Preparation of Nano-SiO2/Al2O3/ZnO-Blended PVDF Cation-Exchange Membranes with Improved Membrane Permselectivity and Oxidation Stability
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials and Reagents
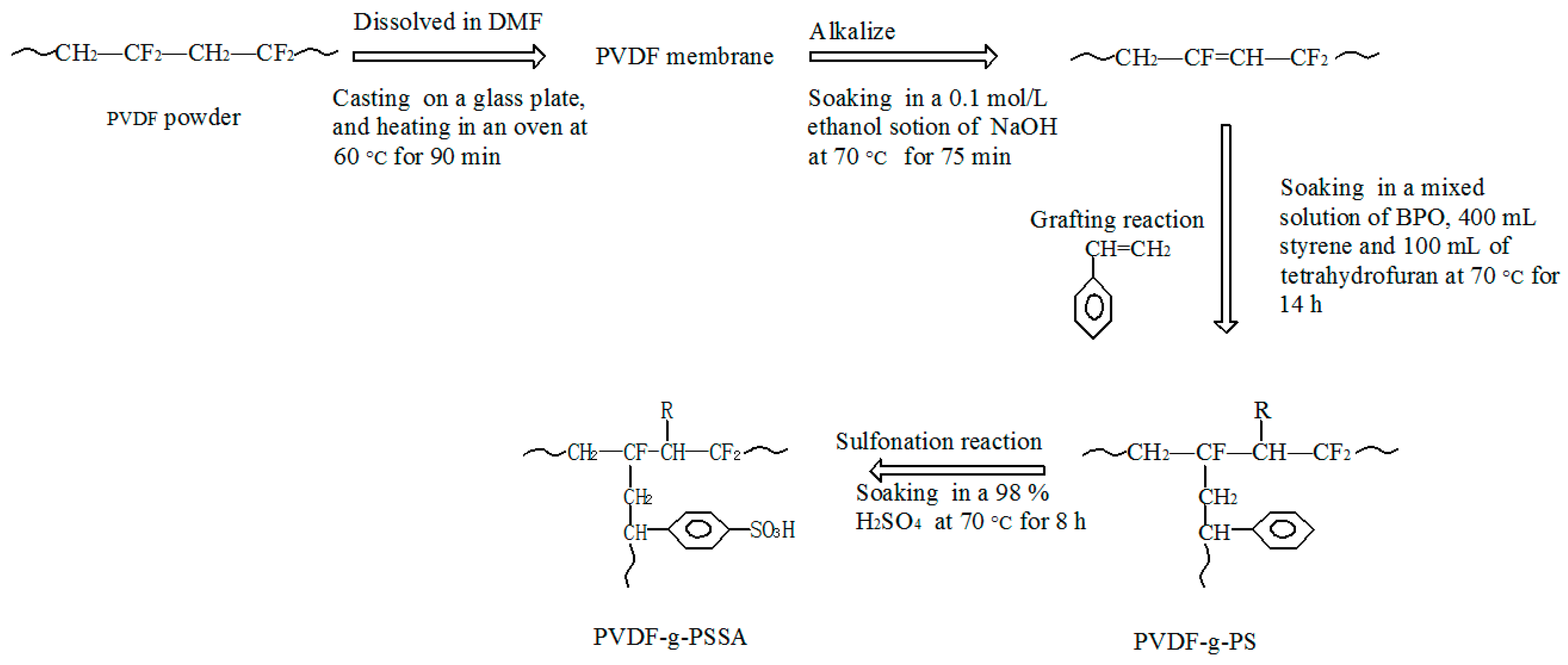
2.2. Preparation of the Membranes
2.3. Membrane Characterization
2.3.1. Transport Number and Membrane Permselectivity
2.3.2. IEC and WU
2.3.3. Membrane Area Resistance
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.6. Membrane Oxidative Stability and Burst Strength
3. Results and Discussion
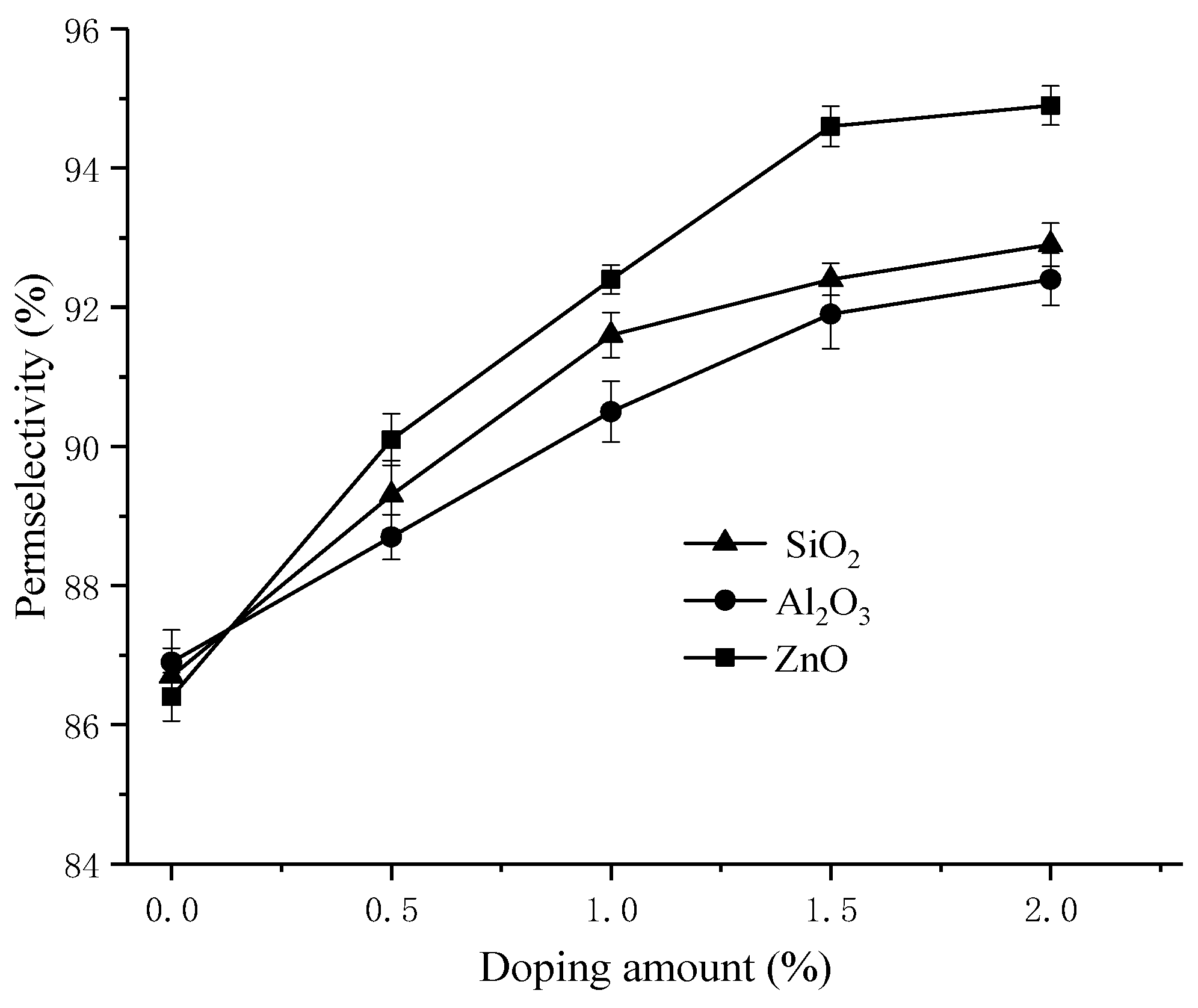
3.1. Transport Number and Membrane Permselectivity
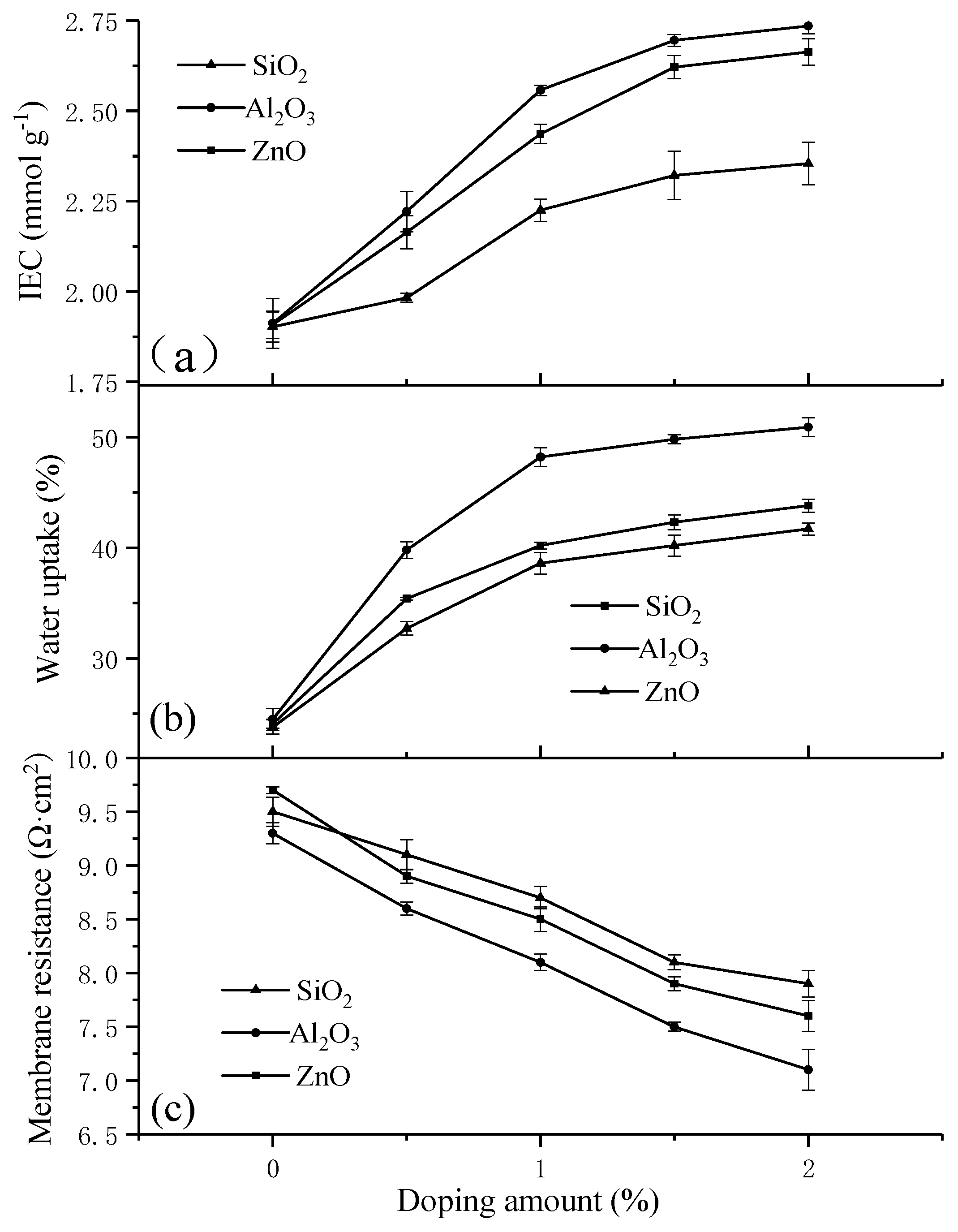
3.2. IEC and WU Properties
3.3. Membrane Area Resistance
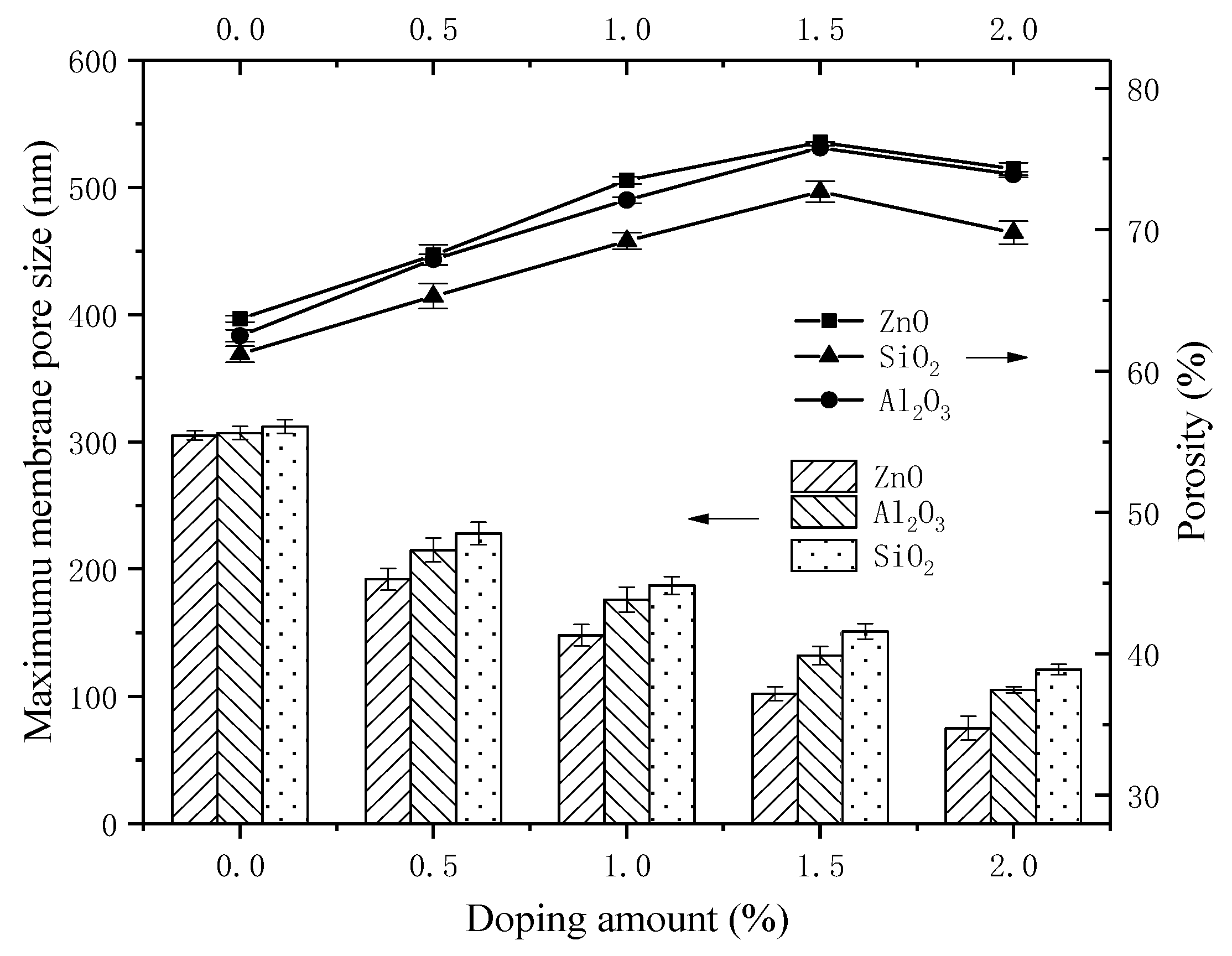
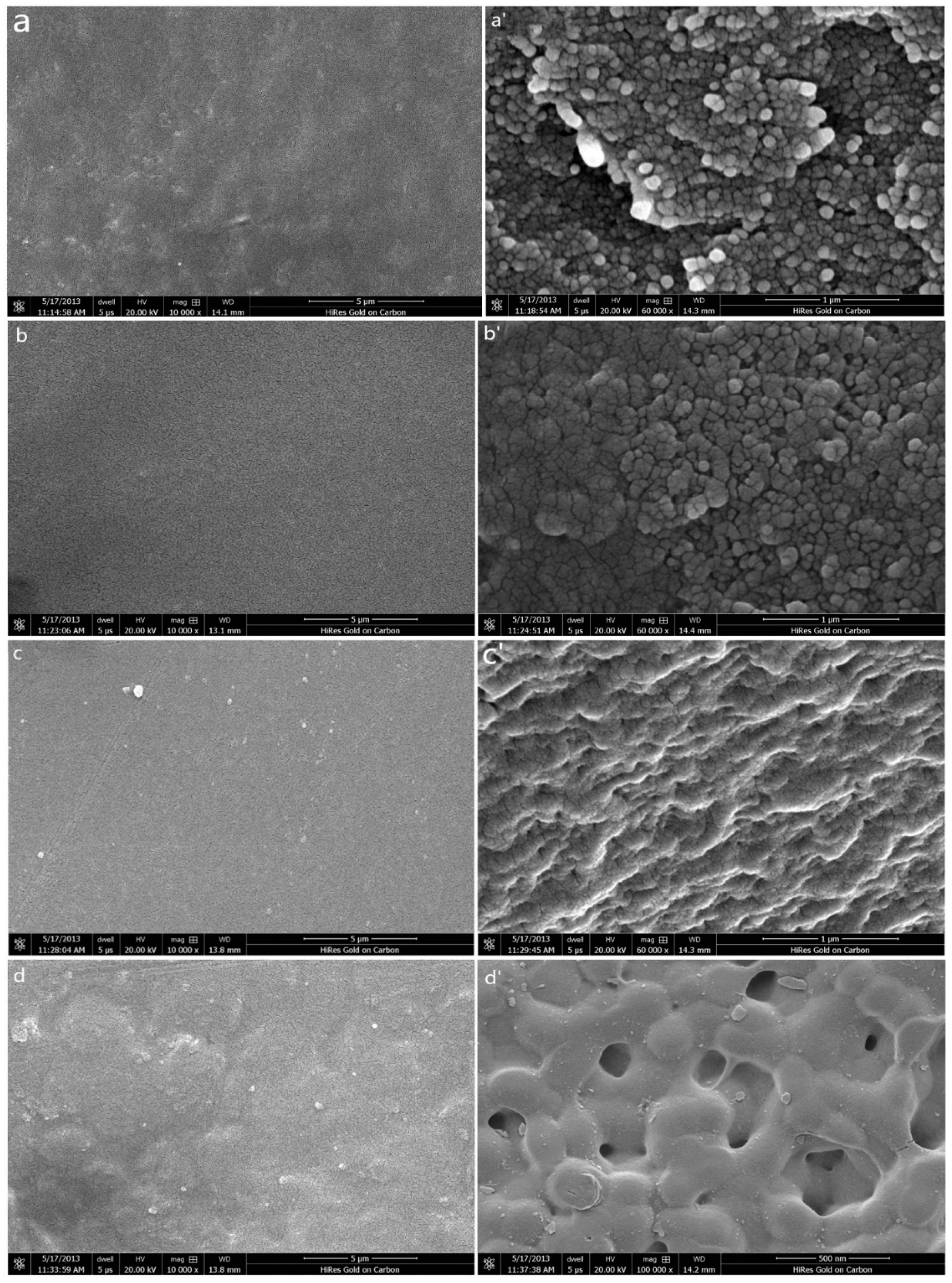
3.4. SEM Studies
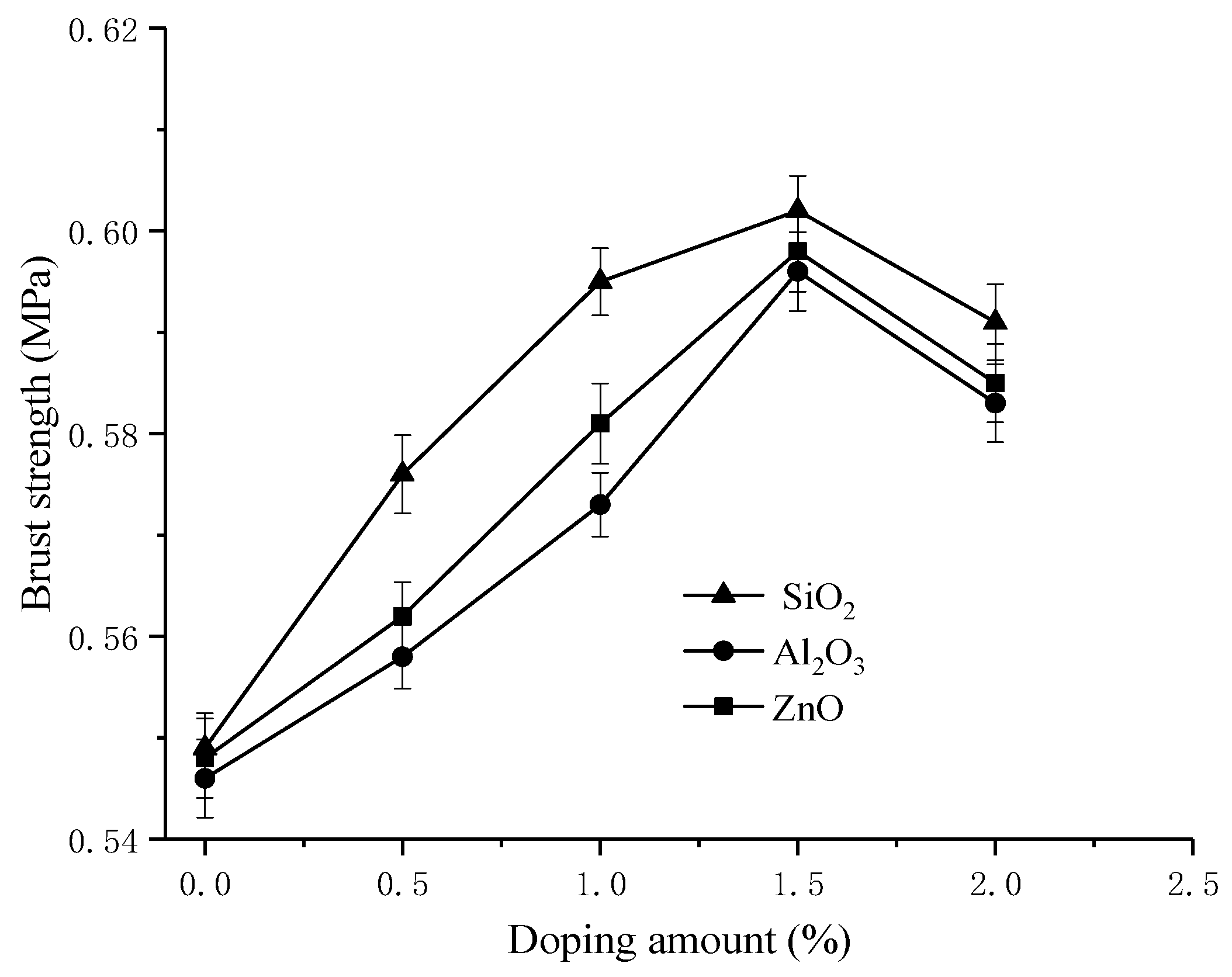
3.5. Membrane Oxidative Stability and Burst Strength
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 26, 1–29. [Google Scholar] [CrossRef]
- Güler, E.; Elizen, R.; Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Performance-determining membrane properties in reverse electrodialysis. J. Membr. Sci. 2013, 446, 266–276. [Google Scholar] [CrossRef]
- Asquith, B.M.; Meier-Haack, J.; Vogel, C.; Butwilowski, W.; Ladewig, B.P. Side-chain sulfonated copolymer cation exchange membranes for electro-driven desalination applications. Desalination 2013, 324, 93–98. [Google Scholar] [CrossRef]
