High Performance Anti-Corrosion Coatings of Poly (Vinyl Butyral) Composites with Poly N-(vinyl)pyrrole and Carbon Black Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. The Synthesis of PNVPY Nanoparticles
2.3. The Preparation of Composite Coating and Coated Zinc
2.4. Characterization Methods
3. Results and Discussion
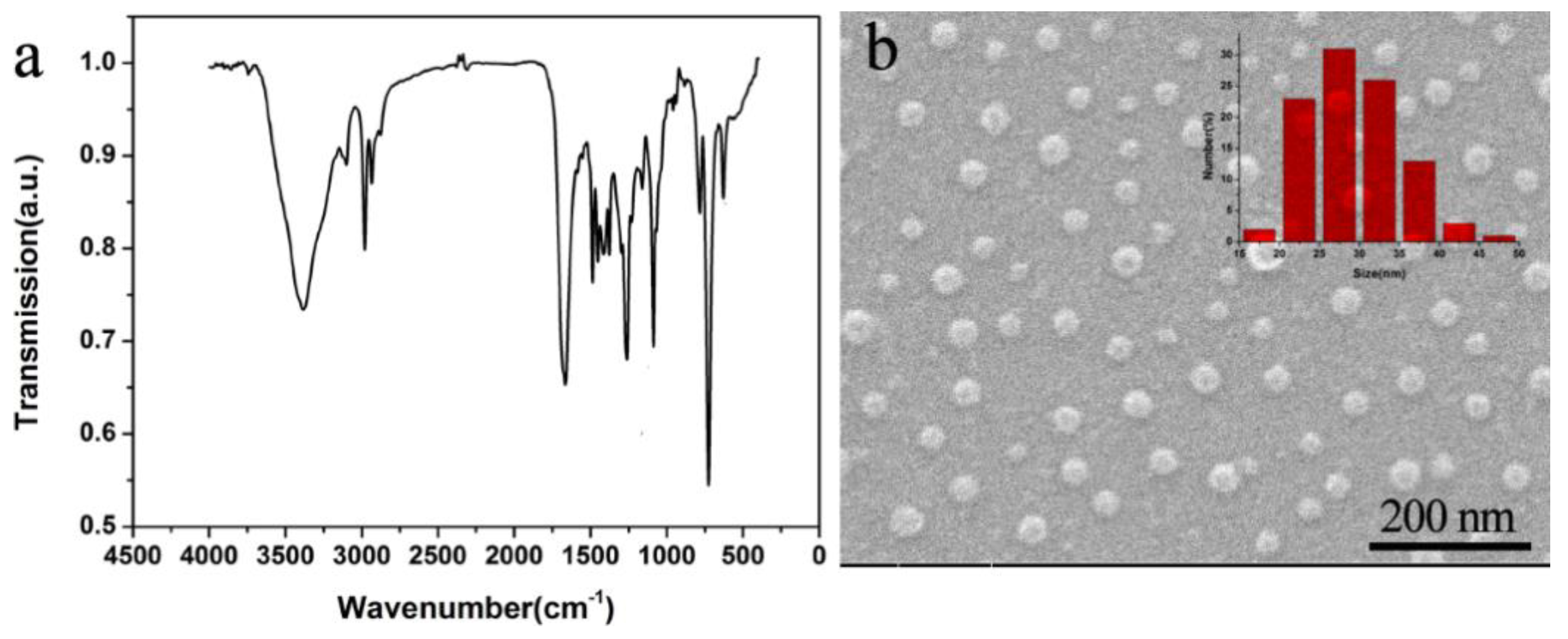
3.1. Characterization of PNVPY Nanoparticles
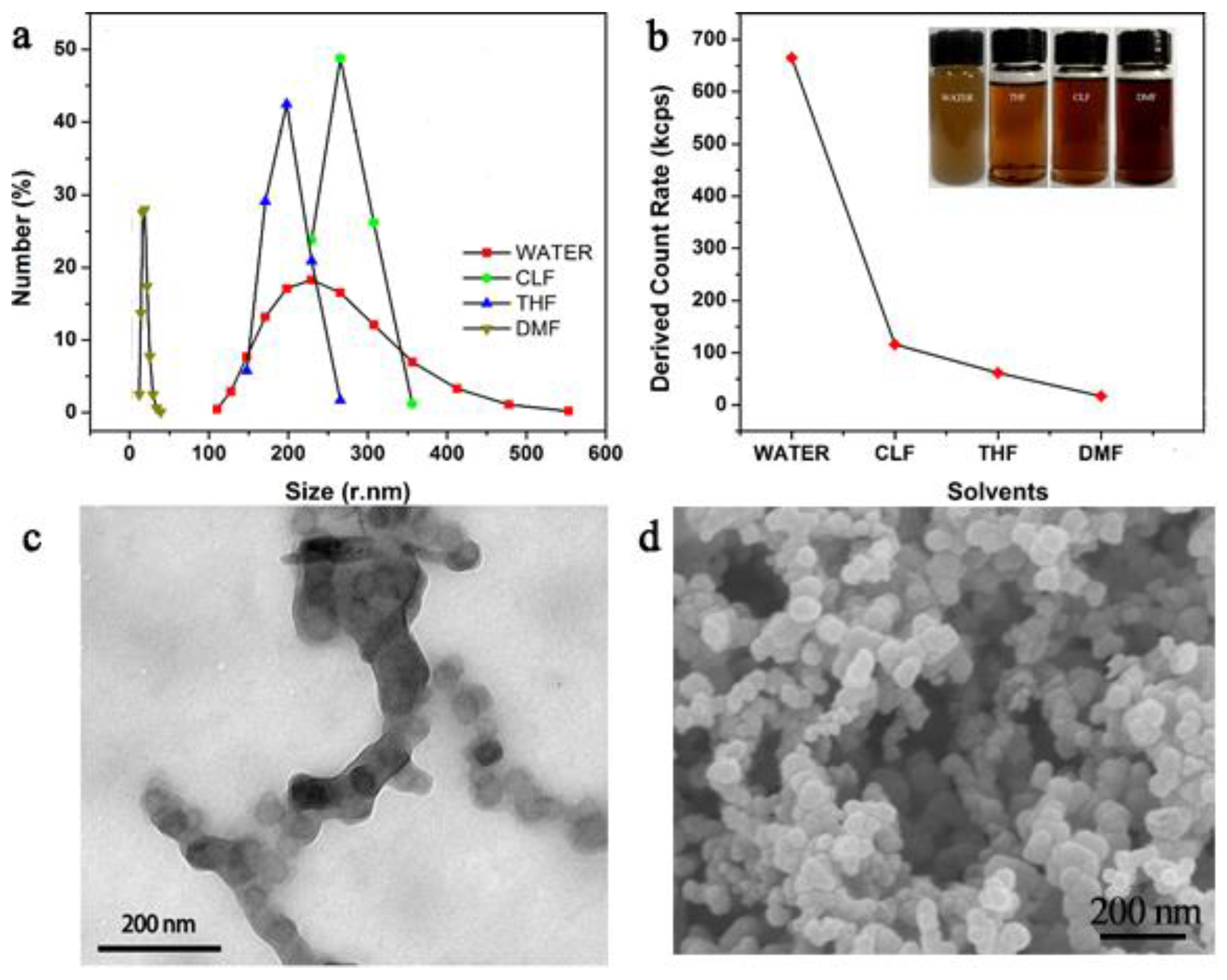
3.2. Morphology Analysis of Coatings
3.3. Corrosion Studies by Electrochemical Method
3.3.1. Open Circuit Potential (OCP) Measurement
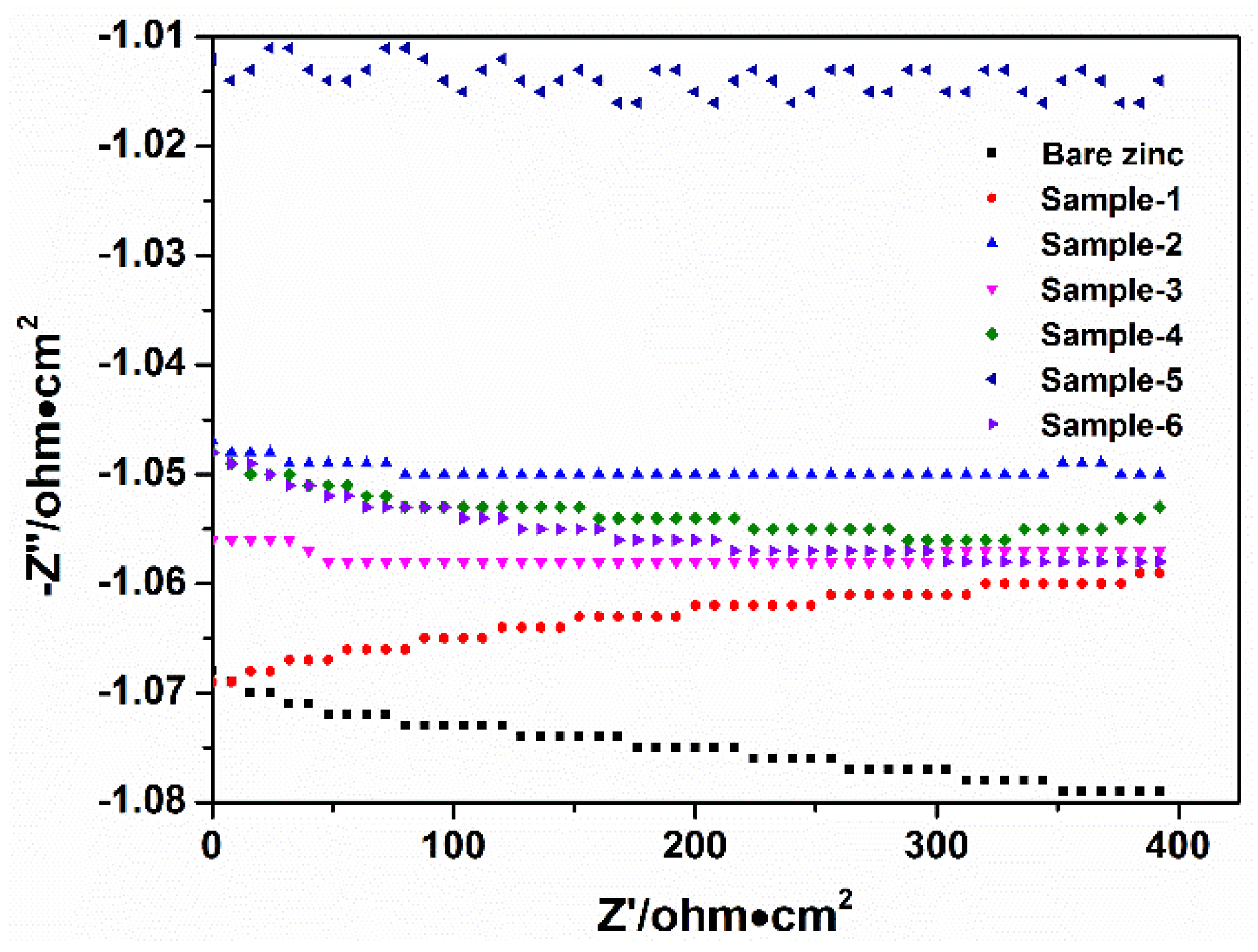
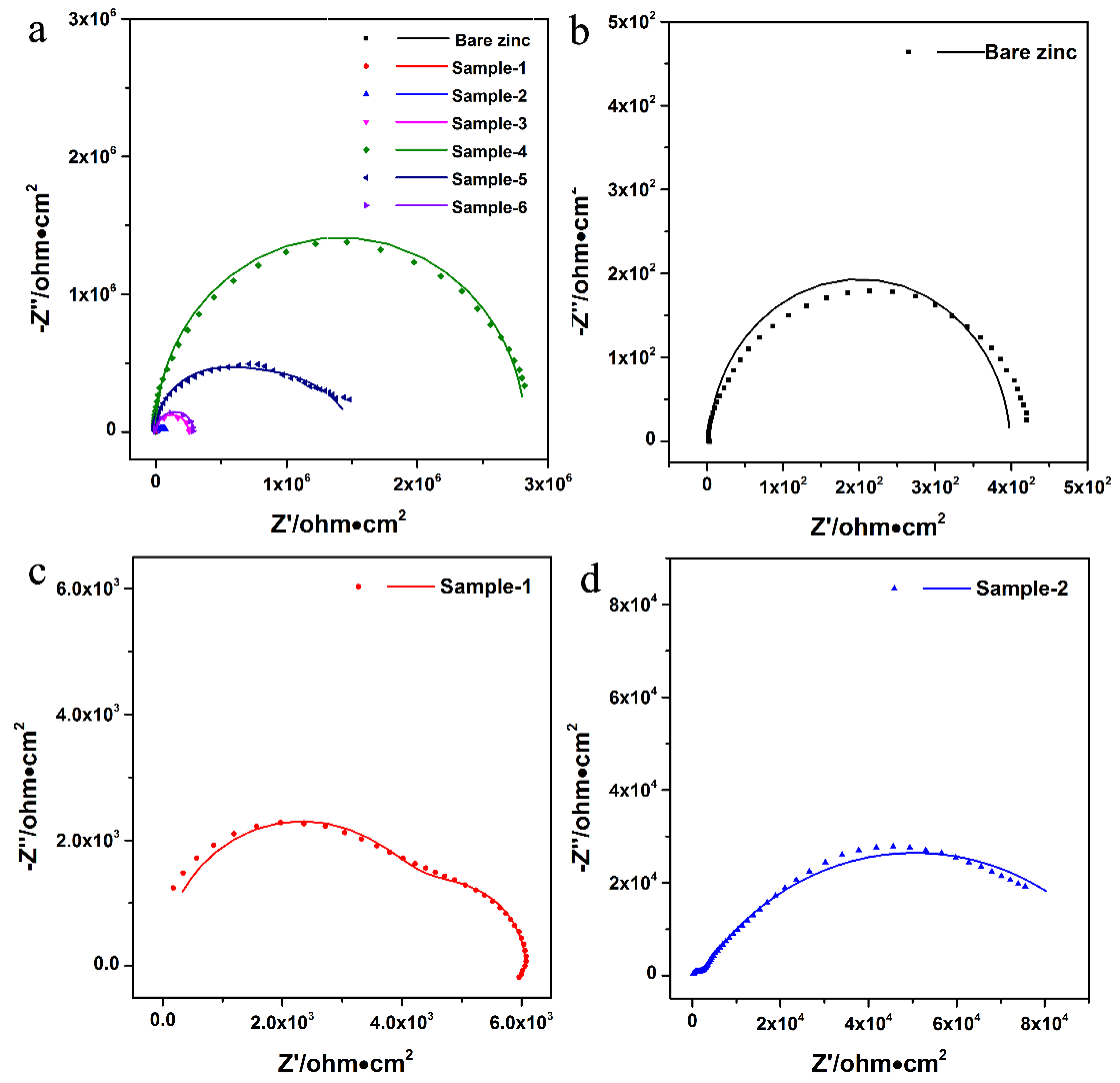
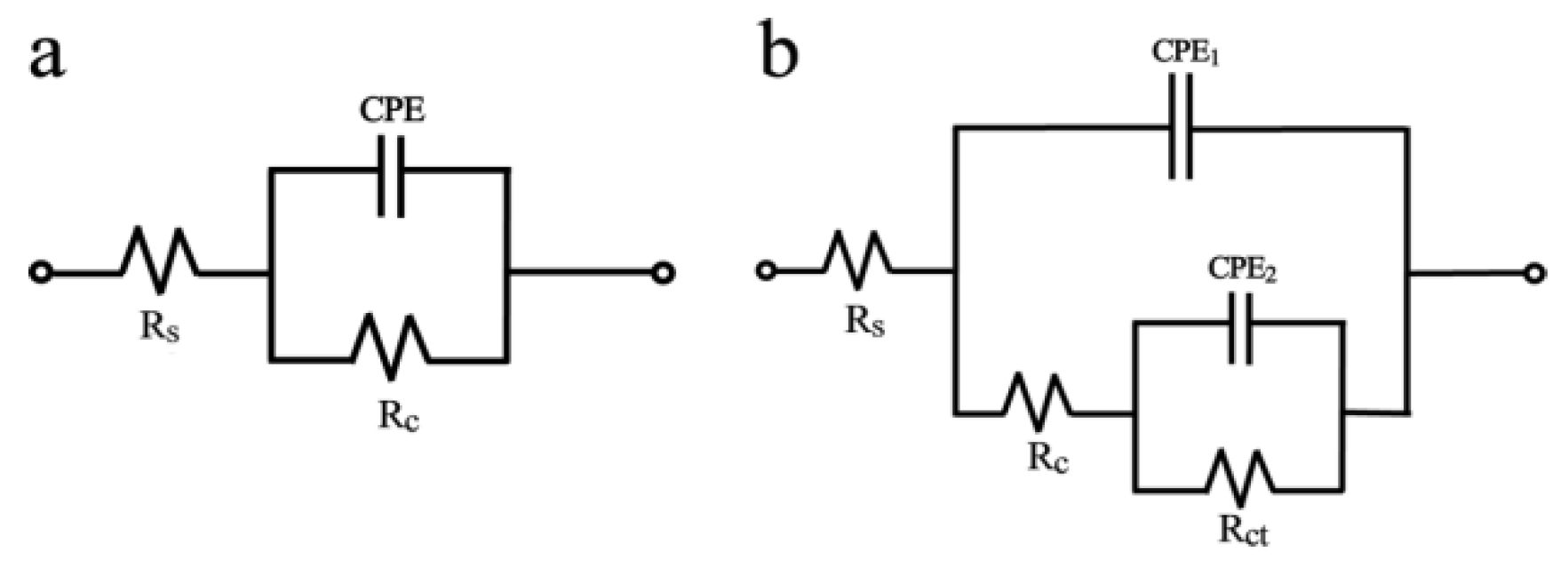
3.3.2. Electrochemical Impedance Spectroscopy
3.3.3. Potentiodynamic Polarization
3.4. Immersion Test of the Coatings without Defect
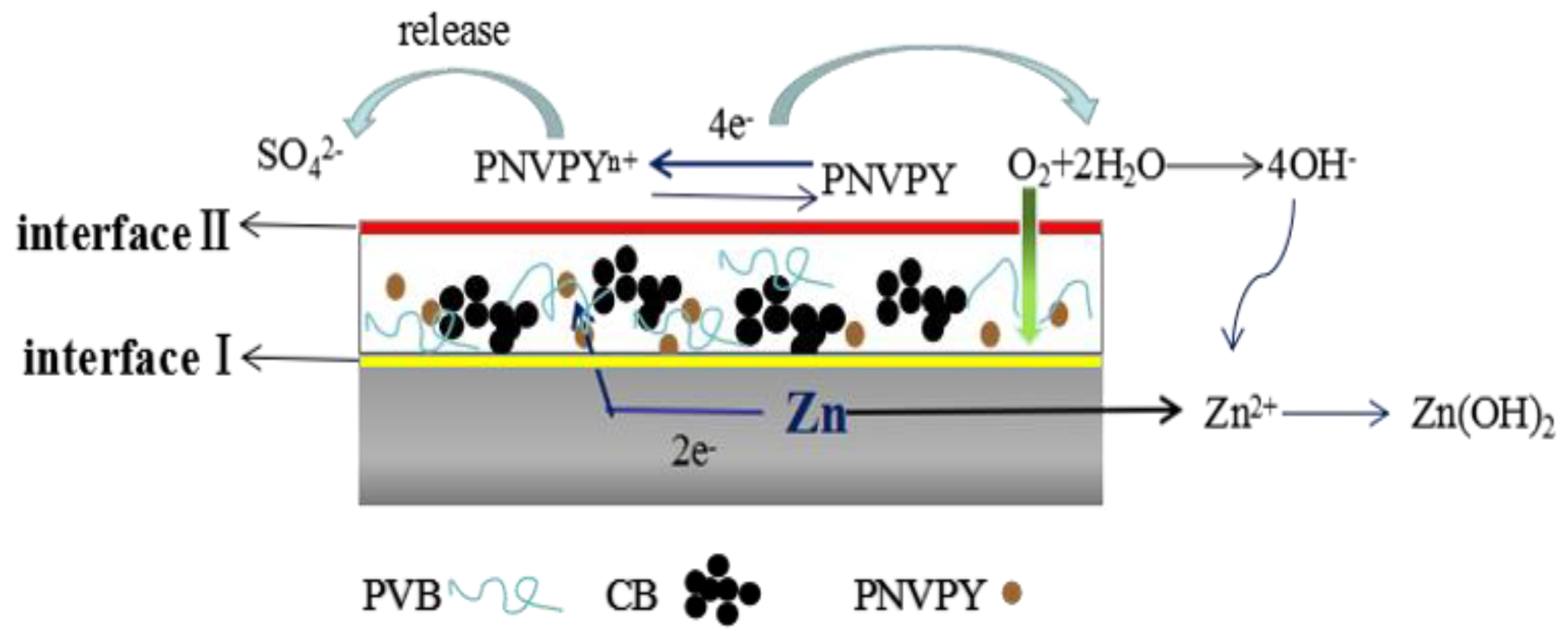
3.5. Anti-Corrosion Mechanism Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yao, B.; Wang, G.; Ye, J.; Li, X. Corrosion inhibition of carbon steel by polyaniline nanofibers. Mater. Lett. 2008, 62, 1775–1778. [Google Scholar] [CrossRef]
- Aramaki, K. Treatment of zinc surface with cerium(III) nitrate to prevent zinc corrosion in aerated 0.5 M NaCl. Corros. Sci. 2001, 43, 2201–2215. [Google Scholar] [CrossRef]
