Effect of Plasma Treatment and Its Post Process Duration on Shear Bonding Strength and Antibacterial Effect of Dental Zirconia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. NTAPP
2.3. Resin Cement
2.4. Surface Analysis
2.4.1. Surface Roughness
2.4.2. X-ray Diffraction
2.4.3. Energy-Dispersive X-ray Spectroscopy and X-ray Photoelectron Spectroscopy
2.4.4. Apparent Contact Angle
2.5. SBS Testing
2.6. S. mutans Biofilm Formation
2.6.1. Preparation
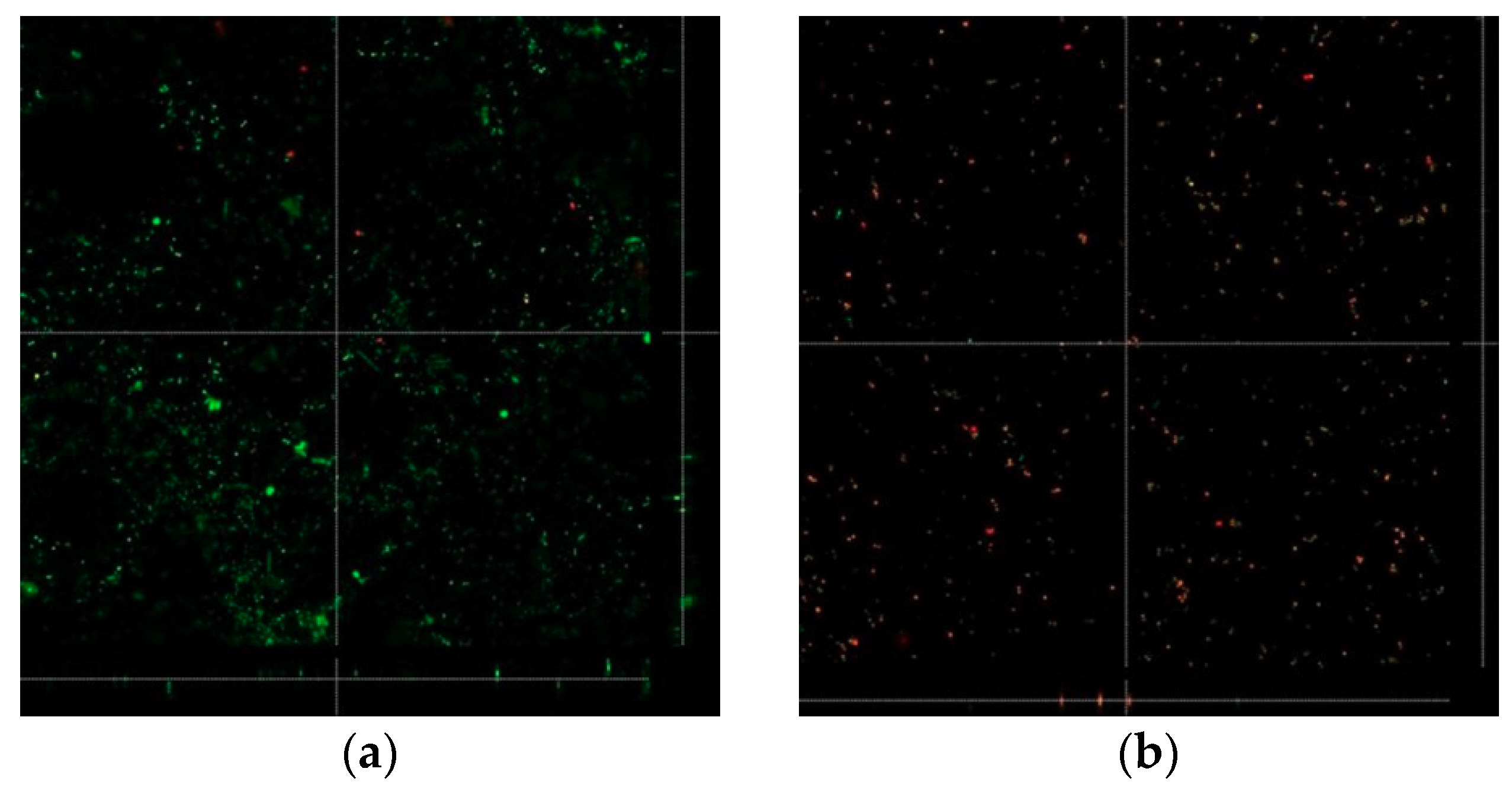
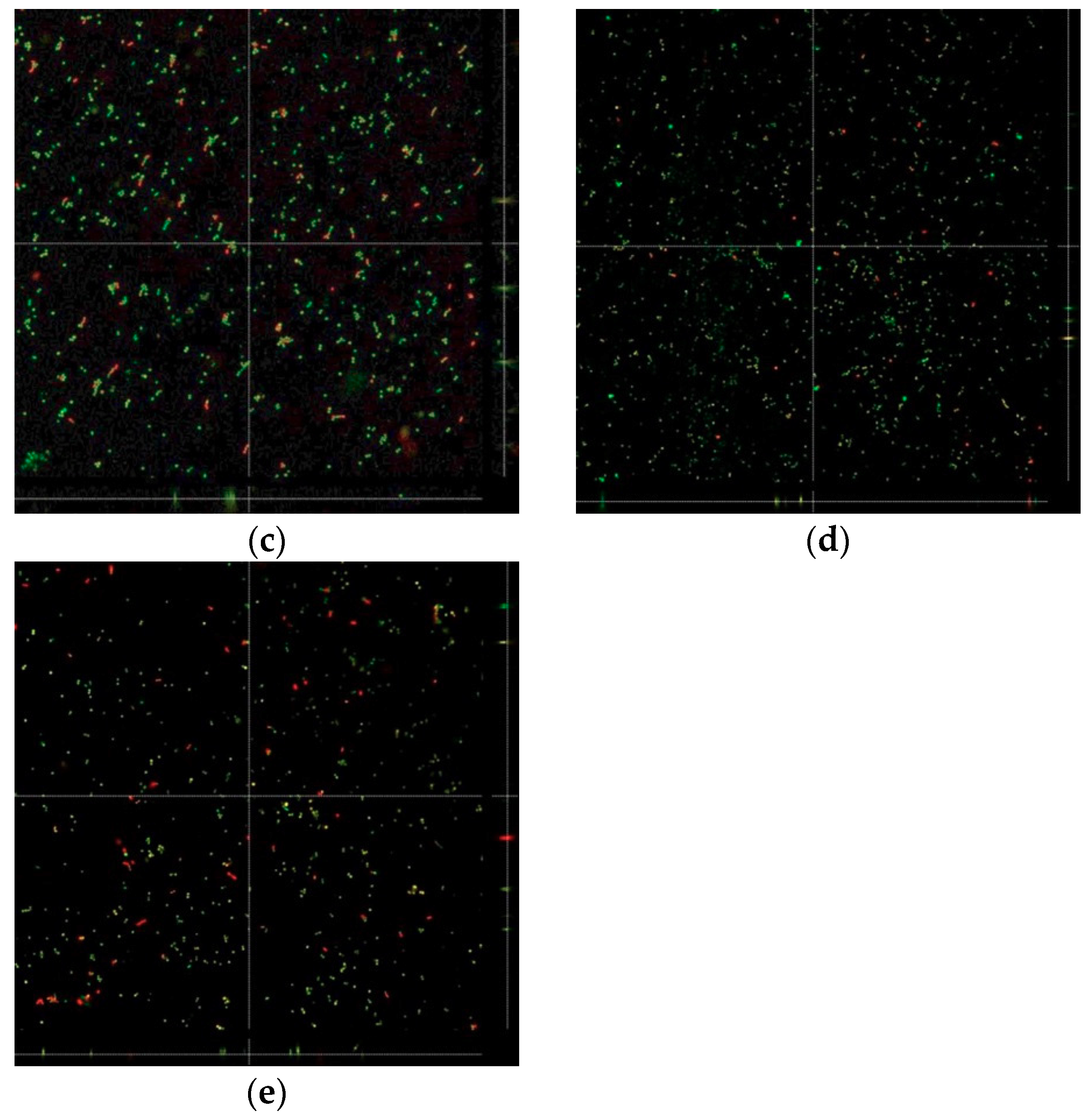
2.6.2. Confocal Laser Microscopy Evaluation of Biofilm Formation
2.7. Statistical Analysis
3. Results
3.1. Surface Analysis
3.1.1. Surface Roughness
3.1.2. XRD
3.1.3. EDS and XPS
3.1.4. Apparent Contact Angle
3.2. SBS Test
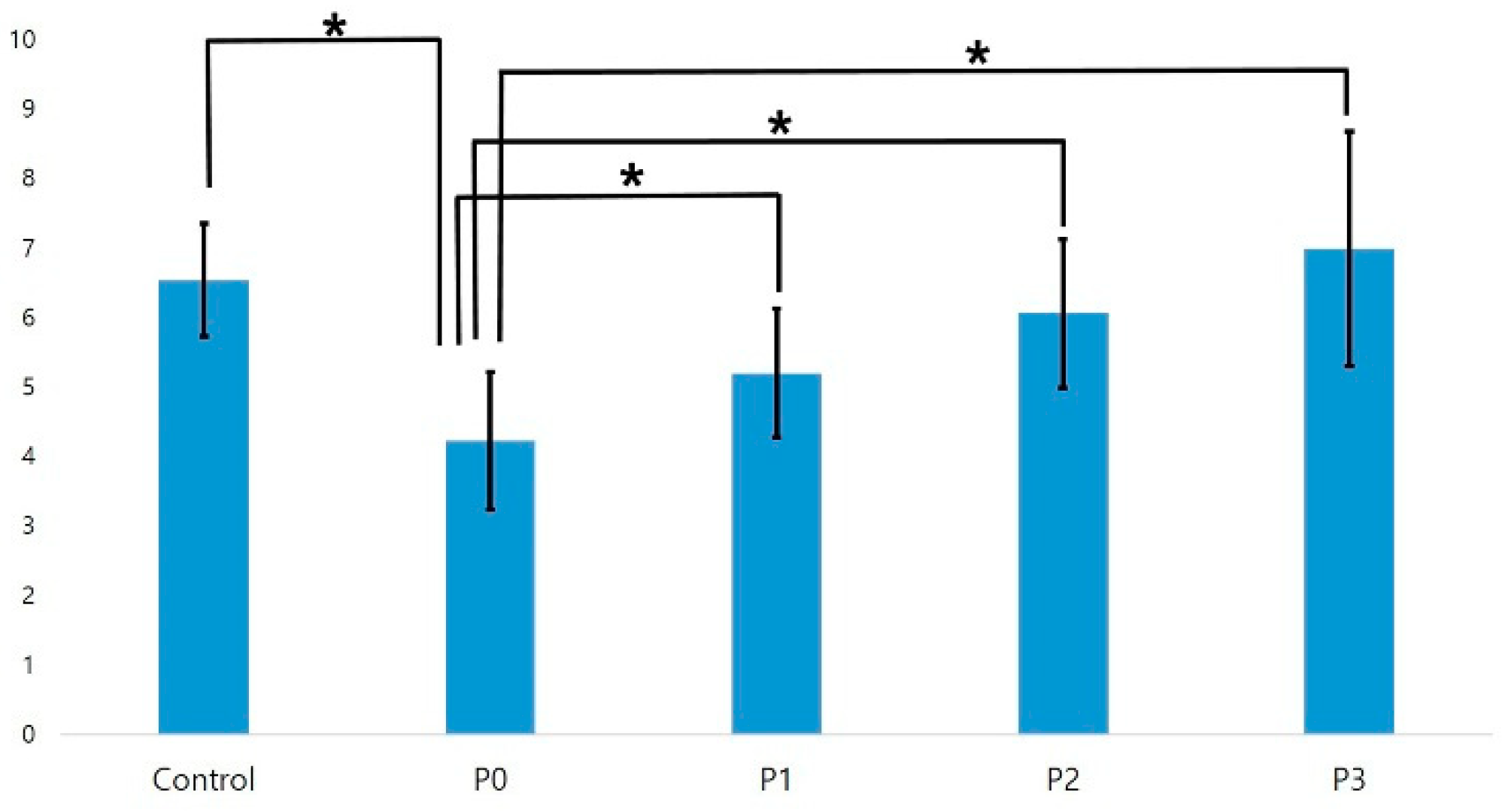
3.2.1. SBS Test with Panavia F2.0
3.2.2. SBS Test with RelyXTM U200
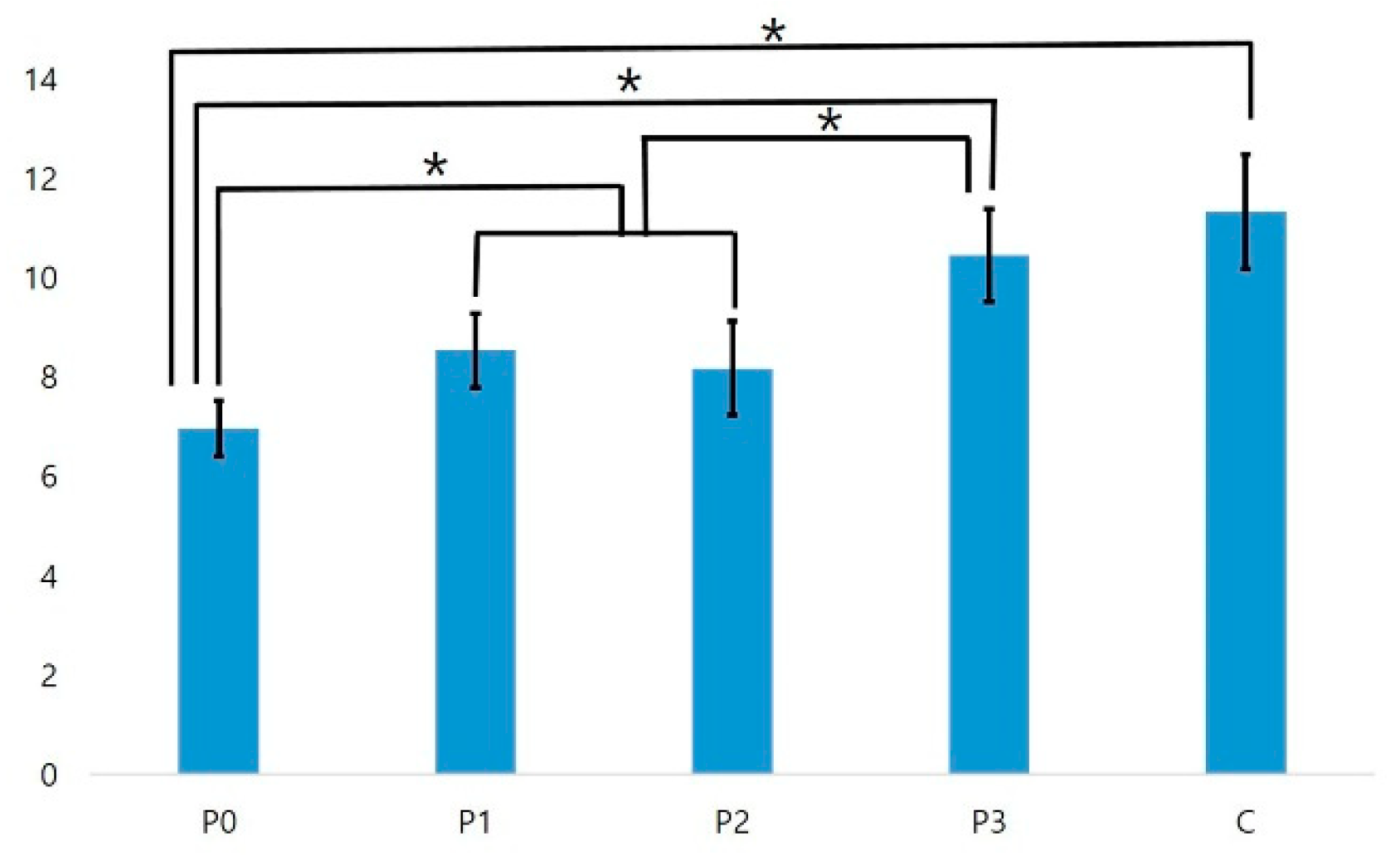
3.3. Biofilm Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic steel? Nature 1975, 258, 703–704. [Google Scholar] [CrossRef]
- Garvie, R.C.; Nicholson, P.S. Phase analysis in zirconia systems. J. Am. Ceram. Soc. 1972, 55, 303–305. [Google Scholar] [CrossRef]
- Deville, S.; Chevalier, J.; Gremillard, L. Influence of surface finish and residual stresses on the ageing sensitivity of biomedical grade zirconia. Biomaterials 2006, 27, 2186–2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Bagno, A.; Di Bello, C. Surface treatments and roughness properties of Ti-based biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.F.; Takabe, J.; Guo, J.; Abron, A.; Holmen, A.; Ellingsen, J.E. Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials 2006, 27, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Matinlinna, J.P.; Lassila, L.V.; Vallittu, P.K. The effect of five silane coupling agents on the bond strength of a luting cement to a silica-coated titanium. Dent. Mater. 2007, 23, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Matinlinna, J.P.; Lassila, L.V.; Kangasniemi, I.; Vallittu, P.K. Isocyanato- and methacryloxysilanes promote bis-GMA adhesion to titanium. J. Dent. Res. 2005, 84, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Eckert, G.J.; Platt, J.A. A statistical evaluation of microtensile bond strength methodology for dental adhesives. Dent. Mater. 