A Comprehensive Approach to Powder Feedstock Characterization for Powder Bed Fusion Additive Manufacturing: A Case Study on AlSi7Mg
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Powder Morphology and Particle Size Distribution
3.2. Density and Flow Characteristics
3.3. Spread Density
3.4. Particle Segregation in A Powder Bed
3.5. Water Vapor Adsorption Characteristics
3.6. Multiple Cycle Flowability Test
3.7. Particle Cohesiveness
3.8. Static and Dynamic Angle of Repose
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rüßmann, M.; Lorenz, M.; Gerbert, P.; Waldner, M.; Justus, J.; Engel, P.; Harnisch, M. Industry 4.0: The Future of Productivity and Growth in Manufacturing Industries; Boston Consulting Group: Boston, MA, USA, 2015; Volume 9. [Google Scholar]
- Ding, Y.; Muñiz-Lerma, J.A.; Trask, M.; Chou, S.; Walker, A.; Brochu, M. Microstructure and mechanical property considerations in additive manufacturing of aluminum alloys. MRS Bull. 2016, 41, 745–751. [Google Scholar] [CrossRef]
- Olakanmi, E.O.; Cochrane, R.F.; Dalgarno, K.W. A review on selective laser sintering/melting (SLS/SLM) of aluminium alloy powders: Processing, microstructure, and properties. Prog. Mater. Sci. 2015, 74, 401–477. [Google Scholar] [CrossRef] [Green Version]
- Strondl, A.; Lyckfeldt, O.; Brodin, H.; Ackelid, U. Characterization and control of powder properties for additive manufacturing. Jom 2015, 67, 549–554. [Google Scholar] [CrossRef]
- German, R.M. Particle Packing Characteristics; Metal Powder Industries Federation: Princeton, NJ, USA, 1989. [Google Scholar]
- Olakanmi, E. Selective laser sintering/melting (SLS/SLM) of pure Al, Al-Mg, and Al-Si powders: Effect of processing conditions and powder properties. J. Mater. Process. Technol. 2013, 213, 1387–1405. [Google Scholar] [CrossRef]
- Carson, J.W.; Pittenger, B.H. Bulk properties of powders. In ASM Handbook, Volume 7: Powder Metal Technologies and Applications; Lee, P.W.T., Iacocca, R., German, R.M., Ferguson, B.L., Eisen, W.B., Moyer, K., Madan, D., Sanderow, H., Eds.; ASM International: Almere, The Netherlands, 1998; pp. 287–301. [Google Scholar]
- Mindt, H.W.; Megahed, M.; Lavery, N.P.; Holmes, M.A.; Brown, S.G.R. Powder bed layer characteristics: the overseen first-order process input. Metall. Mater. Trans. A 2016, 47, 3811–3822. [Google Scholar] [CrossRef]
- Zielinski, J.; Vervoort, S.; Mindt, H.-W.; Megahed, M. Influence of powder bed characteristics on material quality in additive manufacturing. BHM Berg Hüttenmänn. Monatshefte 2017, 162, 192–198. [Google Scholar] [CrossRef]
- Spierings, A.B.; Herres, N.; Levy, G. Influence of the particle size distribution on surface quality and mechanical properties in AM steel parts. Rapid Prototyp. J. 2011, 17, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Wildman, R.; Tuck, C.; Ashcroft, I.; Hague, R. Investigation the effect of particle size distribution on processing parameters optimisation in selective laser melting process. Addit. Manuf. Res. Group Loughb. Univ. 2011, 84, 227–238. [Google Scholar]
- Simchi, A. The role of particle size on the laser sintering of iron powder. Met. Mater. Trans. B 2004, 35, 937–948. [Google Scholar] [CrossRef]
- Parteli, E.J.R.; Pöschel, T. Particle-based simulation of powder application in additive manufacturing. Powder Technol. 2016, 288, 96–102. [Google Scholar] [CrossRef]
- Yoshimi, K.; Hirozumi, A.; Satoshi, Y.; Hisashi, S.; Soya, T. Phenomenological studies in laser cladding. Part I. Time-resolved measurements of the absorptivity of metal powder. Jpn. J. Appl. Phys. 1993, 32, 205. [Google Scholar]
- Boley, C.D.; Khairallah, S.A.; Rubenchik, A.M. Calculation of laser absorption by metal powders in additive manufacturing. Appl. Opt. 2015, 54, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Aboulkhair, N.T.; Everitt, N.M.; Ashcroft, I.; Tuck, C. Reducing porosity in AlSi10Mg parts processed by selective laser melting. Addit. Manuf. 2014, 1, 77–86. [Google Scholar] [CrossRef]
- Equispheres. Available online: https://equispheres.com/powder/ (accessed on 28 August 2018).
