The Influence of Stored Energy on Grain Boundary Chemistry and Intergranular Corrosion Development in AA2024-T3 Alloy
Abstract
:1. Introduction
2. Experimental Methods
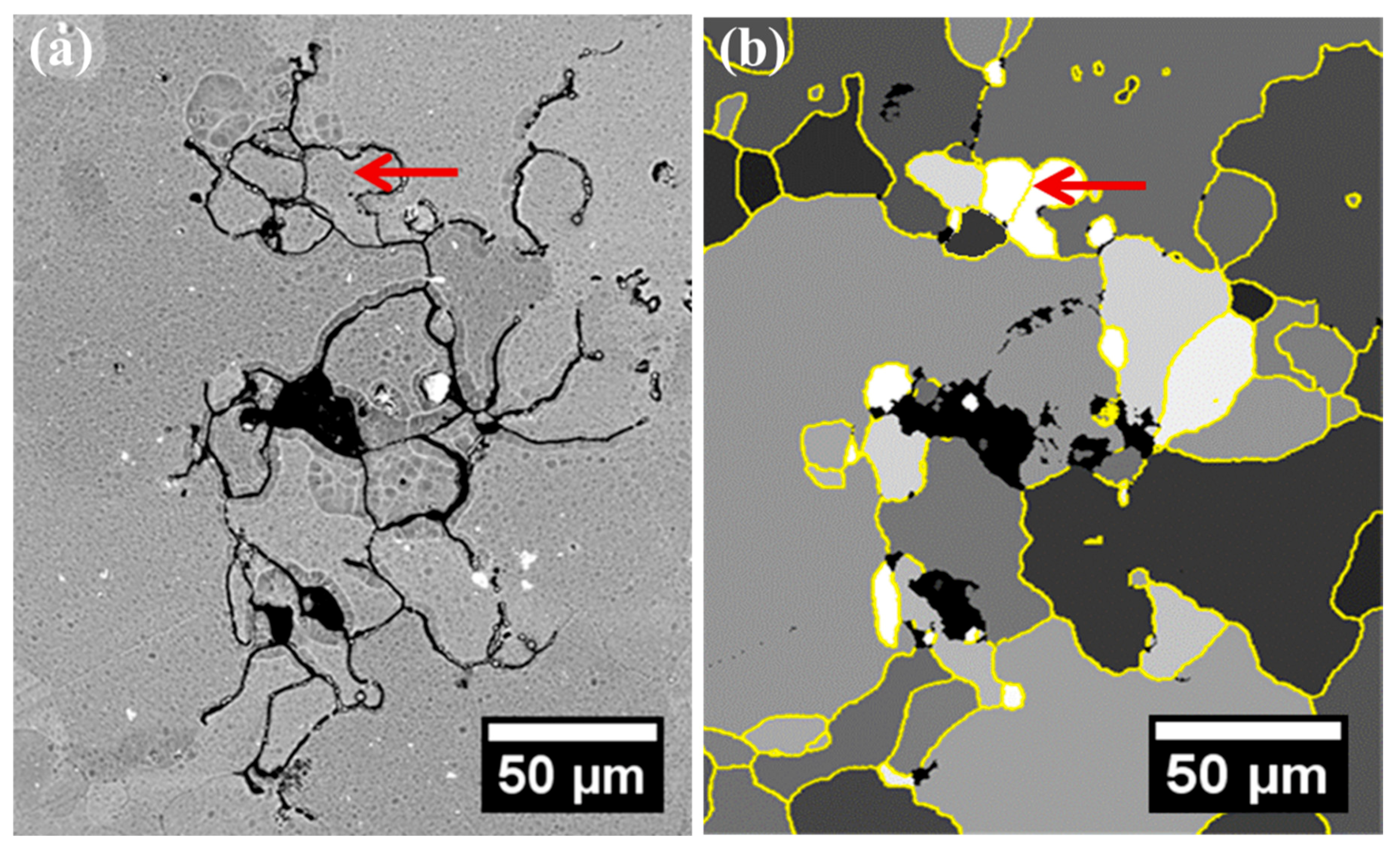
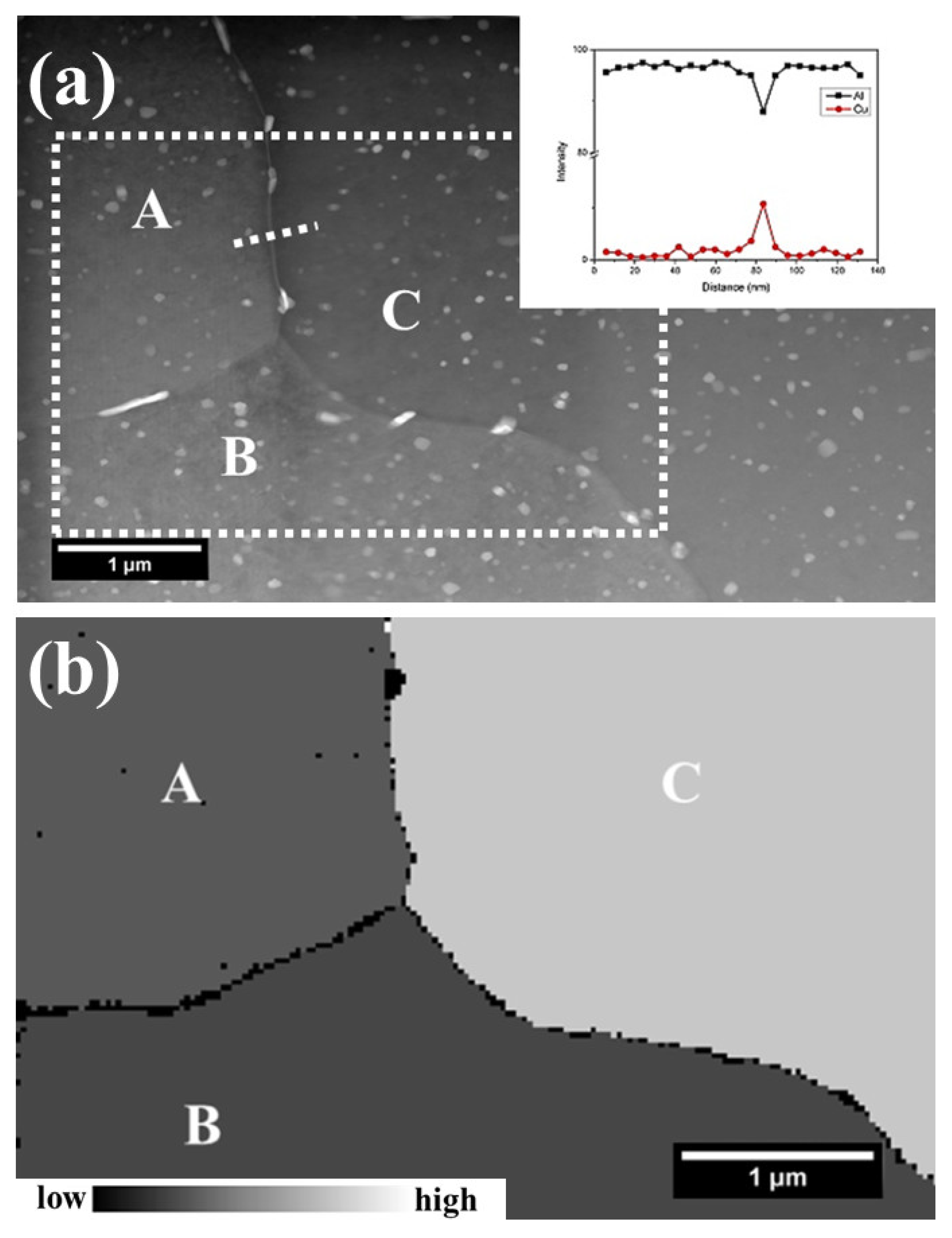
3. Results
4. Discussion
4.1. Microstructural Evolution
4.2. Intergranular Corrosion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Zhou, X.; Hashimoto, T.; Liu, B. Localized corrosion in AA2024-T351 aluminium alloy: Transition from intergranular corrosion to crystallographic pitting. Mater. Charact. 2017, 130, 230–236. [Google Scholar] [CrossRef]
- Malladi, S.R.K.; Xu, Q.; Tichelaar, F.D.; Zandbergen, H.W.; Hannour, F.; Mol, J.M.C.; Terryn, H. Early stages during localized corrosion of AA2024 TEM specimens in chloride environment. Surf. Interface Anal. 2013, 45, 1619–1625. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, C.; Hashimoto, T.; Hughes, A.E.; Thompson, G.E. Study of localized corrosion in AA2024 aluminium alloy using electron tomography. Corros. Sci. 2012, 58, 299–306. [Google Scholar] [CrossRef]
- Glenn, A.M.; Hughes, A.E.; Torpy, A.; Nolze, G.; Birbilis, N. Defect density associated with constituent particles in AA2024-T3 and its role in corrosion. Surf. Interface Anal. 2016, 48, 780–788. [Google Scholar] [CrossRef]
- Kyzioł, K.; Mędala, M.; Kaczmarek, Ł. Influence of plasmochemical modification of Al-Cu-Mg alloys on surface structure and functional properties. Vacuum 2014, 105, 52–58. [Google Scholar] [CrossRef]
- Kyzioł, K.; Kaczmarek, L.; Stanisława, J. Surfaces modification of Al-Cu alloys by plasma-assisted CVD. Solid State Phenom. 2013, 199, 496–501. [Google Scholar] [CrossRef]
- Taweesub, K.; Visuttipitukul, P.; Tungkasmita, S.; Paosawatyanyong, B. Nitridation of Al-6%Cu alloy by RF plasma process. Surf. Coat. Technol. 2010, 204, 3091–3095. [Google Scholar] [CrossRef]
- Sun, F.; Nash, G.L.; Li, Q.; Liu, E.; He, C.; Shi, C.; Zhao, N. Effect of Sc and Zr additions on microstructures and corrosion behavior of Al-Cu-Mg-Sc-Zr alloys. J. Mater. Sci. Technol. 2017, 33, 1015–1022. [Google Scholar] [CrossRef]