2007, 23, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Isgro, G.; Pallav, P.; van der Zel, J.M.; Feilzer, A.J. The influence of the veneering porcelain and different surface treatments on the biaxial flexural strength of a heat-pressed ceramic. J. Prosthet. Dent. 2003, 90, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Dundar, M.; Ozcan, M.; Comlekoglu, E.; Gungor, M.A.; Artunc, C. Bond strengths of veneering ceramics to reinforced ceramic core materials. Int. J. Prosthodont. 2005, 18, 71–72. [Google Scholar] [PubMed]
- Duarte, P.M.; Reis, A.F.; de Freitas, P.M.; Ota-Tsuzuki, C. Bacterial adhesion on smooth and rough titanium surfaces after treatment with different instruments. J. Periodontol. 2009, 80, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold atmospheric plasma: Methods of production and application in dentistry and oncology. Med. Gas Res. 2013, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Vardar, E.; Endogan, T.; Kiziltay, A.; Hasirci, V.; Hasirci, N. Effect of oxygen plasma on surface properties and biocompatibility of PLGA films. Surf. Interface Anal. 2010, 42, 486–491. [Google Scholar]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Yoo, E.M.; Uhm, S.H.; Kwon, J.S.; Choi, H.S.; Choi, E.H.; Kim, K.M.; Kim, K.N. The study on inhibition of planktonic bacterial growth by non-thermal atmospheric pressure plasma jet treated surfaces for dental application. J. Biomed. Nanotechnol. 2015, 11, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Jung-Hwan, L.; Jae-Sung, K.; Ji-yeon, O.; Yong-Hee, K.; Eun-Ha, C.; Kwang-Mahn, K.; Kim, K.N. Cell immobilization on polymer by air atmospheric pressure plasma jet treatment. Jpn. J. Appl. Phys. 2014, 53, 086202. [Google Scholar]
- Ritts, A.C.; Li, H.; Yu, Q.; Xu, C.; Yao, X.; Hong, L.; Wang, Y. Dentin surface treatment using a non-thermal argon plasma brush for interfacial bonding improvement in composite restoration. Eur. J. Oral Sci. 2010, 118, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Okawa, T.; Fukumoto, T.; Tsurumi, A.; Tatsuta, M.; Fuji, T. Influence of atmospheric pressure low-temperature plasma treatment on the shear bond strength between zirconia and resin cement. J. Prosthodont. Res. 2016, 60, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Park, Y.S. Plasma in dentistry. Clin. Plasma Med. 2014, 2, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Lee, M.A.; Han, G.J.; Cho, B.H. Plasma in dentistry: A review of basic concepts and applications in dentistry. Acta Odontol. Scand. 