- German, R.M.; Park, S.J. Mathematical Relations in Particulate Materials Processing: Ceramics, Powder Metals, Cermets, Carbides, Hard Materials, and Minerals; Wiley: Hoboken, NJ, USA, 2008; p. 419. [Google Scholar]
- ASTM B213-17. Standard Test Methods for Flow Rate of Metal Powders Using the Hall Flowmeter Funnel; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM B964-16. Standard Test Methods for Flow Rate of Metal Powders Using the Carney Funnel; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM B212-17. Standard Test Method for Apparent Density of Free-Flowing Metal Powders Using the Hall Flowmeter Funnel; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM B417-13. Standard Test Method for Apparent Density of Non-Free-Flowing Metal Powders Using the Carney Funnel; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM B703-10. Standard Test Method for Apparent Density of Metal Powders and Related Compounds Using the Arnold Meter; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Object Research Systems (ORS) Inc., Montreal, Canada. Dragonfly 3.1 (Computer Software). 2016. Available online: http://www.theobjects.com/dragonfly (accessed on 7 November 2018).
- Johnson, K.; Kendall, K.; Roberts, A. Surface energy and the contact of elastic solids. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1971, 324, 301–313. [Google Scholar] [CrossRef]
- Voelkel, A.; Strzemiecka, B.; Adamska, K.; Milczewska, K. Inverse gas chromatography as a source of physiochemical data. J. Chromatogr. A 2009, 1216, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Jam, S.; Waters, K.E. Inverse gas chromatography applications: A review. Adv. Colloid Interface Sci. 2014, 212, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Lumay, G.; Boschini, F.; Traina, K.; Bontempi, S.; Remy, J.C.; Cloots, R.; Vandewalle, N. Measuring the flowing properties of powders and grains. Powder Technol. 2012, 224, 19–27. [Google Scholar] [CrossRef]
- Spierings, A.B.; Voegtlin, M.; Bauer, T.; Wegener, K. Powder flowability characterisation methodology for powder-bed-based metal additive manufacturing. Prog. Addit. Manuf. 2016, 1, 9–20. [Google Scholar] [CrossRef]
- Cunningham, R.; Nicolas, A.; Madsen, J.; Fodran, E.; Anagnostou, E.; Sangid, M.D.; Rollett, A.D. Analyzing the effects of powder and post-processing on porosity and properties of electron beam melted Ti-6Al-4V. Mater. Res. Lett. 2017, 5, 516–525. [Google Scholar] [CrossRef]