- Klaysom, C.; Marschall, R.; Moon, S.H.; Ladewig, B.P.; Lu, G.M.; Wang, L. Preparation of porous composite ion-exchange membranes for desalination application. J. Mater. Chem. 2011, 21, 7401–7409. [Google Scholar] [CrossRef]
- Vallejo, E.; Pourcelly, G.; Gavach, C.; Mercier, R.; Pineri, M. Sulfonated polyimides as proton conductor exchange membranes. Physicochemical properties and separation H+/Mz+ by electrodialysis comparison with a perfluorosulfonic membrane. J. Membr. Sci. 1999, 160, 127–137. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Nemati, M.; Jeddi, F.; Salehi, E.; Khodabakhshi, A.R.; Madaeni, S.S. Fabrication of mixed matrix heterogeneous cation exchange membrane modified by titanium dioxide nanoparticles: Mono/bivalent ionic transport property in desalination. Desalination 2015, 359, 167–175. [Google Scholar] [CrossRef]
- Zuo, X.; Shi, W.; Yu, S.; He, J. Fundamental characteristics study of anion-exchange PVDF–SiO2 membranes. Water Sci. Technol. 2012, 66, 2343–2348. [Google Scholar] [CrossRef]
- Cseri, L.; Baugh, J.; Alabi, A.; AlHajaj, A.; Zou, L.; Dryfe, R.; Budd, P.M.; Szekely, G. Graphene oxide–polybenzimidazolium nanocomposite anion exchange membranes for electrodialysis. J. Mater. Chem. A 2018. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Vogel, C.; Meier-Haack, J. Preparation of ion-exchange materials and membranes. Desalination 2014, 342, 156–174. [Google Scholar] [CrossRef]
- Liu, Q.F.; Li, F.; Guo, Y.; Dong, Y.; Liu, J.; Shao, H.; Fu, Z. Preparation and characterization of PVDF/alkali-treated-PVDF blend membranes. Membr. Water Treat. 2016, 7, 417–431. [Google Scholar] [CrossRef] [Green Version]