2014, 72, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Yu, Q.S.; Wang, Y. Non-thermal atmospheric plasmas in dental restoration. J. Dent. Res. 2016, 95, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Dobslaw, D.; Schulz, A.; Helbich, S.; Dobslaw, C.; Engesser, K.H. VOC removal and odor abatement by a low-cost plasma enhanced biotrickling filter process. J. Environ. Chem. Eng. 2017, 5, 5501–5511. [Google Scholar] [CrossRef]
- Holub, M.; Brandenburg, R.; Grosch, H.; Weinmann, S.; Hansel, B. Plasma supported odour removal from waste air in water treatment plants: An industrial case study. Aerosol Air Qual. Res. 2014, 14, 697–707. [Google Scholar] [CrossRef]
- Schiavon, M.; Schiorlin, M.; Torretta, V.; Brandenburg, R.; Ragazzi, M. Non-thermal plasma assisting the biofiltration of volatile organic compounds. J. Clean. Prod. 2017, 148, 498–508. [Google Scholar] [CrossRef]
- Wei, Z.S.; Li, H.Q.; He, J.C.; Ye, Q.H.; Huang, Q.R.; Luo, Y.W. Removal of dimethyl sulfide by the combination of non-thermal plasma and biological process. Bioresour. Technol. 2013, 146, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.B.; Ayres, A.P.A.; Lopes, L.B.; Negreiros, W.M.; Giannini, M. The effect of atmospheric plasma treatment of dental zirconia ceramics on the apparent contact angle of water. Appl. Adhes Sci. 2014, 2, 17. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Sky Driver, M.; Caruso, A.N.; Yu, Q.; Wang, Y. Surface modification of several dental substrates by non-thermal, atmospheric plasma brush. Dent. Mater. 2013, 28, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Tallarico, M.; Gautier, G.; Mussano, F.; Botticelli, D. Plasma of argon affects the earliest biological response of different implant surfaces: An in vitro comparative study. J. Dent. Res. 2016, 95, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Duske, K.; Koban, I.; Kindel, E.; Schröder, K.; Nebe, B.; Holtfreter, B.; Jablonowski, L.; Weltmann, K.D.; Kocher, T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J. Clin. Periodontol. 