CVD Diamond Interaction with Fe at Elevated Temperatures
Abstract
:1. Introduction
2. Materials and Methods
- An initial 3 µm layer was evaporated with ion source assistance using an Ar+ ion energy of 500 eV.
- Subsequently, Fe deposition was carried out without ion source assistance. The total Fe film thickness was 10 ± 0.5 µm.
3. Results and discussion
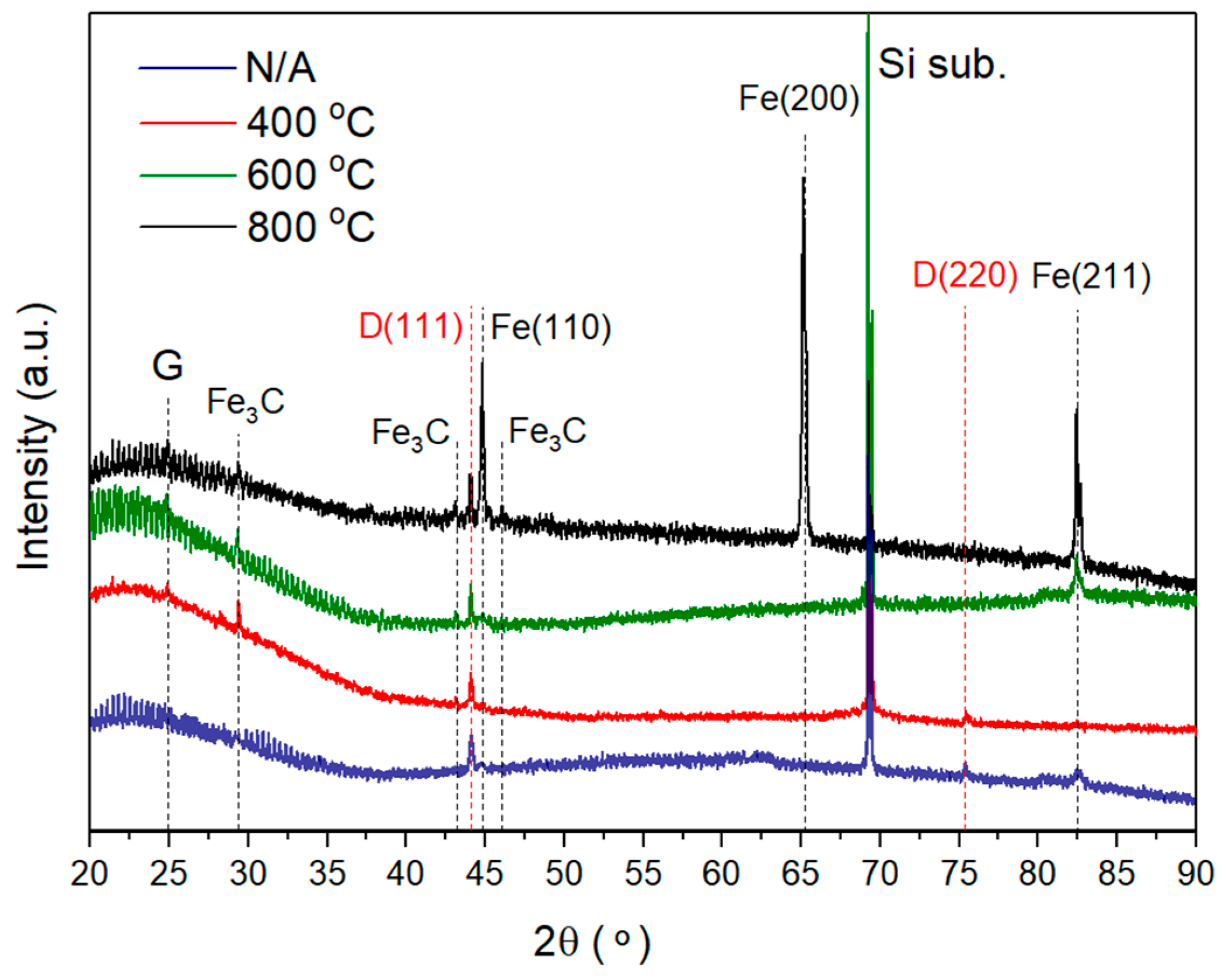
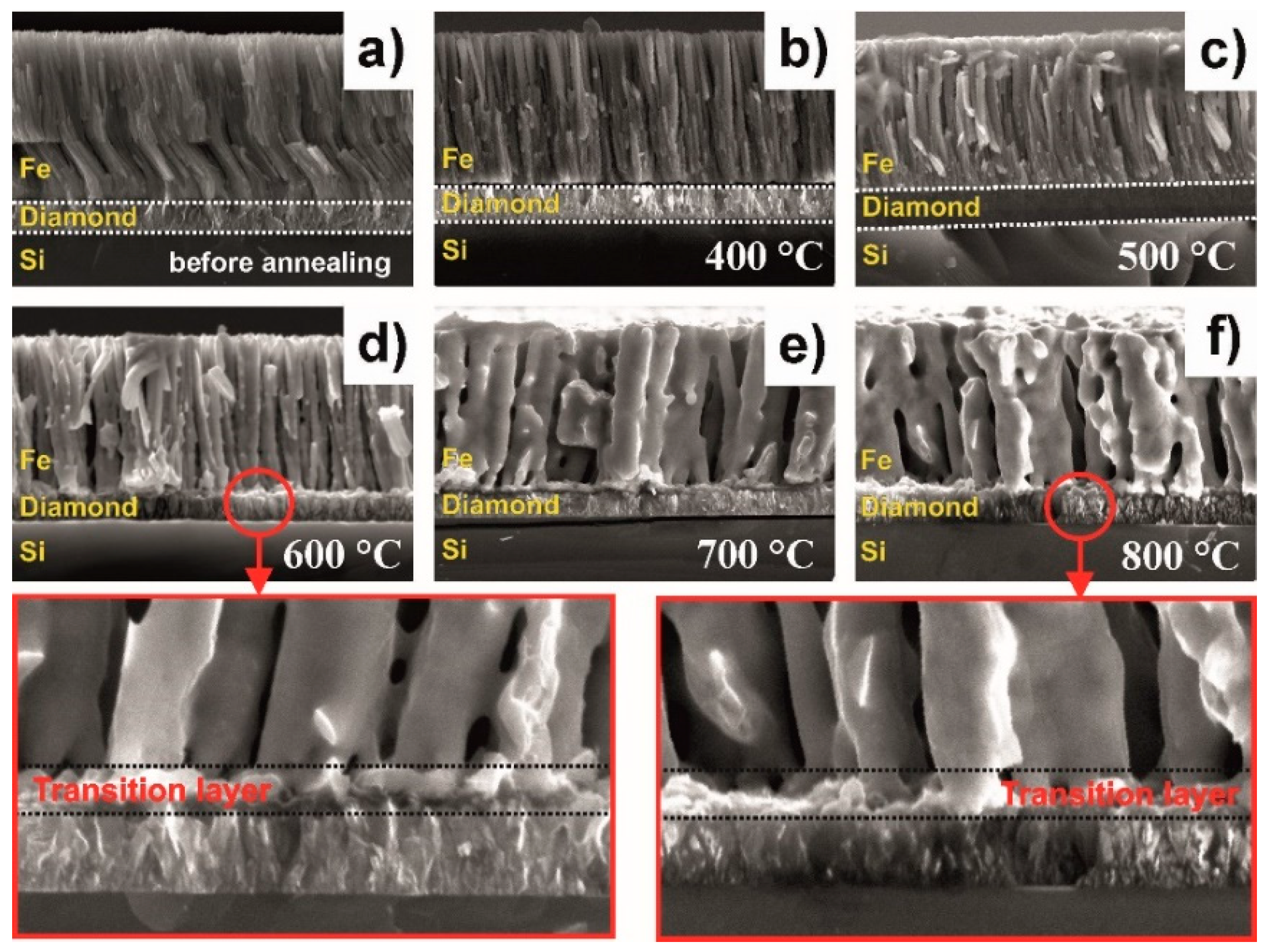
- Recrystallization of the thermally evaporated Fe film, starting at 600 °C, in agreement with XRD measurements.
- Formation of an Fe–C transition layer due to the interdiffusion that takes place in the diamond–Fe system in the temperature range 600–800 °C. The thickness of this transition layer is relatively low (approx. 0.5 µm) due to the limited ability of Fe3C to form. Diffusion of carbon is undetectable on the SEM cross-sections.