- Navaser, M.; Atapour, M. Effect of friction stir processing on pitting corrosion and intergranular attack of 7075 aluminum alloy. J. Mater. Sci. Technol. 2017, 33, 155–165. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, B.; Zhou, X.; Wang, J.; Luo, C.; Sun, Z.; Tang, Z.; Lu, F. Corrosion behavior of friction stir welded 2A97 Al-Cu-Li alloy. Corrosion 2017, 73, 988–997. [Google Scholar] [CrossRef]
- Svenningsen, G.; Larsen, M.H.; Nordlien, J.H.; Nisancioglu, K. Effect of thermomechanical history on intergranular corrosion of extruded AlMgSi(Cu) model alloy. Corros. Sci. 2006, 48, 3969–3987. [Google Scholar] [CrossRef]
- Svenningsen, G.; Larsen, M.H.; Walmsley, J.C.; Nordlien, J.H.; Nisancioglu, K. Effect of artificial aging on intergranular corrosion of extruded AlMgSi alloy with small Cu content. Corros. Sci. 2006, 48, 1528–1543. [Google Scholar] [CrossRef]
- Svenningsen, G.; Larsen, M.H.; Nordlien, J.H.; Nisancioglu, K. Effect of high temperature heat treatment on intergranular corrosion of AlMgSi(Cu) model alloy. Corros. Sci. 2006, 48, 258–272. [Google Scholar] [CrossRef]
- Svenningsen, G.; Lein, J.E.; Bjorgum, A.; Nordlien, J.H.; Yu, Y.D.; Nisancioglu, K. Effect of low copper content and heat treatment on intergranular corrosion of model AlMgSi alloys. Corros. Sci. 2006, 48, 226–242. [Google Scholar] [CrossRef]
- Hashimoto, T.; Zhang, X.; Zhou, X.; Skeldon, P.; Haigh, S.J.; Thompson, G.E. Investigation of dealloying of S phase (Al2CuMg) in AA 2024-T3 aluminium alloy using high resolution 2D and 3D electron imaging. Corros. Sci. 2016, 103, 157–164. [Google Scholar] [CrossRef]
- Zhang, X.; Hashimoto, T.; Lindsay, J.; Zhou, X. Investigation of the de-alloying behaviour of θ-phase (Al2Cu) in AA2024-T351 aluminium alloy. Corros. Sci. 2016, 108, 85–93. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, B.; Zhou, X.; Wang, J.; Hashimoto, T.; Luo, C.; Sun, Z.; Tang, Z.; Lu, F. Laser welding introduced segregation and its influence on the corrosion behaviour of Al-Cu-Li alloy. Corros. Sci. 2018, 135, 177–191. [Google Scholar] [CrossRef]