- Magalhães, A.A.O.; Margarit, I.C.P.; Mattos, O.R. Molybdate conversion coatings on zinc surfaces. J. Electroanal. Chem. 2004, 572, 433–440. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Liu, J.; Chen, P. A two-step roll coating phosphate/molybdate passivation treatment for hot-dip galvanized steel sheet. Corros. Sci. 2010, 52, 3385–3393. [Google Scholar] [CrossRef]
- Silva, C.G.; Margarit-Mattos, I.C.P.; Mattos, O.R. The molybdate–zinc conversion process. Corros. Sci. 2009, 51, 151–158. [Google Scholar] [CrossRef]
- Armelin, E.; Álvaro, M.; Ferreira, C.A.; Alemán, C. Polyaniline, polypyrrole and poly(3,4-ethylenedioxythiophene) as additives of organic coatings to prevent corrosion. Surf. Coat. Technol. 2009, 203, 3763–3769. [Google Scholar] [CrossRef]
- Elhalawany, N.; Mossad, M.A.; Zahran, M.K. Novel water based coatings containing some conducting polymers nanoparticles (CPNs) as corrosion inhibitors. Prog. Org. Coat. 2014, 77, 725–732. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, J.G.; Lucio-García, M.A.; Nicho, M.E.; Cruz-Silva, R.; Casales, M.; Valenzuela, E. Improvement on the corrosion protection of conductive polymers in pemfc environmets by adhesives. J. Power Sources 2007, 168, 184–189. [Google Scholar] [CrossRef]
- De Berry, D.W. Modification of the electrochemical and corrosion behavior of stainless steels with an electroactive coating. J. Electrochem. Soc. 1985, 132, 1022–1026. [Google Scholar] [CrossRef]