- Couture, G.; Alaaeddine, A.; Boschet, F.; Ameduri, B. Polymeric materials as anion-exchange membranes for alkaline fuel cells. Prog. Polym. Sci. 2011, 36, 1521–1557. [Google Scholar] [CrossRef]
- Kim, D.J.; Jeong, M.K.; Sang, Y.N. Research trends in ion exchange membrane processes and practical applications. Appl. Chem. Eng. 2015, 26, 1–16. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A. Preparation and characterization of novel porous PVDF-ZrO2 composite membranes. Desalination 2002, 146, 35–40. [Google Scholar] [CrossRef]
- Shi, B.L.; Su, X.; He, J.; Wang, L.L. Surface hydrophilicity modification of PVDF membranes with an external electric field in the phase inversion process. Membr. Water Treat. 2015, 6, 351–363. [Google Scholar] [CrossRef]
- Alabi, A.; AlHajaj, A.; Cseri, L.; Szekely, G.; Budd, P.; Zou, L. Review of nanomaterials-assisted ion exchange membranes for electromembrane desalination. NPJ Clean Water 2018, 1, 10. [Google Scholar] [CrossRef]
- Shamsaei, E.; Nasef, M.M.; Saidi, H.; Yahaya, A.H. Parametric investigations on proton conducting membrane by radiation induced grafting of 4-vinylpyridine onto poly (vinylidene fluoride) and phosphoric acid doping. Radiochim. Acta 2014, 102, 351–362. [Google Scholar] [CrossRef]
- Ike, I.A.; Dumée, L.F.; Groth, A.; Orbell, J.D.; Duke, M. Effects of dope sonication and hydrophilic polymer addition on the properties of low pressure PVDF mixed matrix membranes. J. Membr. Sci. 2017, 540, 200–211. [Google Scholar] [CrossRef]