4. Conclusions
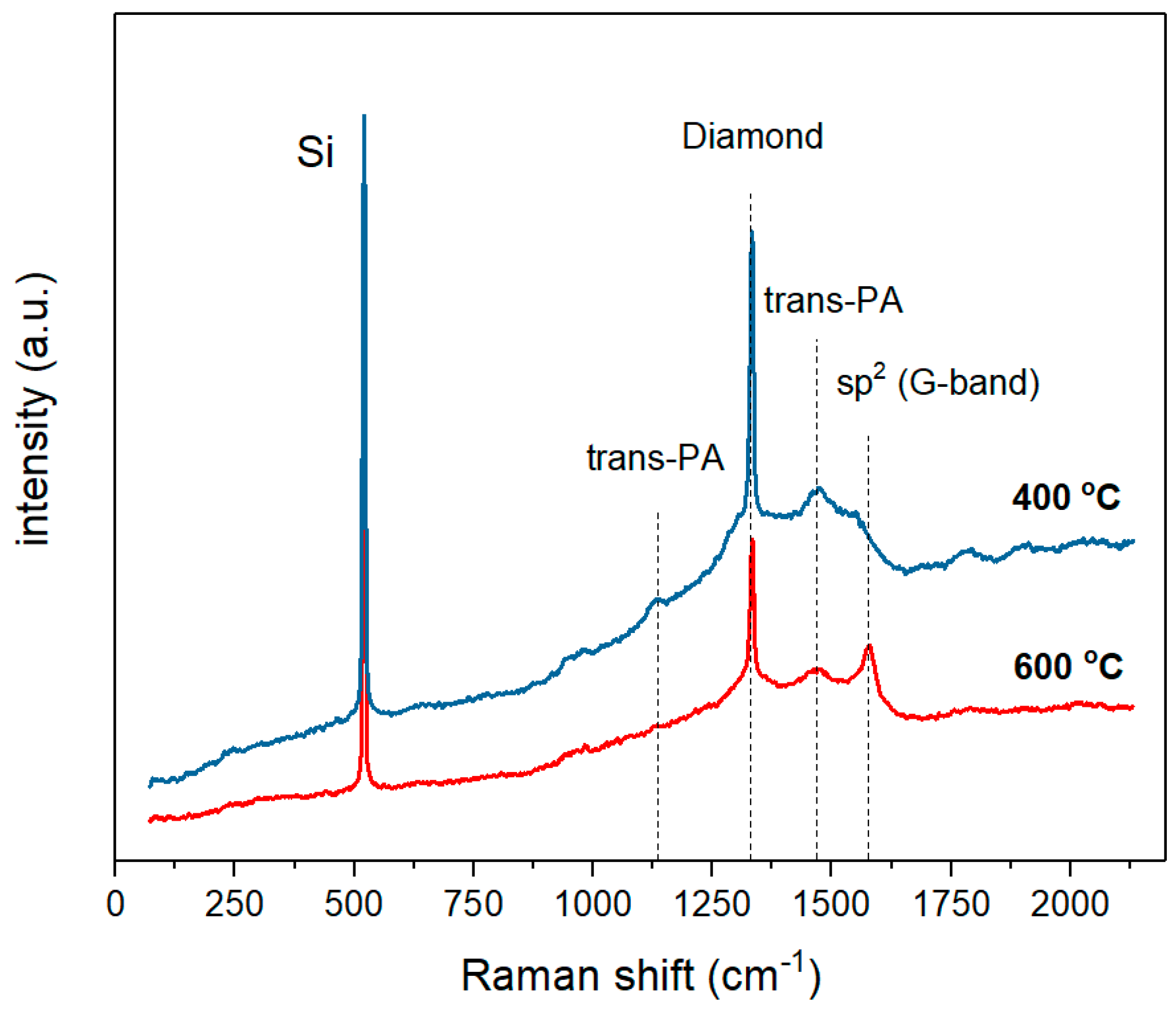
- The CVD diamond–Fe interaction in the range of 400–800 °C is significantly lower in comparison with previously reported data for the range of temperatures 900–1300 °C. Diamond etching and graphitization in the range of 400–800 °C are undetectable during microscopic measurements and can be detected only by XRD and Raman measurements.
- Annealing of the diamond in contact with Fe slightly reduces the quality parameter in the range of 98.7–99.4%, confirming a slight interaction of diamond–Fe at the selected temperature range.
- Strong diffusion of carbon into the Fe occurs even at low-temperature annealing conditions of 400 °C.
- Formation of an Fe–C transition layer due to the interdiffusion process in the diamond–Fe system can be detected in the temperature range 600–800 °C. The thickness of this transition layer is relatively low (approx. 0.5 µm) due to the limited ability of the Fe3C to form.