- Pethrick, R.A.; Hayward, D.; Jeffrey, K.; Affrossman, S.; Wilford, P. Investigation of the hydration and dehydration of aluminium oxide-hydroxide using high frequency dielectric measurements between 300 kHz-3 GHz. J. Mater. Sci. 1996, 31, 2623–2629. [Google Scholar] [CrossRef]
- Litvintsev, A.I.; Arbuzova, L.A. Kinetics of degassing of aluminum powders. Sov. Powder Met. Met. Ceram. 1967, 6, 1–10. [Google Scholar] [CrossRef]
- Pietsch, W. Agglomeration Processes: Phenomena, Technologies, Equipment; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Hao, T. Analogous viscosity equations of granular powders based on Eyring’s rate process theory and free volume concept. RSC Adv. 2015, 5, 95318–95333. [Google Scholar] [CrossRef]
- de Ryck, A.; Condotta, R.; Dodds, J.A. Shape of a cohesive granular heap. Powder Technol. 2005, 157, 72–78. [Google Scholar] [CrossRef]
- Lumay, G.; Fiscina, J.; Ludewig, F.; Vandewalle, N. Influence of cohesive forces on the macroscopic properties of granular assemblies. AIP Conf. Proc. 2013, 1542, 995–998. [Google Scholar]