- Syed, J.A.; Lu, H.; Tang, S. Enhanced corrosion protective PANI-PAA/PEI multilayer composite coatings for 316SS by spin coating technique. Appl. Surf. Sci. 2015, 325, 160–169. [Google Scholar] [CrossRef]
- Wang, L.X.; Li, X.G.; Yang, Y.L. Preparation, properties and applications of polypyrroles. React. Funct. Polym. 2007, 47, 125–139. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Adelojus, B.; Wallaee, G.G. In situ electrochemical studies on the redox properties of polypyrrole in aqueous solutions. Eur. Polym. J. 1999, 35, 1761–1772. [Google Scholar] [CrossRef]
- Jakubiec, B.; Marois, Y.; Zhang, Z. In vitro cellular response to polypyrrole-coated woven polyester fabrics: Potential benefits of electrical conductivity. J. Biomed. Mater. Res. 1998, 41, 519–526. [Google Scholar] [CrossRef]
- Ryu, H.; Sheng, N.; Ohtsuk, T.; Fujita, S.; Kajiyama, H. Polypyrrole Film on 55% Al–Zn-Coated Steel for Corrosion Prevention. Corros. Sci. 2012, 56, 67–77. [Google Scholar] [CrossRef]
- Ruhi, G.; Bhandari, H.; Dhawan, S.K. Designing of corrosion resistant epoxy coatings embedded with polypyrrole/SiO2 composite. Prog. Org. Coat. 2014, 77, 1484–1498. [Google Scholar] [CrossRef]