2012, 39, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeong, W.S.; Seo, S.J.; Kim, H.W.; Kim, K.N.; Choi, E.H.; Kim, K.M. Non-thermal atmospheric pressure plasma functionalized dental implant for enhancement of bacterial resistance and osseointegration. Dent. Mater. 2017, 33, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.M.; da Silva, E.V.; Vechiato-Filho, A.J.; Cesar, P.F.; Rangel, E.C.; da Cruz, N.C.; Goiato, M.C. Aging effect of atmospheric air on lithium disilicate ceramic after non-thermal plasma treatment. J. Prosthet. Dent. 2016, 115, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Marmur, A.; Della Volpe, C.; Siboni, S.; Amirfazli, A.; Drelich, J.W. Contact angles and wettability: Towards common and accurate terminology. Surf. Innov. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Akgungor, G.; Sen, D.; Aydin, M. Influence of different surface treatments on the short-term bond strength and durability between a zirconia post and a composite resin core material. J. Prosthet. Dent. 2008, 99, 388–399. [Google Scholar] [CrossRef]
- Piascik, J.R.; Thompson, J.Y.; Swift, E.J.; Grego, S.; Stoner, B.R. Surface modification for enhanced silanation of high strength ceramics. Dent. Mater. 2009, 25, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Vallittu, P.K. Effect of surface conditioning methods on the bond strength of luting cement to ceramics. Dent. Mater. 2003, 19, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Kern, M.; Wegner, S.M. Bonding to zirconia ceramic: Adhesion methods and their durability. Dent. Mater. 1998, 14, 64–71. [Google Scholar] [CrossRef]
- Vechiato-Filho, A.J.; da Silva Vieira Marques, I.; Dos Santos, D.M.; Matos, A.O.; Rangel, E.C.; da Cruz, N.C.; Barão, V.A.R. Effect of nonthermal plasma treatment on surface chemistry of commercially-pure titanium and shear bond strength to autopolymerizing acrylic resin. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.E.F.; Bastos, D.C.; Da Silva, M.L.V.J.; Thire, R.M.S.M.; Simao, R.A. Chemical analysis of a cornstarch film surface modified by SF 6 plasma treatment. Carbohydr. Polym. 2012, 87, 2217–2222. [Google Scholar] [CrossRef]