- Foster, B.; Reutzel, E.; Nassar, A.; Hall, B.; Brown, S.; Dickman, C. Optical, layerwise monitoring of powder bed fusion. In Proceedings of the 6th International Solid Freeform Fabrication Symposium, Austin, TX, USA, 10–12 August 2015. [Google Scholar]
- Baitimerov, R.; Lykov, P.; Zherebtsov, D.; Radionova, L.; Shultc, A.; Prashanth, K. Influence of powder characteristics on processability of AlSi12 alloy fabricated by selective laser melting. Materials 2018, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.L.; Davé, R.N. Dynamic simulation of particle packing influenced by size, aspect ratio and surface energy. Granul. Matter 2013, 15, 401–415. [Google Scholar] [CrossRef]
- Yu, A.B.; Bridgwater, J.; Burbidge, A. On the modelling of the packing of fine particles. Powder Technol. 1997, 92, 185–194. [Google Scholar] [CrossRef]
- Parteli, E.J.; Schmidt, J.; Blümel, C.; Wirth, K.-E.; Peukert, W.; Pöschel, T. Attractive particle interaction forces and packing density of fine glass powders. Sci. Rep. 2014, 4, 6227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiscina, J.E.; Lumay, G.; Ludewig, F.; Vandewalle, N. Compaction dynamics of wet granular assemblies. Phys. Rev. Lett. 2010, 105, 048001. [Google Scholar] [CrossRef] [PubMed]