- Rohwerder, M.; Michalik, A. Conducting polymers for corrosion protection: What makes the difference between failure and success? Electrochim. Acta 2007, 53, 1300–1313. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting polymers for corrosion protection: A review. J. Coat. Technol. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- Michalik, A.; Rohwerder, M. Conducting polymers for corrosion protection: A critical view. Z. Phys. Chem. 2005, 219, 1547–1559. [Google Scholar] [CrossRef]
- Rohwerder, M.; Isik-Uppenkamp, S.; Amarnath, C.A. Application of the Kelvin Probe method for screening the interfacial reactivity of conducting polymer based coatings for corrosion protection. Electrochim. Acta 2011, 56, 1889–1893. [Google Scholar] [CrossRef]
- Vimalanandan, A.; Lv, L.P.; Tran, T.H.; Landfester, K.; Crespy, D.; Rohwerder, M. Redox-responsive self-healing for corrosion protection. Adv. Mater. 2013, 25, 6980–6984. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Tran, T.H.; Yu, D.; Vimalanandan, A.; Hu, X.; Rohwerder, M. Novel conducting polymer based composite coatings for corrosion protection of zinc. Corros. Sci. 2015, 95, 110–116. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, K.; Lv, G.; Wang, G.; Yu, D.; Shao, J. UV-Catalytic Preparation of Polypyrrole Nanoparticles Induced by H2O2. J. Phys. Chem. C 2015, 119, 18707–18718. [Google Scholar] [CrossRef]
