Solidification and Segregation Behaviors of Superalloy IN718 at a Slow Cooling Rate
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Experimental Material and Compositions
2.2. In Situ Observation
2.3. Element Determination and Microstructural Analysis
3. Results and Discussion
3.1. Solidification Behavior
3.1.1. DSC Measurement
3.1.2. CLSM Observations
3.2. Microsegregation Analysis
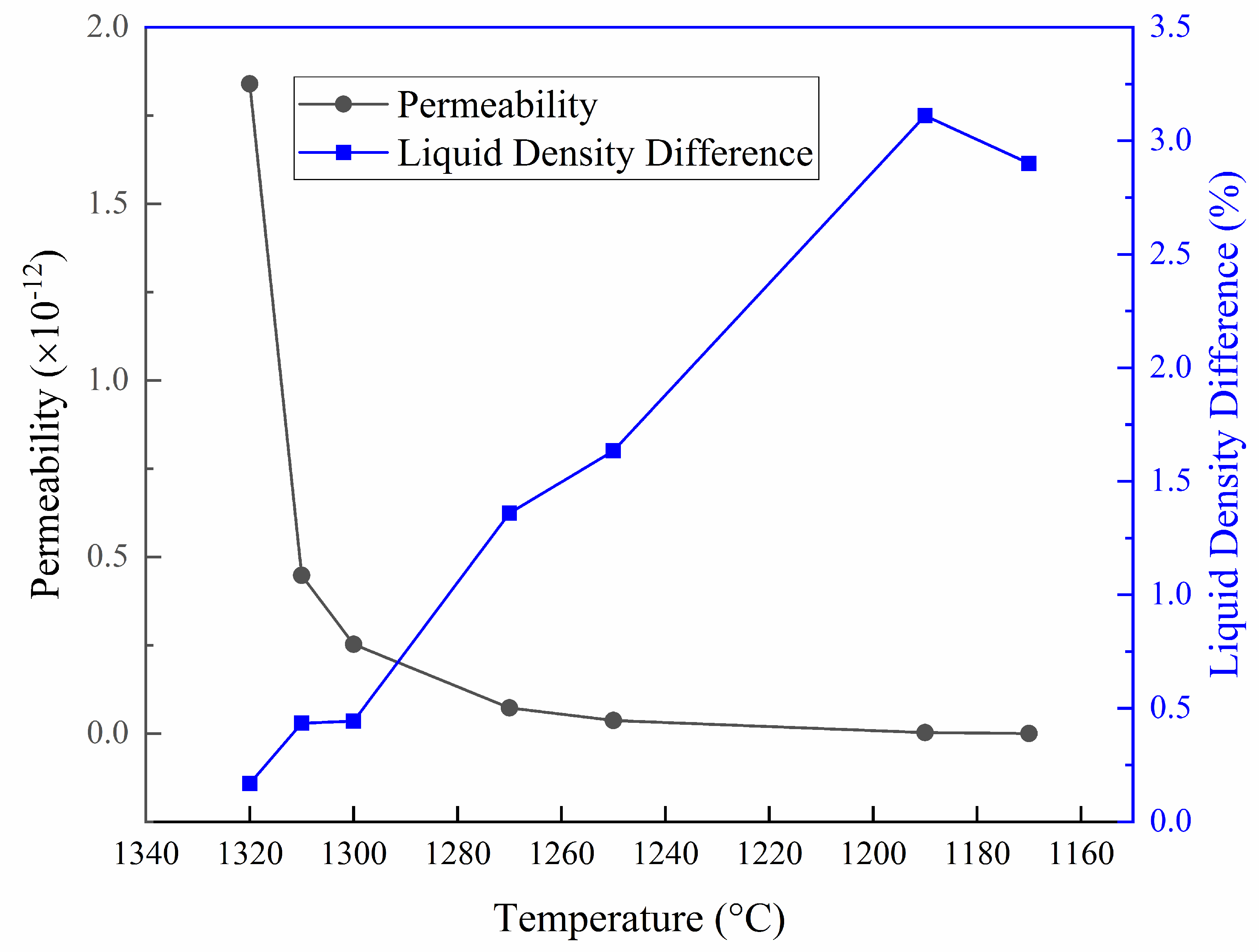
3.3. Macrosegregation Prediction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paulonis, D.F.; Schirra, J.J. Alloy 718 at Pratt & Whitney-historical Perspective and Future Challenges. In Proceedings of the Superalloys 718, 625, 706 and Various Derivatives, Pittsburgh, PA, USA, 17–20 June 2001; TMS: Pittsburgh, PA, USA, 2001; pp. 13–23. [Google Scholar]
- Schafrik, R.E.; Ward, D.D.; Groh, J.R. Application of alloy 718 in GE aircraft engines: past, present and next five years. In Proceedings of the Superalloys 718, 625, 706 and Various Derivatives, Pittsburgh, PA, USA, 17–20 June 2001; TMS: Pittsburgh, PA, USA, 2001; pp. 1–11. [Google Scholar]
- Ping, D.H.; Gu, Y.F.; Cui, C.Y.; Harada, H. Grain boundary segregation in a Ni-Fe-based (Alloy 718) superalloy. Mater. Sci. Eng. A 2007, 456, 99–102. [Google Scholar] [CrossRef]
- Thomas, A.; El-Wahabi, M.; Cabrera, J.M.; Prado, J.M. High temperature deformation of Inconel 718. J. Mater. Process Tech. 2006, 177, 469–472. [Google Scholar] [CrossRef]
- Liu, Y.C.; Guo, Q.Y.; Li, C.; Mei, Y.P.; Zhou, X.S.; Huang, Y.; Li, H.J. Recent progress on evolution of precipitates in Inconel 718 superalloy. Acta. Metall. Sin. 2016, 52, 1259–1266. [Google Scholar]
- Jones, R.M.F.; Jackman, L.A. The structural evolution of superalloy ingots during hot working. JOM 1999, 51, 27–31. [Google Scholar] [CrossRef]
- Shi, X.; Duan, S.C.; Yang, W.S.; Guo, H.J.; Guo, J. Effect of Cooling Rate on Microsegregation during Solidification of Superalloy INCONEL 718 Under Slow-Cooled Conditions. Metall. Mater. Trans. B 2018, 49, 1883–1897. [Google Scholar] [CrossRef]
- Ling, L.; Han, Y.; Zhou, W.; Gao, H.Y.; Shu, D.; Wang, J.; Kang, M.D.; Sun, B.D. Study of Microsegregation and Laves Phase in INCONEL718 Superalloy Regarding Cooling Rate During Solidification. Metall. Mater. Trans. A 2015, 46, 1–8. [Google Scholar] [CrossRef]