- Shi, H.W.; Tian, Z.H.; Hu, T.H.; Liu, F.C.; Han, E.H.; Taryba, M.; Lamaka, S.V. Simulating corrosion of Al2CuMg phase by measuring ionic currents, chloride concentration and pH. Corros. Sci. 2014, 88, 178–186. [Google Scholar] [CrossRef]
- Hashimoto, T.; Zhou, X.; Skeldon, P.; Thompson, G.E. Structure of the copper-enriched layer introduced by anodic oxidation of copper-containing aluminium alloy. Electrochim. Acta 2015, 179, 394–401. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Hashimoto, T.; Liu, B.; Luo, C.; Sun, Z.; Tang, Z.; Lu, F.; Ma, Y. Corrosion behaviour of 2A97-T6 Al-Cu-Li alloy: The influence of non-uniform precipitation. Corros. Sci. 2018, 132, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Hashimoto, T.; Lindsay, J.; CillCa, O.; Luo, C.; Sun, Z.; Zhang, X.; Tang, Z. The influence of grain structure on the corrosion behaviour of 2A97-T3 Al-Cu-Li alloy. Corros. Sci. 2017, 116, 14–21. [Google Scholar] [CrossRef]
- Sha, G.; Marceau, R.K.W.; Gao, X.; Muddle, B.C.; Ringer, S.P. Nanostructure of aluminium alloy 2024: Segregation, clustering and precipitation processes. Acta Mater. 2011, 59, 1659–1670. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, C.; Ma, Y.; Hashimoto, T.; Thompson, G.E.; Hughes, A.E.; Skeldon, P. Grain-stored energy and the propagation of intergranular corrosion in AA2xxx aluminium alloys. Surf. Interface Anal. 2013, 45, 1543–1547. [Google Scholar] [CrossRef]
- Luo, C.; Zhou, X.; Thompson, G.E.; Hughes, A.E. Observations of intergranular corrosion in AA2024-T351: The influence of grain stored energy. Corros. Sci. 2012, 61, 35–44. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Liao, Y.; Yi, Y.; Wu, H.; Wang, Z.; Huang, W. Localized corrosion in AA 2099-T83 aluminium-lithium alloy: The role of grain orientation. Corros. Sci. 2016, 107, 41–48. [Google Scholar] [CrossRef]
