Valve Dis. 2000, 9, 561–566. [Google Scholar] [PubMed]
- Berglund, J.D.; Mohseni, M.M.; Nerem, R.M.; Sambanis, A. A biological hybrid model for collagen-based tissue engineered vascular constructs. Biomaterials 2003, 24, 1241–1254. [Google Scholar] [CrossRef]
- Hardin-Young, J.; Carr, R.M.; Downing, G.J.; Condon, K.D.; Termin, P.L. Modification of native collagen reduces antigenicity but preserves cell compatibility. Biotechnol. Bioeng. 1996, 49, 675–682. [Google Scholar] [CrossRef]
- Lee, J.H.; Nho, Y.C.; Lim, Y.M.; Son, T.I. Prevention of surgical adhesions with barriers of carboxymethylcellulose and poly (ethylene glycol) hydrogels synthesized by irradiation. J. Appl. Polym. Sci. 2005, 96, 1138–1145. [Google Scholar] [CrossRef]
- Harjula, A.; Nickels, J.; Mattila, S. Histological study of gluytaraldehyde-processed vascular grafts of biological origin. Ann. Chir. Gynaecol. 1980, 6, 256–262. [Google Scholar]
- Cooke, A.; Oliver, R.F.; Edward, M. An in vitro cytotoxicity study of aldehyde-treated pig dermal collagen. Br. J. Exp. Pathol. 1983, 64, 172–176. [Google Scholar]
- Wach, R.A.; Mitomo, H.; Nagasawa, N.; Yoshii, F. Radiation crosslinking of methylcellulose and hydroxyethylcellulose in concentrated aqueous solutions. Nucl. Instr. Meth. Phys. Res. B 2003, 211, 533–544. [Google Scholar] [CrossRef]
- Chapiro, A. Radiation chemistry of polymers. Radiat. Res. Suppl. 1964, 4, 179–191. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Ulański, P. Synthesis of hydrogels by irradiation of polymers in aqueous solution. Radiat. Phys. Chem. 1999, 55, 139–151. [Google Scholar] [CrossRef]
- Leavitt, F.C. Crosslinking of cellulosics by high energy radiation II. J. Polym. Sci. 1961, 51, 349–357. [Google Scholar] [CrossRef]
- Rimdusit, S.; Somsaeng, K.; Kewsuwan, P.; Jubsilp, C.; Tiptipakorn, S. Comparison of Gamma Radiation Crosslinking and Chemical Crosslinking on Properties of Methylcellulose Hydrogel. Agric. Eng. J. 2012, 16, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Lim, Y.M.; Lee, J.H.; Nho, Y.C.; Son, T.I. Preparation of crosslinked carboxymethylcellulose (CMC) by 60 Co γ-ray irradiation and its biodegradable properties. J. Radiat. Ind. 2007, 1, 53–59. [Google Scholar]
- Puleo, D.A.; Nanci, A. Understanding and controlling the boneimplant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, L.; Huang, X.; Wei, S.; Zhai, M. Structural study and preliminary biological evaluation on the collagen hydrogel crosslinked by γ-irradiation. J. Orthop. Res. 2009, 100, 2960–2969. [Google Scholar] [CrossRef]
- Sionkowska, A.; Wisniewski, M.; Skopinska, M.J.; Poggi, G.; Marsano, E.; Maxwell, C.; Wess, T. Thermal and mechanical properties of UV irradiated collagen/chitosan thin films. Polym. Degrad. STable 2006, 91, 3026–3032. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, Y.; Yang, Z.; Wu, Z.; Huang, G.; Lin, L.; Zhang, X. Radiation curing of collagen/divinyl ether enhanced by pyridinium salts. J. Appl. Polym. Sci. 2005, 98, 2094–2100. [Google Scholar] [CrossRef]
- Cataldo, F.; Ursini, O.; Lilla, E.; Angelini, G. Radiation-induced crosslinking of collagen gelatin into a stable hydrogel. J. Radioanal. Nucl. Chem. 2008, 275, 125–131. [Google Scholar] [CrossRef]
- Yoshii, F.; Zhao, L.; Wach, R.A.; Nagasawa, N.; Mitomo, H.; Kume, T. Hydrogels of Polysaccharide Derivatives Crosslinked with Irradiation at Paste-like Condition. Nucl. Instrum. Methods Phys. Res. B 2003, 208, 320–324. [Google Scholar] [CrossRef]