- He, Y.; Hong, J.M. Effect of Nano-Sized ZnO Particle Addition on PVDF Ultrafiltration Membrane Performance. Adv. Mater. Res. 2011, 311, 1818–1821. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Shen, Y.; He, Q.; Guo, S. Tumor-specific disintegratable nanohybrids containing ultrasmall inorganic nanoparticles: from design and improved properties to cancer applications. Mater. Horiz. 2018, 5, 184–205. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, Y.; Xu, J.; Han, Y.; Xu, X. Preparation, performances of PVDF/ZnO hybrid membranes and their applications in the removal of copper ions. Appl. Surf. Sci. 2014, 316, 333–340. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.; Su, Y.; Hou, H.; Du, Q.; Zhang, H.; Qin, X. Enhanced performance of thin-film nanocomposite RO/NWF membrane by adding ZnO nanospheres in aqueous phase during interfacial polymerization process. Membr. Water Treat. 2017, 8, 225–244. [Google Scholar] [CrossRef]
- Sarihan, A.; Eren, E. Novel high performanced and fouling resistant PSf/ZnO membranes for water treatment. Membr. Water Treat. 2017, 8, 563–574. [Google Scholar]
- Lv, L.Y. The Preparation and Properties of Sulfonated Poly (vinidene difluoride) Grafted Polystyrene Ion Exchange Membranes. Master’s Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2007. (In Chinese). [Google Scholar]
- Xu, T.W.; Huang, C.H. Preparation and Application of Ion Exchange Membrane; Chemical Industry Publisher: Beijing, China, 2008. (In Chinese) [Google Scholar]
- Khodabakhshi, A.R.; Madaeni, S.S.; Hosseini, S.M. Comparative studies on morphological, electrochemical, and mechanical properties of S-polyvinyl chloride based heterogeneous cation-exchange membranes with different resin ratio loading. Ind. Eng. Chem. Res. 2010, 49, 8477–8487. [Google Scholar] [CrossRef]