- Hao, L.; Zhu, K.; Zhang, S.; Yu, D. The green preparation of poly N-vinylpyrrole nanoparticles. RSC Adv. 2016, 6, 90354–90359. [Google Scholar] [CrossRef]
- Wang, J.; Neoh, K.G.; Kang, E.T. Comparative study of chemically synthesized and plasma polymerized pyrrole and thiophene thin films. Thin Solid Films 2004, 446, 205–217. [Google Scholar] [CrossRef]
- Pruna, A.; Pilan, L. Electrochemical study on new polymer composite for zinc corrosion protection. Compos. Part B Eng. 2012, 43, 3251–3257. [Google Scholar] [CrossRef]
- Kloprogge, J.; Wharton, D.; Hickey, L.; Frost, R.L. Infrared and Raman study of interlayer anions CO32−, NO3−, SO42− and ClO4− in Mg/Al-hydrotalcite. Am. Miner. 2002, 87, 623–629. [Google Scholar] [CrossRef]
- Ren, S. Electrochemically prepared poly (3-methylthiophene) films for passivation of 430 stainless steel. J. Electrochem. Soc. 1992, 139, 69–74. [Google Scholar] [CrossRef]
- Yağan, A.; Pekmez, N.Ö.; Yildiz, A. Electrochemical synthesis of poly (N-methylaniline) on an iron electrode and its corrosion performance. Electrochim. Acta 2008, 53, 5242–5251. [Google Scholar] [CrossRef]
- Sherif, E.S.M. Fabrication of various epoxy coatings for offshore applications and evaluating their mechanical properties and corrosion behavior. Int. J. Electrochem. Sci. 2013, 8, 3121–3131. [Google Scholar]