- Blecher, J.J.; Palmer, T.A.; Debroy, T. Solidification map of a nickel-base alloy. Metall. Mater. Trans A 2014, 45, 2142–2151. [Google Scholar] [CrossRef]
- Yang, W.H.; Chen, W.; Chang, K.M.; Mannan, S.; DeBarbadillo, J. Freckle criteria for the upward directional solidification of alloys. Metall. Mater. Trans. A 2001, 32, 397–406. [Google Scholar] [CrossRef]
- Frueh, C.; Poirier, D.R.; Erdmann, R.G.; Felicelli, S.D. On the length-scale and location of channel nucleation in directional solidification. Mater. Sci. Eng. A 2003, 345, 72–80. [Google Scholar] [CrossRef]
- Long, Z.D.; Liu, X.B.; Yang, W.H.; Chang, K.M.; Barbero, E. Thermodynamic assessment of liquid composition change during solidification and its effect on freckle formation in superalloys. Mater. Sci. Eng. A 2004, 386, 254–261. [Google Scholar] [CrossRef]
- Manikandan, S.G.K.; Sivakumar, D.; Rao, K.P.; Kamaraj, M. Laves phase in alloy 718 fusion zone—microscopic and calorimetric studies. Mater. Charac. 2015, 100, 192–206. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Y.J.; Dong, J.X.; Zhang, M.C. Effect of cooling rates on segregation and density variation in the mushy zone during solidification of superalloy Inconel 718. Chem. Eng. Commun. 2010, 197, 1571–1585. [Google Scholar] [CrossRef]
- Knorovsky, G.A.; Cieslak, M.J.; Headley, T.J.; Romig, A.D., Jr.; Hammetter, W.F. INCONEL 718: A solidification diagram. Metall. Trans. A 1989, 20, 2149–2158. [Google Scholar] [CrossRef]
- Valdes, J.; Liu, X.B.; King, P.; Cowen, C.; Jablonski, P. Solidification Front Tilt Angle Effect on Potential Nucleation Sites for Freckling in the Remelt of Ni-Base Superalloys. In Proceedings of the Superalloy 718 and Derivatives, Pittsburgh, PA, USA, 10–13 October 2010; TMS: Pittsburgh, PA, USA, 2010; pp. 78–94. [Google Scholar]
- Li, D.J.; Chen, X.Q.; Fu, P.X.; Ma, X.P.; Liu, H.W.; Chen, Y.; Cao, Y.F.; Luan, Y.K.; Li, Y.Y. Inclusion flotation-driven channel segregation in solidifying steels. Nat. Commun. 2014, 6, 6291–6298. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Debarbadillo, J.J.; Morita, K.; Suzuki, K.; Chen, W.; Chang, K.M. A freckle criterion for the solidification of superalloys with a tilted solidification front. JOM 2004, 56, 56–61. [Google Scholar] [CrossRef]