- Guerin, M.; Alexis, J.; Andrieu, E.; Laffont, L.; Lefebvre, W.; Odemer, G.; Blanc, C. Identification of the metallurgical parameters explaining the corrosion susceptibility in a 2050 aluminium alloy. Corros. Sci. 2016, 102, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Bałkowiec, A.; Michalski, J.; Matysiak, H.; Kurzydlowski, K.J. Influence of grain boundaries misorientation angle on intergranular corrosion in 2024-T3 aluminium. Mater. Sci.-Pol. 2011, 29, 305–311. [Google Scholar] [CrossRef]
- Brunner, J.G.; Birbilis, N.; Ralston, K.D.; Virtanen, S. Impact of ultrafine-grained microstructure on the corrosion of aluminium alloy AA2024. Corros. Sci. 2012, 57, 209–214. [Google Scholar] [CrossRef]
- Parvizi, R.; Hughes, A.E.; Tan, M.Y.; Marceau, R.K.W.; Forsyth, M.; Cizek, P.; Glenn, A.M. Probing corrosion initiation at interfacial nanostructures of AA2024-T3. Corros. Sci. 2017, 116, 98–109. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, X.; Zhou, X.; Sun, Z.; Zhang, X.; Tang, Z.; Lu, F.; Thompson, G.E. Characterization of Localized Corrosion in an Al-Cu-Li Alloy. J. Mater. Eng. Perform. 2016, 25, 1811–1819. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Ma, Y.; Thompson, G.E.; Luo, C.; Sun, Z.; Zhang, X.; Tang, Z. The propagation of localized corrosion in Al-Cu-Li alloy. Surf. Interface Anal. 2016, 48, 745–749. [Google Scholar] [CrossRef]
- Hughes, A.E.; Boag, A.; Glenn, A.M.; McCulloch, D.; Muster, T.H.; Ryan, C.; Luo, C.; Zhou, X.; Thompson, G.E. Corrosion of AA2024-T3 Part II Co-operative corrosion. Corros. Sci. 2011, 53, 27–39. [Google Scholar] [CrossRef]
- Glenn, A.M.; Muster, T.H.; Luo, C.; Zhou, X.; Thompson, G.E.; Boag, A.; Hughes, A.E. Corrosion of AA2024-T3 Part III: Propagation. Corros. Sci. 2011, 53, 40–50. [Google Scholar] [CrossRef]
- Boag, A.; Hughes, A.E.; Glenn, A.M.; Muster, T.H.; McCulloch, D. Corrosion of AA2024-T3 Part I. Localised corrosion of isolated IM particles. Corros. Sci. 2011, 53, 17–26. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Nilsson, J.O.; Dong, Z.; Cai, C. Corrosion behaviour of AA6082 Al-Mg-Si alloy extrusion: Recrystallized and non-recrystallized structures. Corros. Sci. 2018, 144, 163–171. [Google Scholar] [CrossRef]