- Li, S.; Jinjin, D. Improvement of hydrophobic properties of silk and cotton by hexafluoropropene plasma treatment. Appl. Surf. Sci. 2007, 253, 5051–5055. [Google Scholar] [CrossRef]
- Choi, S.H.; Jeong, W.S.; Cha, J.Y.; Lee, J.H.; Yu, H.S.; Choi, E.H.; Kim, K.M.; Hwang, C.J. Time-dependent effects of ultraviolet and non-thermal atmospheric pressure plasma on the biological activity of titanium. Sci. Rep. 2016, 6, 33421. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.; Ozcan, M.; Bottino, M.A.; Valandro, L.F. Microtensile bond strength of a resin cement to glass infiltrated zirconia-reinforced ceramic: The effect of surface conditioning. Dent. Mater. 2006, 22, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, K.; Yoshimura, A.; Shibamori, Y.; Kubota, N. Hydrophilic modification of plastic surface by using microwave plasma irradiation. IHI Eng. Rev. 2013, 46, 29–33. [Google Scholar]
- Silva, N.R.; Coelho, P.G.; Valverde, G.B.; Becker, K.; Ihrke, R.; Quade, A.; Thompson, V.P. Surface characterization of Ti and Y-TZP following non-thermal plasma exposure. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Min, B.K.; Son, J.S.; Kwon, T.Y. Influence of different post-plasma treatment storage conditions on the shear bond strength of veneering porcelain to zirconia. Materials 2016, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Tay, F.R.; Zhang, F.; Lu, Y.; Shen, S.; Chen, C. Coupling of 10-methacryloyloxydecyldihydrogenphosphate to tetragonal zirconia: Effect of pH reaction conditions on coordinate bonding. Dent. Mater. 2015, 31, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Silikas, N.; Wincott, P.L.; Vaughan, D.; Watts, D.C.; Eliades, G. Surface characterization of precious alloys treated with thione metal primers. Dent. Mater. 2007, 23, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Barloi, A.; Kern, M. Influence of air-abrasion on zirconia ceramic bonding using an adhesive composite resin. Dent. Mater. 2010, 26, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Magne, P.; Paranhos, M.P.G.; Burnett, L.H. New zirconia primer improves bond strength of resin-based cements. Dent. Mater. 2010, 26, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.; Chaniel, G.; Grynyov, R. Towards understanding hydrophobic recovery of plasma treated polymers: Storing in high polarity liquids suppresses hydrophobic recovery. Appl. Surf. Sci. 2013, 273, 549–553. [Google Scholar] [CrossRef]