- Khodabakhshi, A.R.; Madaeni, S.S.; Hosseini, S.M. Preparation and characterization of monovalent ion-selective poly (vinyl chloride)-blend-poly (styrene-co-butadiene) heterogeneous anion-exchange membranes. Polym. Int. 2011, 60, 466–474. [Google Scholar] [CrossRef]
- Zuo, X.; Yu, S.; Xu, X.; Xu, J.; Bao, R.; Yan, X. New PVDF organic–inorganic membranes: the effect of SiO2 nanoparticles content on the transport performance of anion-exchange membranes. J. Membr. Sci. 2009, 340, 206–213. [Google Scholar] [CrossRef]
- Wilhelm, F.G.; Pünt, I.G.M.; Van Der Vegt, N.F.A.; Strathmann, H.; Wessling, M. Cation permeable membranes from blends of sulfonated poly (ether ether ketone) and poly (ether sulfone). J. Membr. Sci. 2002, 199, 167–176. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Madaeni, S.S.; Khodabakhshi, A.R.; Zendehnam, A. Preparation and surface modification of PVC/SBR heterogeneous cation exchange membrane with silver nanoparticles by plasma treatment. J. Membr. Sci. 2010, 365, 438–446. [Google Scholar] [CrossRef]
- Parsons, R. Handbook of Electrochemical Constants; Butterworths Scientific Publications: London, UK, 1959. [Google Scholar]
- Khodabakhshi, A.R.; Madaeni, S.S.; Xu, T.W.; Wu, L.; Wu, C.; Li, C.; Na, W.; Zolanvari, S.A.; Babayi, A.; Ghasemi, J.; et al. Preparation, optimization and characterization of novel ion exchange membranes by blending of chemically modified PVDF and SPPO. Sep. and Purif. Technol. 2012, 90, 10–21. [Google Scholar] [CrossRef]