- Wang, S.C.; Starink, M.J. Precipitates and intermetallic phases in precipitation hardening Al-Cu-Mg-(Li) based alloys. Int. Mater. Rev. 2005, 50, 193–215. [Google Scholar] [CrossRef]
- Wang, S.C.; Starink, M.J.; Gao, N. Precipitation hardening in Al-Cu-Mg alloys revisited. Scr. Mater. 2006, 54, 287–291. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Miao, F.; Sun, W.; Fang, B.; Song, R.; Zheng, Z. Improving the intergranular corrosion resistance of Al–Mg–Si–Cu alloys without strength loss by a two-step aging treatment. Mater. Sci. Eng. A 2014, 590, 267–273. [Google Scholar] [CrossRef]
- Engler, O.; Schäfer, C.; Brinkman, H.J.; Brecht, J.; Beiter, P.; Nijhof, K. Flexible rolling of aluminium alloy sheet—Process optimization and control of materials properties. J. Mater. Process. Technol. 2016, 229, 139–148. [Google Scholar] [CrossRef]
- de Haas, M.; van Scherpenzeel, S.M.; de Hosson, J.T.M. Grain boundary segregation and precipitation in aluminium alloy AA6061. Mater. Sci. Forum 2006, 519, 467–472. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Huang, W.; Liao, Y.; Chen, X.; Zhang, X.; Thompson, G.E. Crystallographic defects induced localised corrosion in AA2099-T8 aluminium alloy. Corros. Eng. Sci. Technol. 2015, 50, 420–424. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhou, X.; Cai, G.; Yu, Y.; Lu, X.; Jiao, Y.; Dong, Z. The Influence of Stored Energy on Grain Boundary Chemistry and Intergranular Corrosion Development in AA2024-T3 Alloy. Materials 2018, 11, 2299. https://doi.org/10.3390/ma11112299
Zhang X, Zhou X, Cai G, Yu Y, Lu X, Jiao Y, Dong Z. The Influence of Stored Energy on Grain Boundary Chemistry and Intergranular Corrosion Development in AA2024-T3 Alloy. Materials. 2018; 11(11):2299. https://doi.org/10.3390/ma11112299
Chicago/Turabian StyleZhang, Xinxin, Xiaorong Zhou, Guangyi Cai, Yang Yu, Xueqin Lu, Yanbin Jiao, and Zehua Dong. 2018. "The Influence of Stored Energy on Grain Boundary Chemistry and Intergranular Corrosion Development in AA2024-T3 Alloy" Materials 11, no. 11: 2299. https://doi.org/10.3390/ma11112299