- Strobel, M.; Lyons, C.S. An essay on contact angle measurements. Plasma Process. Polym. 2011, 8, 8–13. [Google Scholar] [CrossRef]
- Bormashenko, E. Wetting of real solid surfaces: New glance on well-known problems. Colloid Polym. Sci. 2013, 291, 339–342. [Google Scholar] [CrossRef]
- Grundke, K.; Pöschel, K.; Synytska, A.; Frenzel, R.; Drechsler, A.; Nitschke, M.; Cordeiro, A.L.; Uhlmann, P.; Welzel, P.B. Experimental studies of contact angle hysteresis phenomena on polymer surfaces—Toward the understanding and control of wettability for different applications. Adv. Colloid Interface Sci. 2015, 222, 350–376. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.A.; Stangel, I.; Ellis, T.H.; Zhu, X.X. Oxygen inhibition in dental resins. J. Dent. Res. 2005, 84, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Schutze, A.; Jeong, J.Y.; Babayan, S.E.; Park, J.Y.; Selwyn, G.S.; Hicks, R.F. The atmospheric-pressure plasma jet: A review and comparison to other plasma sources. IEEE Trans. Plasma Sci. 1998, 26, 1685–1694. [Google Scholar] [CrossRef]
- Blatz, M.B.; Chiche, G.; Holst, S.; Sadan, A. Influence of surface treatment and simulated aging on bond strengths of luting agents to zirconia. Quintessence Int. 2007, 38, 745–753. [Google Scholar] [PubMed]
- Aydın, C.; Yılmaz, H.; Bankoğlu, M. A single-tooth, two-piece zirconia implant located in the anterior maxilla: A clinical report. J. Prosthet. Dent. 2013, 109, 70–74. [Google Scholar] [CrossRef]
- Borgonovo, A.E.; Censi, R.; Vavassori, V.; Arnaboldi, O.; Maiorana, C.; Re, D. Zirconia implants in esthetic areas: 4-year follow-up evaluation study. Int. J. Dent. 2015, 41, 415029. [Google Scholar] [CrossRef] [PubMed]
- Thurnheer, T.; Belibasakis, G.N. Incorporation of staphylococci into titanium-grown biofilms: An in vitro “submucosal” biofilm model for peri-implantitis. Clin. Oral Implants Res. 2016, 27, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Schmid, B.; Rutar, A.; Lang, N.P. Persistence patterns of Porphyromonas gingivalis, Prevotella intermedia/nigrescens, and Actinobacillus actinomycetemcomitans after mechanical therapy of periodontal disease. J. Periodontol. 