- Germer, W.; Harms, C.; Tullius, V.; Leppin, J.; Dyck, A. Comparison of conductivity measurement systems using the example of nafion and anion exchange membrane. Solid State Ionics 2015, 275, 71–74. [Google Scholar] [CrossRef]
- Tian, B.; Wang, X.Y.; Zhang, L.N.; Shi, F.N.; Zhang, Y.; Li, S.X. Preparation of PVDF anionic exchange membrane by chemical grafting of GMA onto PVDF macromolecule. Solid State Ionics 2016, 293, 56–63. [Google Scholar] [CrossRef]
- Khodabakhshi, A.R.; Madaeni, S.S.; Hosseini, S.M. Investigation of electrochemical and morphological properties of S-PVC based heterogeneous cation-exchange membranes modified by sodium dodecyl sulphate. Sep. Purif. Technol. 2011, 77, 220–229. [Google Scholar] [CrossRef]
- Wang, S.F.; Sun, S.X.; Lu, J.Y. Mechanism Research of Ion Exchange Membrane Permselectivity and Guided Membrane Modification Methods; National Natural Science Foundation: Beijing, China, 2015. [Google Scholar]
- Khan, M.A.; Khan, M.I.; Zafar, S. Removal of different anionic dyes from aqueous solution by anion exchange membrane. Membr. Water Treat. 2017, 8, 259–277. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhou, J.; Wang, S.F. A Device for Measuring the Pore Size of Ion Exchange Membranes. CN205323551U. 20 June 2016. (In Chinese) [Google Scholar]
- Tavakolmoghadam, M.; Mohammadi, T.; Hemmati, M. Preparation and characterization of PVDF/TiO2 composite ultrafiltration membranes using mixed solvents. Membr. Water Treat. 2016, 7, 377–401. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.S.; Hu, H.D.; Song, W.L. Insight into macroscopic metal-assisted chemical etching for silicon nanowires. Acta Physico-Chimica Sinica 2016, 32, 1019–1028. [Google Scholar]