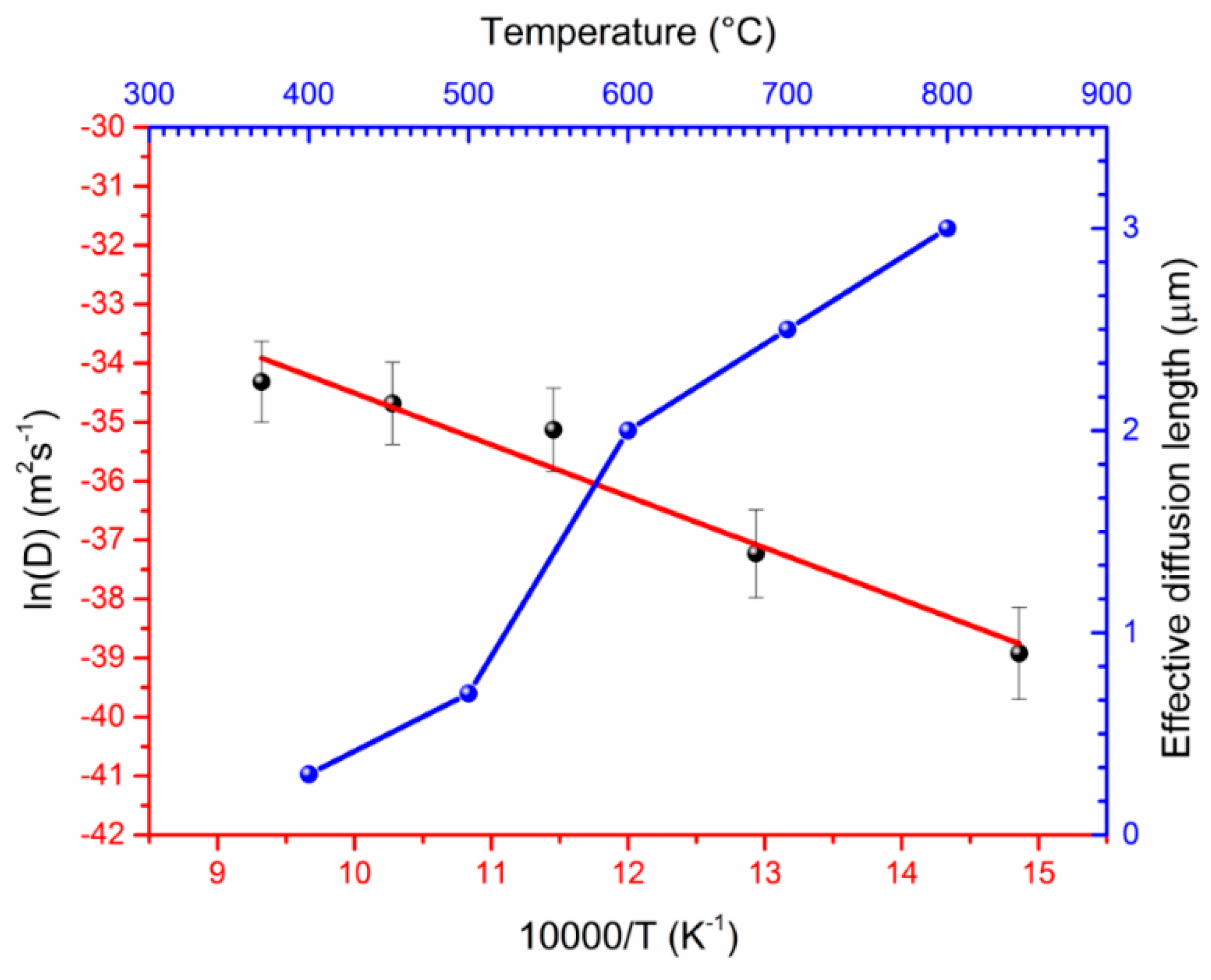
- When annealing at 600 °C, one can detect a diffusion of the Fe into the diamond film. The Fe diffusion coefficient was estimated from 1.25 × 10−17 m2 s−1 at 400 °C up to 1.25 × 10−15 m2 s−1 at 800 °C. The diffusion activation energy of the Fe was determined to be 69.1 kJ/mol.