2000, 71, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, A.; Gröndahl, K.; Bergström, C.; Lekholm, U. Long-term follow-up of osseointegrated titanium implants using clinical, radiographic and microbiological parameters. Clin. Oral Implants Res. 2002, 13, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs)-Part I: Structural and ecological aspects. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Carral, C.; Muñoz, F.; Permuy, M.; Liñares, A.; Dard, M.; Blanco, J. Mechanical and chemical implant decontamination in surgical peri-implantitis treatment: Preclinical “in vivo” study. J. Clin. Periodontol. 2016, 43, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Sahm, N.; Becker, J.; Santel, T.; Schwarz, F. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: A prospective, randomized, controlled clinical study. J. Clin. Periodontol. 2011, 38, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.R.; Duarte, P.M. Surgical anti-infective mechanical therapy for peri-implantitis: A clinical report with a 12-month follow-up. Gen. Dent. 2009, 57, 230–235. [Google Scholar] [PubMed]
- Southwood, L.L.; Baxter, G.M. Instrument sterilization, skin preparation, and wound management. Vet. Clin. N. Am Equine Pract. 1996, 12, 173–194. [Google Scholar] [CrossRef]
- Ayliffe, G. Decontamination of minimally invasive surgical endoscopes and accessories. J. Hosp. Infect. 2000, 45, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Moisan, M.; Barbeau, J.; Moreau, S.; Pelletier, J.; Tabrizian, M.; Yahia, L.H. Low-temperature sterilization using gas plasmas: A review of the experiments and an analysis of the inactivation mechanisms. Int. J. Pharm. 2001, 226, 1–21. [Google Scholar] [CrossRef]
- Laroussi, M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Lackmann, J.W.; Bandow, J.E. Inactivation of microbes and macromolecules by atmospheric-pressure plasma jets. Appl. Microbiol. Biotechnol. 2014, 98, 6205–6213. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Shi, J.; Kong, M.G. Physical mechanisms of inactivation of Bacillus subtilis spores using cold atmospheric plasmas. IEEE Trans. Plasma Sci. 2006, 34, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Radman, M. Phenomenology of an inducible mutagenic DNA repair pathway in Escherichia coli: SOS repair hypothesis. Mol. Environ. Asp. Mutagen. 1974, 6, 128–142. [Google Scholar]