- Iroh, J.O.; Su, W. Corrosion performance of polypyrrole coating applied to low carbon steel by an electrochemical process. Electrochim. Acta 2000, 46, 15–24. [Google Scholar] [CrossRef]
- Wessling, B. Scientific and commercial breakthrough for organic metals. Synth. Met. 1997, 85, 1313–1318. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, T.A.; Pham, M.C.; Piro, B.; Normand, B.; Takenouti, H. Mechanism for protection of iron corrosion by an intrinsically electronic conducting polymer. J. Electroanal. Chem. 2004, 572, 225–234. [Google Scholar] [CrossRef]
- Song, Y.K.; Mansfeld, F. Development of a molybdate–phosphate–silane–silicate (MPSS) coating process for electrogalvanized steel. Corros. Sci. 2006, 48, 154–164. [Google Scholar] [CrossRef]
- Machnikova, E.; Pazderova, M.; Bazzaoui, M. Corrosion study of PVD coatings and conductive polymer deposited on mild steel: Part I: Polypyrrole. Surf. Coat. Technol. 2008, 202, 1543–1550. [Google Scholar] [CrossRef]
- Poornima, T.; Nayak, J.; Shetty, A.N. 3,4-Dimethoxybenzaldehydethiosemicarbazone as corrosion inhibitor for aged 18 Ni 250 grade maraging steel in 0.5 M sulfuric acid. J. Appl. Electrochem. 2011, 41, 223–233. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Muthukrishnan, S.; Venkatachari, G. Performance of polyaniline pigmented vinyl acrylic coating on steel in aqueous solutions. Prog. Org. Coat. 2006, 55, 5–10. [Google Scholar] [CrossRef]