Author Contributions
Funding
Conflicts of Interest
References
- Gracio, J.J.; Fan, Q.H.; Madaleno, J.C. Diamond growth by chemical vapour deposition. J. Phys. D Appl. Phys. 2010, 43, 374017. [Google Scholar] [CrossRef] [Green Version]
- Shimada, S.; Tanaka, H.; Higuchi, M.; Yamaguchi, T.; Honda, S.; Obata, K. Thermo-chemical wear mechanism of diamond tool in machining of ferrous metals. CIRP Ann. 2004, 53, 57–60. [Google Scholar] [CrossRef]
- Nakamura, E.; Hirakuri, K.K.; Ohyama, M.; Friedbacher, G.; Mutsukura, N. High quality chemical vapor deposition diamond growth on iron and stainless steel substrates. J. Appl. Phys. 2002, 92, 3393. [Google Scholar] [CrossRef]
- Mehedi, H.; Arnault, J.-C.; Eon, D.; Hébert, C.; Carole, D.; Omnes, F.; Gheeraert, E. Etching mechanism of diamond by Ni nanoparticles for fabrication of nanopores. Carbon 2013, 59, 448–456. [Google Scholar] [CrossRef]
- Zaitsev, A.M.; Kosaca, G.; Richarz, B.; Raiko, V.; Job, R.; Fries, T.; Fahrner, W.R. Thermochemical polishing of CVD diamond films. Diam. Relat. Mater. 1998, 7, 1108–1117. [Google Scholar] [CrossRef]
- Jin, S.; Graebner, J.E.; Tiefel, T.H.; Kammlott, G.W. Thinning and patterning of CVD diamond films by diffusional reaction. Diam. Relat. Mater. 1993, 2, 1038–1042. [Google Scholar] [CrossRef]
- Tokura, H.; Yang, C.; Yoshikawa, M. Study on the polishing of chemically vapour deposited diamond film. Thin Solid Films 1992, 212, 49–55. [Google Scholar] [CrossRef]
- Jin, S.; Graebner, J.E.; Kammlott, G.W.; Tiefel, T.H.; Kosinski, S.G.; Chen, L.H.; Fastnacht, R.A. Massive thinning of diamond films by a diffusion process. Appl. Phys. Lett. 1992, 60, 1948. [Google Scholar] [CrossRef]
- Ralchenko, V.G.; Kononenko, T.V.; Pimenov, S.M.; Chernenko, N.V.; Loubnin, E.N.; Armeyev, V.Y.; Zlobin, A.Y. Catalytic interaction of Fe, Ni and Pt with diamond films: Patterning applications. Diam. Relat. Mater. 1993, 2, 904–909. [Google Scholar] [CrossRef]
- Giménez, S.; Biest, O.v.d.; Vleugels, J. The role of chemical wear in machining iron based materials by PCD and PCBN super-hard tool materials. Diam. Relat. Mater. 2007, 16, 435–445. [Google Scholar] [CrossRef]
- Girolami, M.; Bellucci, A.; Calvani, P.; Orlando, S.; Valentini, V.; Trucchi, D.M. Raman investigation of femtosecond laser-induced graphitic columns in single-crystal diamond. Appl. Phys. A 2014, 117, 143–147. [Google Scholar] [CrossRef]
- Guillemet, T.; Xie, Z.Q.; Zhou, Y.S.; Park, J.B.; Veillere, A.; Xiong, W.; Heintz, J.M.; Silvain, J.F.; Chandra, N.; Lu, Y.F. Stress and phase purity analyses of diamond films deposited through laser-assisted combustion synthesis. ACS Appl. Mater. Interfaces 2011, 3, 4120–4125. [Google Scholar] [CrossRef]
- Verein Deutscher Eisenhüttenleute. Werkstoffkunde Stahl. Band 1: Grundlagen; Springer-Verlag GmbH: Berlin/Heidelberg, German, 1984. (In Germany) [Google Scholar]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenkin, S.; Gaydaychuk, A.; Okhotnikov, V.; Linnik, S. CVD Diamond Interaction with Fe at Elevated Temperatures. Materials 2018, 11, 2505. https://doi.org/10.3390/ma11122505
Zenkin S, Gaydaychuk A, Okhotnikov V, Linnik S. CVD Diamond Interaction with Fe at Elevated Temperatures. Materials. 2018; 11(12):2505. https://doi.org/10.3390/ma11122505
Chicago/Turabian StyleZenkin, Sergei, Aleksandr Gaydaychuk, Vitaly Okhotnikov, and Stepan Linnik. 2018. "CVD Diamond Interaction with Fe at Elevated Temperatures" Materials 11, no. 12: 2505. https://doi.org/10.3390/ma11122505