- Mehrabian, R.; Keane, M.; Flemings, M.C. Interdendritic fluid flow and macrosegregation; Influence of gravity. Metall Mater Trans. 1970, 5, 1209–1220. [Google Scholar] [CrossRef]
- Valdes, J.; Shang, S.L.; Liu, Z.K.; King, P.; Liu, X.B. Quenching Differential Thermal Analysis and Thermodynamic Calculation to Determine Partition Coefficients of Solute Elements in Simplified Ni-Base Superalloys. Metall. Mater. Trans. A 2010, 41, 487–498. [Google Scholar] [CrossRef]
- Auburtin, P.; Wang, T.; Cockcroft, S.L.; Mitchell, A. Freckle formation and freckle criterion in superalloy castings. Metall. Mater. Trans. B 2000, 31, 801–811. [Google Scholar] [CrossRef]
- Antonsson, T.; Fredriksson, H. The effect of cooling rate on the solidification of Inconel 718. Metall. Mater. Trans. B 2005, 36, 85–96. [Google Scholar] [CrossRef]
- Miao, Z.J.; Shan, A.D.; Wang, W.; Lu, J.; Xu, W.L.; Song, H.W. Solidification process of conventional superalloy by confocal scanning laser microscope. Trans. Nonferrous Met. Soc. China 2011, 21, 236–242. [Google Scholar] [CrossRef]
- Li, J.; Yu, L.X.; Sun, W.R.; Zhang, W.H.; Liu, F.; Qi, F.; Guo, S.R.; Hu, Z.L. Effect of solidification rates on microstructures and segregation of IN718 alloy. Chin. J. Mater. Res. 2010, 24, 118–122. [Google Scholar]
- Dupont, J.N. Solidification of an alloy 625 weld overlay. Metall. Mater. Trans. A 1996, 27, 3612–3620. [Google Scholar] [CrossRef]
- Hirai, M. Estimation of viscosities of liquid alloys. ISIJ Int. 1993, 33, 251–258. [Google Scholar] [CrossRef]
- Mukai, K.; Li, Z.; Mills, K.C. Prediction of the densities of liquid Ni-based superalloys. Metall. Mater. Trans. B. 2005, 36, 255–262. [Google Scholar] [CrossRef]
- Poirier, D.R. Permeability for flow of interdendritic liquid in columnar-dendritic alloys. Metall. Trans. B 1987, 18, 245–255. [Google Scholar] [CrossRef]