- Kinlen, P.J.; Silverman, D.C.; Jeffreys, C.R. Corrosion protection using polyaniline coating formulations. Synth. Met. 1997, 85, 1327–1332. [Google Scholar] [CrossRef]
- Schauer, T.; Joos, A.; Dulog, L.; Eisenbach, C.D. Protection of iron against corrosion with polyaniline primers. Prog. Org. Coat. 1998, 33, 20–27. [Google Scholar] [CrossRef]



| Sample | PNVPY (wt.%) | CB (wt.%) | PVB (wt.%) |
|---|---|---|---|
| 1 | 0 | 0 | 100 |
| 2 | 2 | 0 | 98 |
| 3 | 0 | 2.3 | 97.7 |
| 4 | 1.9 | 2.3 | 95.8 |
| 5 | 6.4 | 2.2 | 91.4 |
| 6 | 8.9 | 2.1 | 89 |
| Sample | CPE1 | n1 | Rc (Ω cm2) | CPE2 | n2 | Rct (Ω cm2) |
|---|---|---|---|---|---|---|
| Bare zinc | 1.48 × 105 ± 5.8% | 0.98 ± 2.0% | 395 ± 5.4% | - | - | - |
| 1 | 1.51 × 109 ± 1.6% | 0.95 ± 2.0% | 4.59 × 103 ± 2.9% | 5.21 × 108 ± 1.7% | 0.98 ± 0.8% | 1.48 × 103 ± 3.5% |
| 2 | 1.54 × 108 ± 4.0% | 0.88 ± 5.2% | 3.89 × 103 ± 2.8% | 2.24 × 106 ± 2.9% | 0.63 ± 3.3% | 8.94 × 104 ± 4.4% |
| 3 | 4.24 × 1010 ± 3.6% | 0.96 ± 4.2% | 2.16 × 105 ± 5.5% | 3.08 × 106 ± 2.1% | 0.66 ± 5.8% | 2.86 × 104 ± 3.9% |
| 4 | 3.55 × 1010 ± 2.3% | 0.95 ± 3.5% | 2.71 × 106 ± 2.0% | 1.57 × 107 ± 1.8% | 0.78 ± 4.6% | 2.12 × 105 ± 2.7% |
| 5 | 1.67 × 1010 ± 2.2% | 0.94 ± 3.6% | 1.65 × 106 ± 1.9% | 7.27 × 109 ± 4.0% | 0.76 ± 2.5% | 1.42 × 105 ± 3.1% |
| 6 | 3.61 × 1010 ± 5.0% | 0.92 ± 3.4% | 2.65 × 105 ± 6.1% | 6.26 × 106 ± 2.5% | 0.82 ± 4.3% | 2.60 × 104 ± 2.7% |