- Yin, X.; Zhou, Z. Resilient Ion Exchange Membranes. U.S. Patent 20,130,313,187, 28 November 2013. [Google Scholar]
- Siracusano, S.; Trocino, S.; Briguglio, N.; Baglio, V.; Aricò, A. Electrochemical impedance spectroscopy as a diagnostic tool in polymer electrolyte membrane electrolysis. Materials 2018, 11, 1368. [Google Scholar] [CrossRef]
- Li, K.D.; Chen, P.W.; Chang, K.S.; Hsu, S.C.; Jan, D.J. Indium-Zinc-Tin-Oxide Film Prepared by Reactive Magnetron Sputtering for Electrochromic Applications. Materials 2018, 11, 2221. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.; Zhang, Y.; Zhou, R. Comparison study of the effect of blending method on PVDF/PPTA blend membrane structure and performance. Membr. Water Treat. 2015, 6, 205–224. [Google Scholar] [CrossRef]
- Zaitsev, S.Y.; Solovieva, D.O.; Zaitsev, I.S. Membrane and Films Based on Novel Crown-Containing Dyes as Promising Chemosensoring Materials. Materials 2010, 3, 5293–5310. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Chen, G. Preparation and assessment of heat-treated α-chitin nanowhiskers reinforced poly (viny alcohol) film for packaging application. Materials 2018, 11, 1883. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Li, Y. The effects of using aluminum oxide nanoparticles as heat transfer fillers on morphology and thermal performances of form-stable phase change fibrous membranes based on capric–palmitic–stearic acid ternary eutectic/polyacrylonitrile composite. Materials 2018, 11, 1785. [Google Scholar] [CrossRef] [PubMed]