| Element | Ni | Cr | Nb | Mo | Al | Ti | C | Si | P | S | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMS Specification [22] | 50.0–55.0 | 17.0–21.0 | 4.75–5.50 | 2.80–3.30 | 0.20–0.80 | 0.65–1.15 | ≤0.08 | ≤0.35 | ≤0.015 | ≤0.015 | balance |
| Measured Results | 52.72 | 18.26 | 4.92 | 3.13 | 0.43 | 1.13 | 0.02 | 0.20 | 0.01 | 0.01 | 19.00 |
| Quenching Temperature (°C) | 1320 | 1310 | 1300 | 1270 | 1250 | 1190 | 1170 |
|---|---|---|---|---|---|---|---|
| Solid Fraction | 0.71 | 0.81 | 0.84 | 0.89 | 0.91 | 0.96 | 0.99 |
| Average Cr Content in Liquid (wt %) | 14.86 | 15.01 | 14.89 | 14.41 | 14.03 | 13.88 | 13.44 |
| Average Fe Content in Liquid (wt %) | 11.55 | 11.40 | 11.46 | 11.16 | 11.35 | 11.18 | 10.95 |
| Average Al Content in Liquid (wt %) | 0.345 | 0.355 | 0.352 | 0.354 | 0.341 | 0.326 | 0.319 |
| Average Nb Content in Liquid (wt %) | 10.44 | 13.09 | 14.39 | 17.60 | 21.08 | 24.25 | 22.21 |
| Average Mo Content in Liquid (wt %) | 4.42 | 4.55 | 4.90 | 5.42 | 5.51 | 6.22 | 4.96 |
| Average Ti Content in Liquid (wt %) | 1.77 | 1.84 | 1.88 | 2.08 | 1.88 | 1.71 | 1.69 |
| Elements | N | C | Cr | Fe | Ni | Al | Ti | Nb | Mo |
|---|---|---|---|---|---|---|---|---|---|
| Spectrum 1 | 10.14 | - | 0.97 | 0.78 | 1.97 | 5.02 | 72.07 | 9.05 | - |
| Spectrum 2 | 10.78 | - | 0.86 | - | 1.20 | 7.48 | 69.16 | 10.53 | - |
| Spectrum 3 | - | 24.15 | 3.23 | 2.37 | 9.79 | - | 4.15 | 53.78 | - |
| Spectrum 4 | - | 30.52 | 0.74 | - | 1.39 | - | 5.94 | 61.41 | - |
| Spectrum 5 | - | 34.55 | 0.55 | - | 1.29 | - | 4.19 | 59.43 | - |
| Spectrum 6 | - | - | 13.91 | 12.14 | 36.41 | 0.07 | 0.81 | 29.07 | 6.30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Duan, S.; Yang, W.; Guo, H.; Guo, J. Solidification and Segregation Behaviors of Superalloy IN718 at a Slow Cooling Rate. Materials 2018, 11, 2398. https://doi.org/10.3390/ma11122398
Shi X, Duan S, Yang W, Guo H, Guo J. Solidification and Segregation Behaviors of Superalloy IN718 at a Slow Cooling Rate. Materials. 2018; 11(12):2398. https://doi.org/10.3390/ma11122398
Chicago/Turabian StyleShi, Xiao, Shengchao Duan, Wensheng Yang, Hanjie Guo, and Jing Guo. 2018. "Solidification and Segregation Behaviors of Superalloy IN718 at a Slow Cooling Rate" Materials 11, no. 12: 2398. https://doi.org/10.3390/ma11122398