| Sample | Ecorr (V) | icorr (A/cm2) | % P.E. |
|---|---|---|---|
| Bare zinc | −1.320 ± 4.3% | 2.665 × 10−4 ± 3.6% | ----------- |
| 1 | −1.069 ± 3.5% | 8.278 × 10−6 ± 6.1% | 96.89 |
| 2 | −1.015 ± 3.8% | 4.868 × 10−8 ± 6.1% | 99.98 |
| 3 | −1.009 ± 6.6% | 2.231 × 10−7 ± 3.7% | 99.92 |
| 4 | −0.821 ± 5.0% | 1.245 × 10−9 ± 5.2% | 99.99 |
| 5 | −1.030 ± 7.6% | 5.349 × 10−9 ± 6.3% | 99.98 |
| 6 | −0.930 ± 4.9% | 1.924 × 10−7 ± 4.0% | 99.93 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, L.; Lv, G.; Zhou, Y.; Zhu, K.; Dong, M.; Liu, Y.; Yu, D. High Performance Anti-Corrosion Coatings of Poly (Vinyl Butyral) Composites with Poly N-(vinyl)pyrrole and Carbon Black Nanoparticles. Materials 2018, 11, 2307. https://doi.org/10.3390/ma11112307
Hao L, Lv G, Zhou Y, Zhu K, Dong M, Liu Y, Yu D. High Performance Anti-Corrosion Coatings of Poly (Vinyl Butyral) Composites with Poly N-(vinyl)pyrrole and Carbon Black Nanoparticles. Materials. 2018; 11(11):2307. https://doi.org/10.3390/ma11112307
Chicago/Turabian StyleHao, Lu, Guowei Lv, Yaqian Zhou, Kaiming Zhu, Mochen Dong, Yuhang Liu, and Demei Yu. 2018. "High Performance Anti-Corrosion Coatings of Poly (Vinyl Butyral) Composites with Poly N-(vinyl)pyrrole and Carbon Black Nanoparticles" Materials 11, no. 11: 2307. https://doi.org/10.3390/ma11112307