| Membranes | Membrane Potential (mV) | Transport Number (%) |
|---|---|---|
| PVDF-g-PSSA | 45.61 | 91.83 |
| PVDF/0.5% SiO2-g-PSSA | 46.20 | 93.66 |
| PVDF/1.0% SiO2-g-PSSA | 46.59 | 94.88 |
| PVDF/1.5% SiO2-g-PSSA | 46.75 | 95.36 |
| PVDF/2.0% SiO2-g-PSSA | 46.85 | 95.67 |
| PVDF/0.5% Al2O3-g-PSSA | 46.02 | 93.11 |
| PVDF/1.0% Al2O3-g-PSSA | 46.38 | 94.21 |
| PVDF/1.5% Al2O3-g-PSSA | 46.65 | 95.06 |
| PVDF/2.0% Al2O3-g-PSSA | 46.75 | 95.36 |
| PVDF/0.5% ZnO-g-PSSA | 46.30 | 93.96 |
| PVDF/1.0% ZnO-g-PSSA | 46.75 | 95.36 |
| PVDF/1.5% ZnO-g-PSSA | 47.18 | 96.71 |
| PVDF/2.0% ZnO-g-PSSA | 47.24 | 96.89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhou, J.; Zou, X.; Wang, Z.; Chu, Y.; Wang, S. Preparation of Nano-SiO2/Al2O3/ZnO-Blended PVDF Cation-Exchange Membranes with Improved Membrane Permselectivity and Oxidation Stability. Materials 2018, 11, 2465. https://doi.org/10.3390/ma11122465
Zhang X, Zhou J, Zou X, Wang Z, Chu Y, Wang S. Preparation of Nano-SiO2/Al2O3/ZnO-Blended PVDF Cation-Exchange Membranes with Improved Membrane Permselectivity and Oxidation Stability. Materials. 2018; 11(12):2465. https://doi.org/10.3390/ma11122465
Chicago/Turabian StyleZhang, Xuemin, Jian Zhou, Xin Zou, Zhongyu Wang, Yunchen Chu, and Sanfan Wang. 2018. "Preparation of Nano-SiO2/Al2O3/ZnO-Blended PVDF Cation-Exchange Membranes with Improved Membrane Permselectivity and Oxidation Stability" Materials 11, no. 12: 2465. https://doi.org/10.3390/ma11122465