- Liu, B.; Harrell, R.; Davis, R.H.; Dresden, M.H.; Spira, M. The effect of gamma irradiation on injectable human amnion collagen. J. Biomed. Mater. Res. 1989, 23, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Krampera, M.; Pizzolo, G.; Aprili, G.; Franchini, M. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone 2006, 39, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biometerials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivares-Navarrete, R.; Hyzy, S.L.; Hutton, D.L.; Erdman, C.P.; Wieland, M.; Boyan, B.D.; Schwartz, Z. Direct and indirect effects of micro-structured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials 2010, 31, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Raines, A.L.; Wieland, M.; Schwartz, Z.; Boyan, B.D. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials 2007, 28, 2821–2829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wall, I.; Donos, N.; Carlqvist, K.; Jones, F.; Brett, P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone 2011, 45, 17–26. [Google Scholar] [CrossRef]
- Bernhardt, R.; Kuhlisch, E.; Schulz, M.C.; Eckelt, U.; Stadlinger, B. Comparison of bone-implant contact and bone-implant volume between 2D-histological sections and 3D-SRμCT slices. Eur. Cells Mater. 2012, 23, 237–248. [Google Scholar] [CrossRef]
- Rammelt, S.; Schulze, E.; Bernhardt, R.; Hanisch, U.; Scharnweber, D.; Worch, H.; Biewener, A. Coating of titanium implants with type-I collagen. J. Orthop. Res. 2004, 22, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Schliephake, H.; Aref, A.; Scharnweber, D.; Bierbaum, S.; Roessler, S.; Sewing, A. Effect of immobilized bone morphogenic protein 2 coating of titanium implants on peri-implant bone formation. Clin. Oral Implants Res. 2005, 16, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.; Giavaresi, G.; Parrilli, A.; Ferrari, A.; Aldini, N.N.; Morra, M.; Cassinelli, C.; Bollati, D.; Fini, M. Collagen type I coating stimulates bone regeneration and osteointegration of titanium implants in the osteopenic rat. Int. Orthop. 2015, 39, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.; Langer, F. Nonparametric analysis of ordered categorical data in designs with longitudinal observations and small sample sizes. Biom. J. 2000, 42, 663–675. [Google Scholar] [CrossRef]



| Group | Mean ± SD | Median |
|---|---|---|
| Control | 5.55 ± 0.48 | 5.55 |
| GA | 7.50 ± 1.14 | 7.81 |
| 25 kGy | 6.87 ± 2.01 | 7.47 |
| p-value | 0.006 ** | |
| Group | Mean ± SD | Median |
|---|---|---|
| Control | 35.21 ± 3.98 | 35.60 |
| GA | 49.66 ± 13.84 | 54.22 |
| 25 kGy | 9.66 ± 10.47 | 50.19 |
| p-value | 0.019 * | |
| Target Genes | Sequences |
|---|---|
| Runx2 | F: 5′-TGCTTTGGTCTTGAAATCACA-3′ |
| R: 5′- TCTTAGAACAAATTCTGCCCTTT-3′ | |
| BMP-2 | F: 5′-AACACTGTGCGCAGCTTCC-3′ |
| R: 5′-CTCCGGGTTGTTTTCCCAC-3′ | |
| ALP | F: 5′-ATTTCTCTTGGGCAGGCAGAGAGT-3′ |
| R: 5′-ATCCAGAATGTTCCACGGAGGCTT-3′ | |
| OCN | F: 5′-CAGCGAGGTAGTGAAGAGAC-3′ |
| R: 5′-TGAAAGCCGATGTGGTCAG-3′ | |
| OPN | F: 5′-AGACACATATGATGGCCGAGG-3′ |
| R: 5′-GGCCTTGTATGCACCATTCAA-3′ | |
| Actin | F: 5′-ACTCTTCCAGCCTTCCTTCC-3′ |
| R: 5′-TGTTGGCGTACAGGTCTTTG-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, E.-B.; Yoo, J.-H.; Jeong, S.-I.; Kim, M.-S.; Lim, Y.-M.; Ahn, J.-J.; Lee, J.-J.; Lee, S.-H.; Kim, H.-J.; Huh, J.-B. Effect of Titanium Implants Coated with Radiation-Crosslinked Collagen on Stability and Osseointegration in Rat Tibia. Materials 2018, 11, 2520. https://doi.org/10.3390/ma11122520
Bae E-B, Yoo J-H, Jeong S-I, Kim M-S, Lim Y-M, Ahn J-J, Lee J-J, Lee S-H, Kim H-J, Huh J-B. Effect of Titanium Implants Coated with Radiation-Crosslinked Collagen on Stability and Osseointegration in Rat Tibia. Materials. 2018; 11(12):2520. https://doi.org/10.3390/ma11122520
Chicago/Turabian StyleBae, Eun-Bin, Ji-Hyun Yoo, Sung-In Jeong, Min-Su Kim, Youn-Mook Lim, Jong-Ju Ahn, Jin-Ju Lee, So-Hyoun Lee, Hyung-Joon Kim, and Jung-Bo Huh. 2018. "Effect of Titanium Implants Coated with Radiation-Crosslinked Collagen on Stability and Osseointegration in Rat Tibia" Materials 11, no. 12: 2520. https://doi.org/10.3390/ma11122520