- Harnby, N. Chapter 5—The mixing of cohesive powders. In Mixing in the Process Industries, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1992; pp. 79–98. [Google Scholar]
- Chaudhuri, B.; Mehrotra, A.; Muzzio, F.J.; Tomassone, M.S. Cohesive effects in powder mixing in a tumbling blender. Powder Technol. 2006, 165, 105–114. [Google Scholar] [CrossRef]

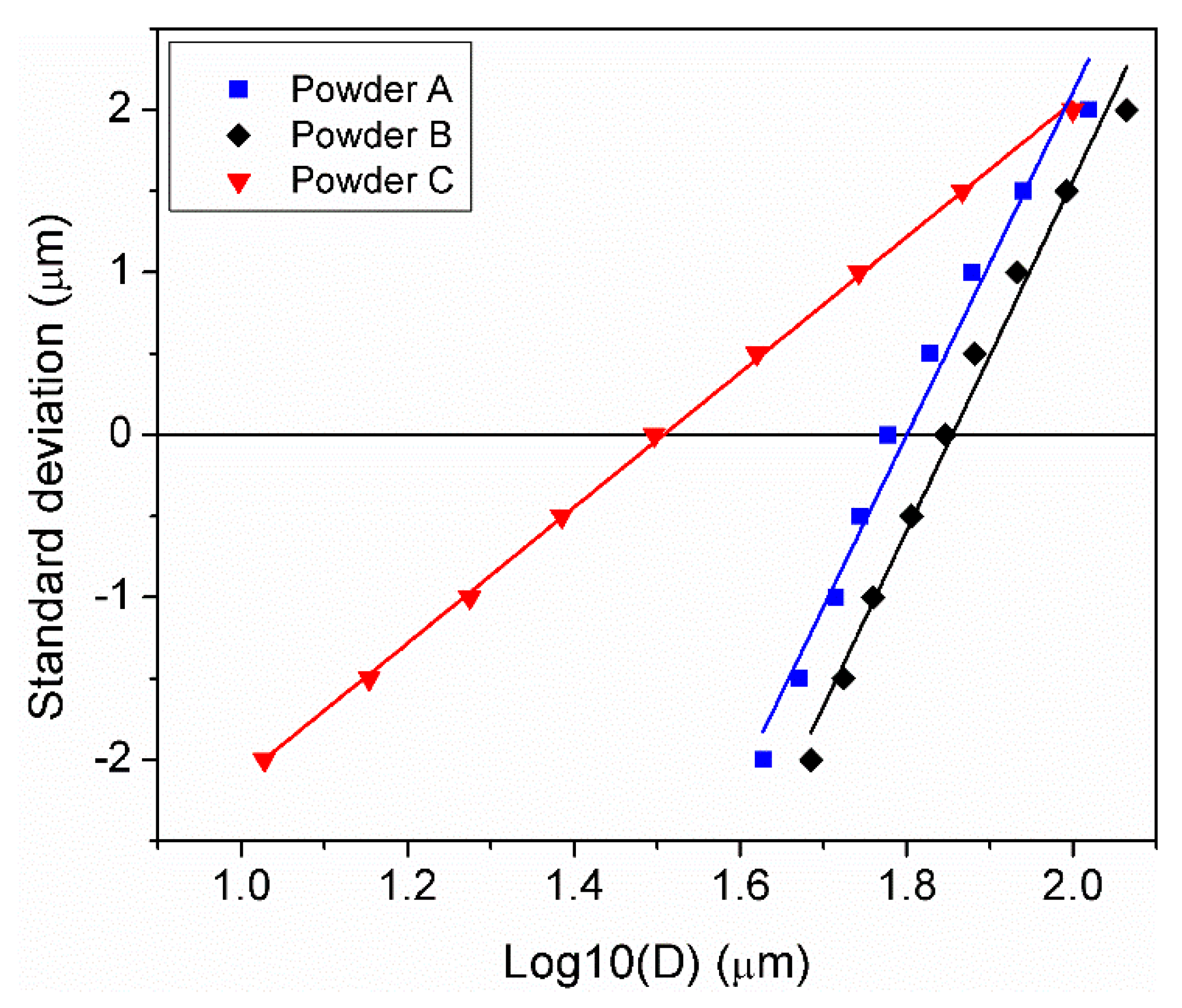


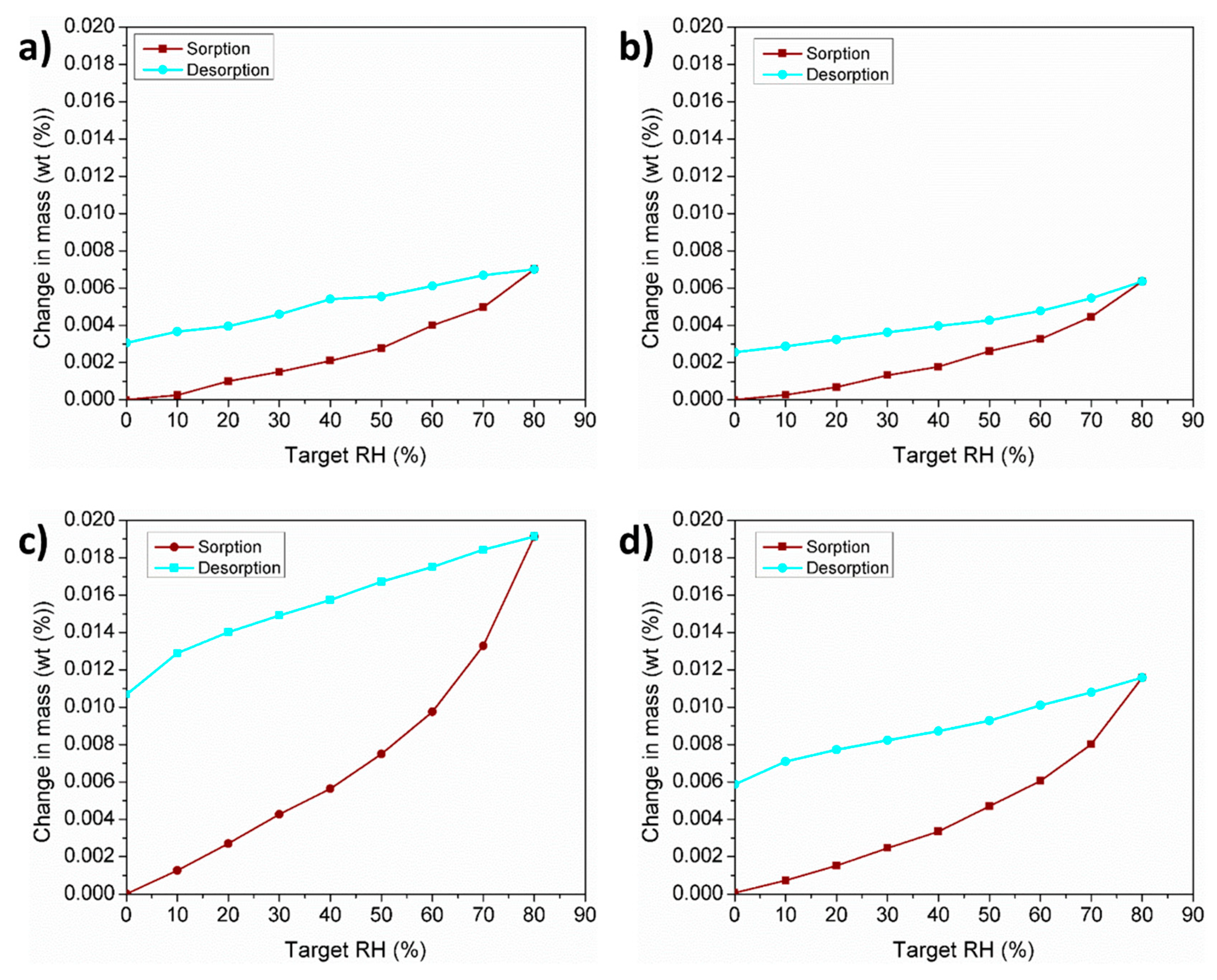
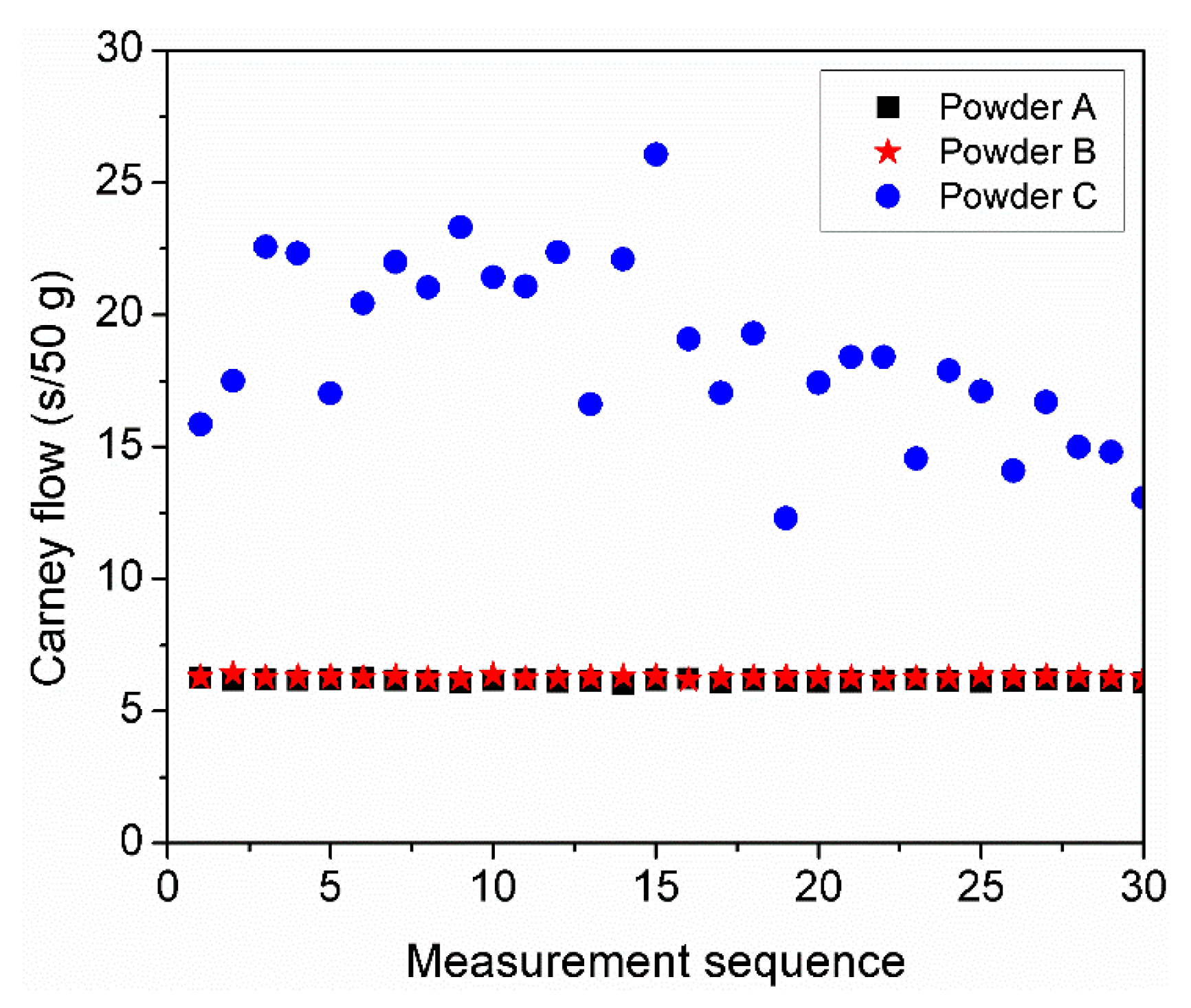
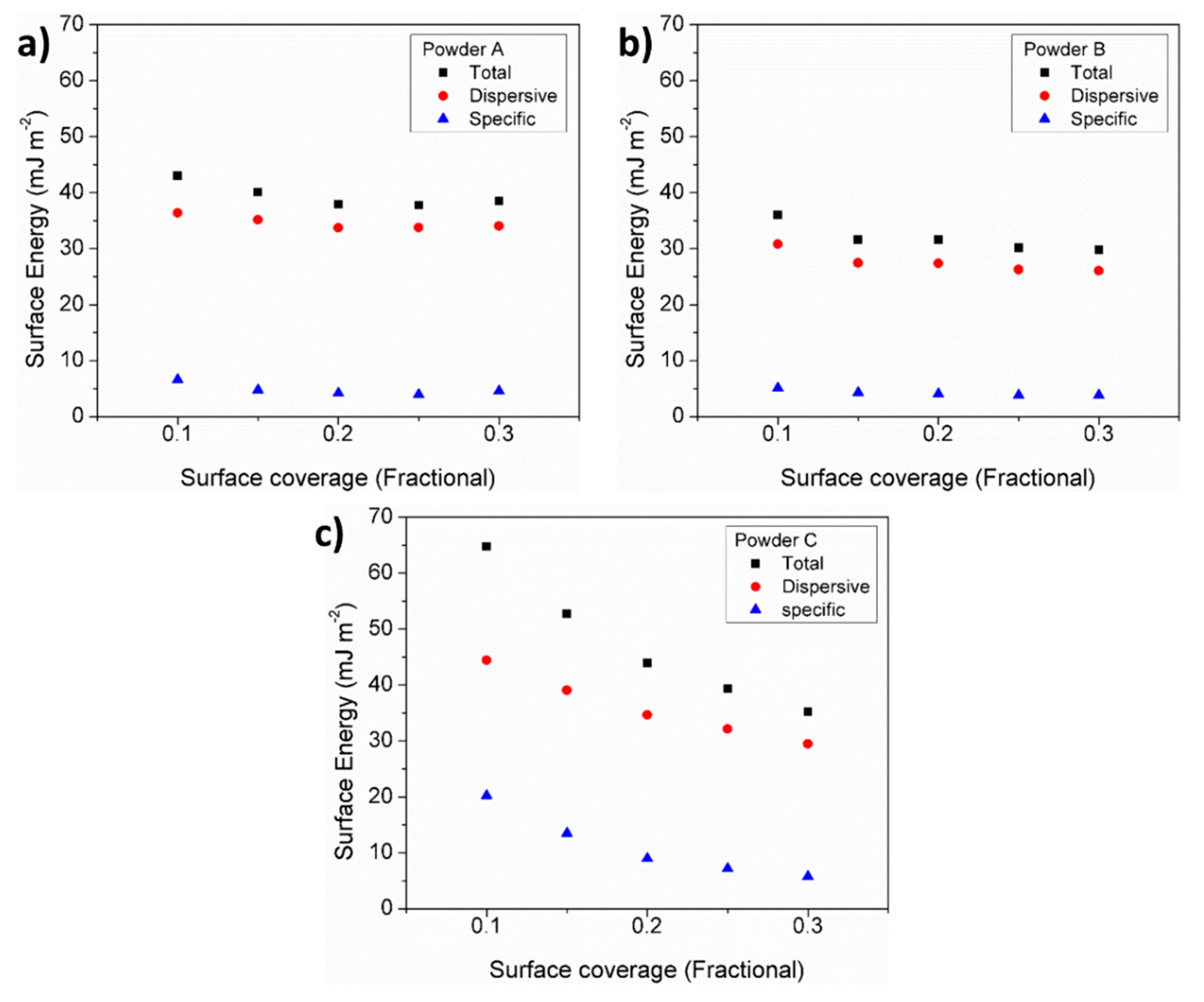


| Powder | D10 (µm) | D50 (µm) | D90 (µm) | Sw |
|---|---|---|---|---|
| Powder A | 48 | 63 | 83 | 10.6 |
| Powder B | 54 | 70 | 91 | 10.8 |
| Powder C | 14 | 31 | 58 | 4.2 |
| Powder | Hall Flow (s/50 g) | Carney Flow (s/50 g) |
|---|---|---|
| Powder A | 33.0 ± 0.4 | 6.1 ± 0.1 |
| Powder B | 32.7 ± 0.7 | 6.1 ± 0.1 |
| Powder C | No flow | 15.3 ± 0.4 |
| Powder | Hall Apparent Density (%) | Carney Apparent Density (%) | Arnold Apparent Density (%) |
|---|---|---|---|
| Powder A | 54.8 ± 0.5 | 55.5 ± 0.1 | 56.3 ± 0.1 |
| Powder B | 53.3 ± 0.5 | 54.8 ± 0.1 | 55.8 ± 0.1 |
| Powder C | - | 51.6 ± 0.4 | 52.7 ± 0.2 |
| Powder | Static Angle of Repose (°) |
|---|---|
| Powder A | 30 ± 4 |
| Powder B | 26 ± 3 |
| Powder C | 39 ± 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñiz-Lerma, J.A.; Nommeots-Nomm, A.; Waters, K.E.; Brochu, M. A Comprehensive Approach to Powder Feedstock Characterization for Powder Bed Fusion Additive Manufacturing: A Case Study on AlSi7Mg. Materials 2018, 11, 2386. https://doi.org/10.3390/ma11122386
Muñiz-Lerma JA, Nommeots-Nomm A, Waters KE, Brochu M. A Comprehensive Approach to Powder Feedstock Characterization for Powder Bed Fusion Additive Manufacturing: A Case Study on AlSi7Mg. Materials. 2018; 11(12):2386. https://doi.org/10.3390/ma11122386
Chicago/Turabian StyleMuñiz-Lerma, Jose Alberto, Amy Nommeots-Nomm, Kristian Edmund Waters, and Mathieu Brochu. 2018. "A Comprehensive Approach to Powder Feedstock Characterization for Powder Bed Fusion Additive Manufacturing: A Case Study on AlSi7Mg" Materials 11, no. 12: 2386. https://doi.org/10.3390/ma11122386
APA StyleMuñiz-Lerma, J. A., Nommeots-Nomm, A., Waters, K. E., & Brochu, M. (2018). A Comprehensive Approach to Powder Feedstock Characterization for Powder Bed Fusion Additive Manufacturing: A Case Study on AlSi7Mg. Materials, 11(12), 2386. https://doi.org/10.3390/ma11122386