- Preedy, E.; Perni, S.; Nipic, D.; Bohinc, K.; Prokopovich, P. Surface roughness mediated adhesion forces between borosilicate glass and gram-positive bacteria. Langmuir 2014, 30, 9466–9476. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Ueno, T.; Migita, S.; Tsutsumi, Y.; Doi, H.; Ogawa, T.; Hanawa, T.; Wakabayashi, N. Hydrocarbon deposition attenuates osteoblast activity on titanium. J. Dent. Res. 2014, 93, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Hauser-Gerspach, I.; Kulik, E.M.; Weiger, R.; Decker, E.M.; Von Ohle, C.; Meyer, J. Adhesion of Streptococcus sanguinis to dental implant and restorative materials in vitro. Dent. Mater. J. 2007, 26, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.S.; Kwon, J.S.; Lee, J.H.; Uhm, S.H.; Choi, E.H.; Kim, K.M. Bacterial attachment on titanium surfaces is dependent on topography and chemical changes induced by non-thermal atmospheric pressure plasma. Biomed. Mater. 2017, 12, 045015. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Ikeda, T.; Tsukimura, N.; Ishijima, M.; Minamikawa, H.; Sugita, Y.; Yamada, M.; Wakabayashi, N.; Ogawa, T. Novel antioxidant capability of titanium induced by UV light treatment. Biomaterials 2016, 108, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laroussi, M. Non-thermal decontamination of biological media by atmospheric-pressure plasmas: Review, analysis, and prospects. IEEE Trans. Plasma Sci. 2002, 30, 1409–1415. [Google Scholar] [CrossRef]
- Yu, Q.S.; Huang, C.; Hsieh, F.H.; Huff, H.; Duan, Y. Bacterial inactivation using a low-temperature atmospheric plasma brush sustained with argon gas. J. Biomed. Mater. Res. B 2007, 80, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Vechiato-Filho, A.J.; Matos, A.O.; Landers, R.; Goiato, M.C.; Rangel, E.C.; De Souza, G.M. Surface analysis and shear bond strength of zirconia on resin cements after non-thermal plasma treatment and/or primer application for metallic alloys. Mater. Sci. Eng. C 2017, 72, 284–292. [Google Scholar] [CrossRef] [PubMed]

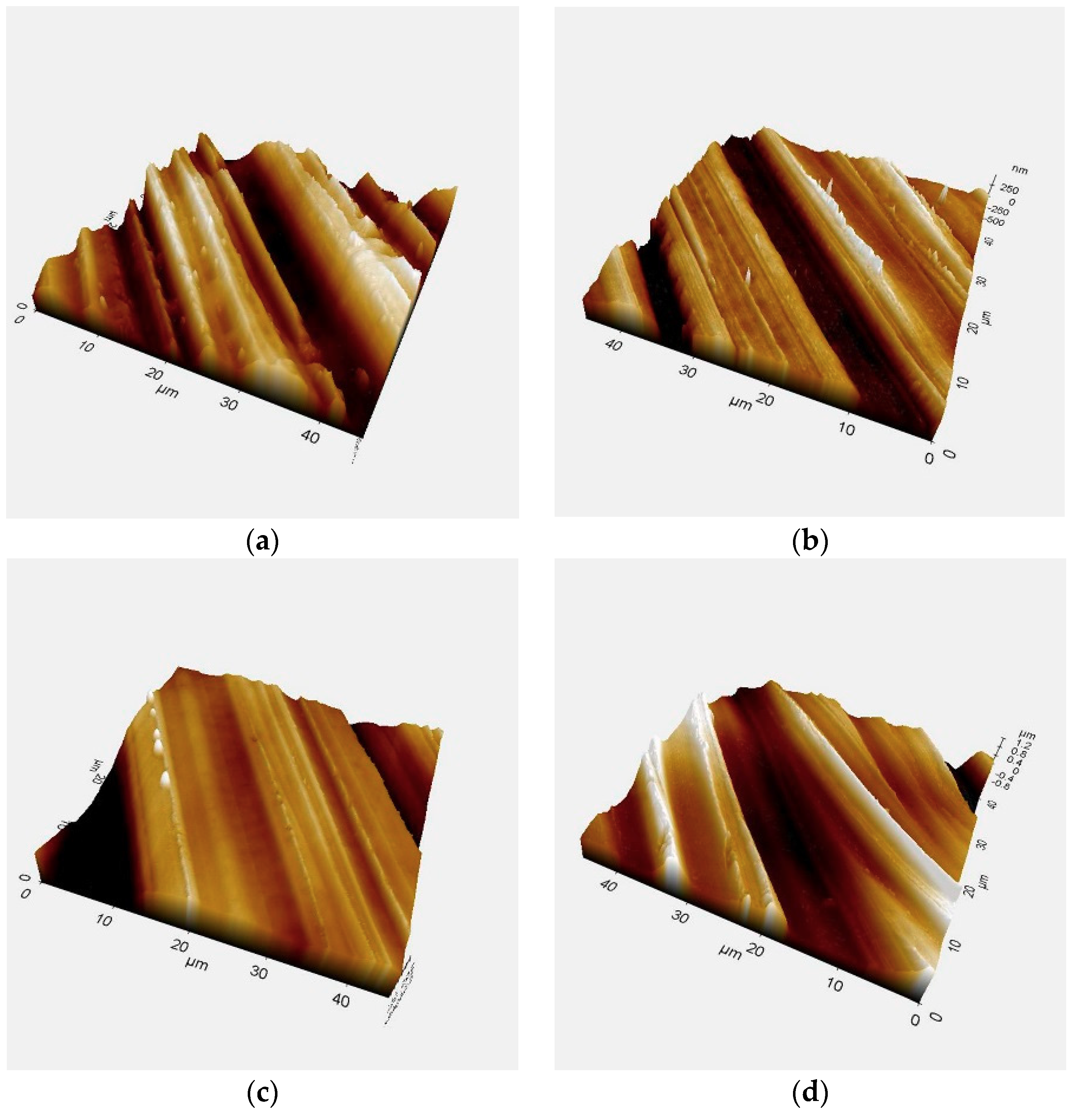


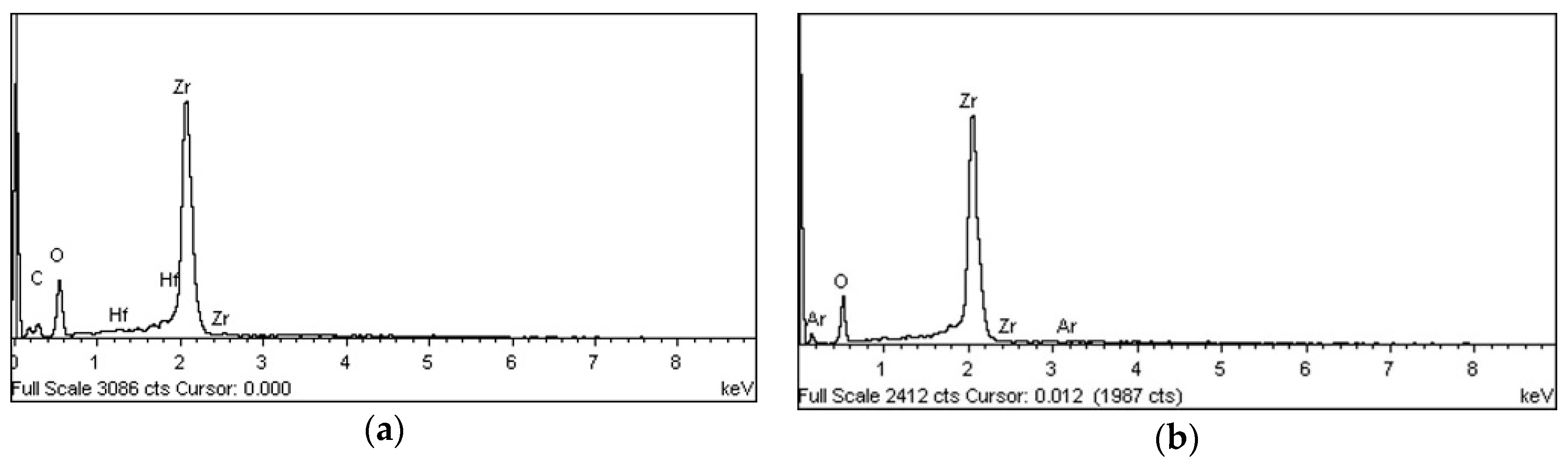
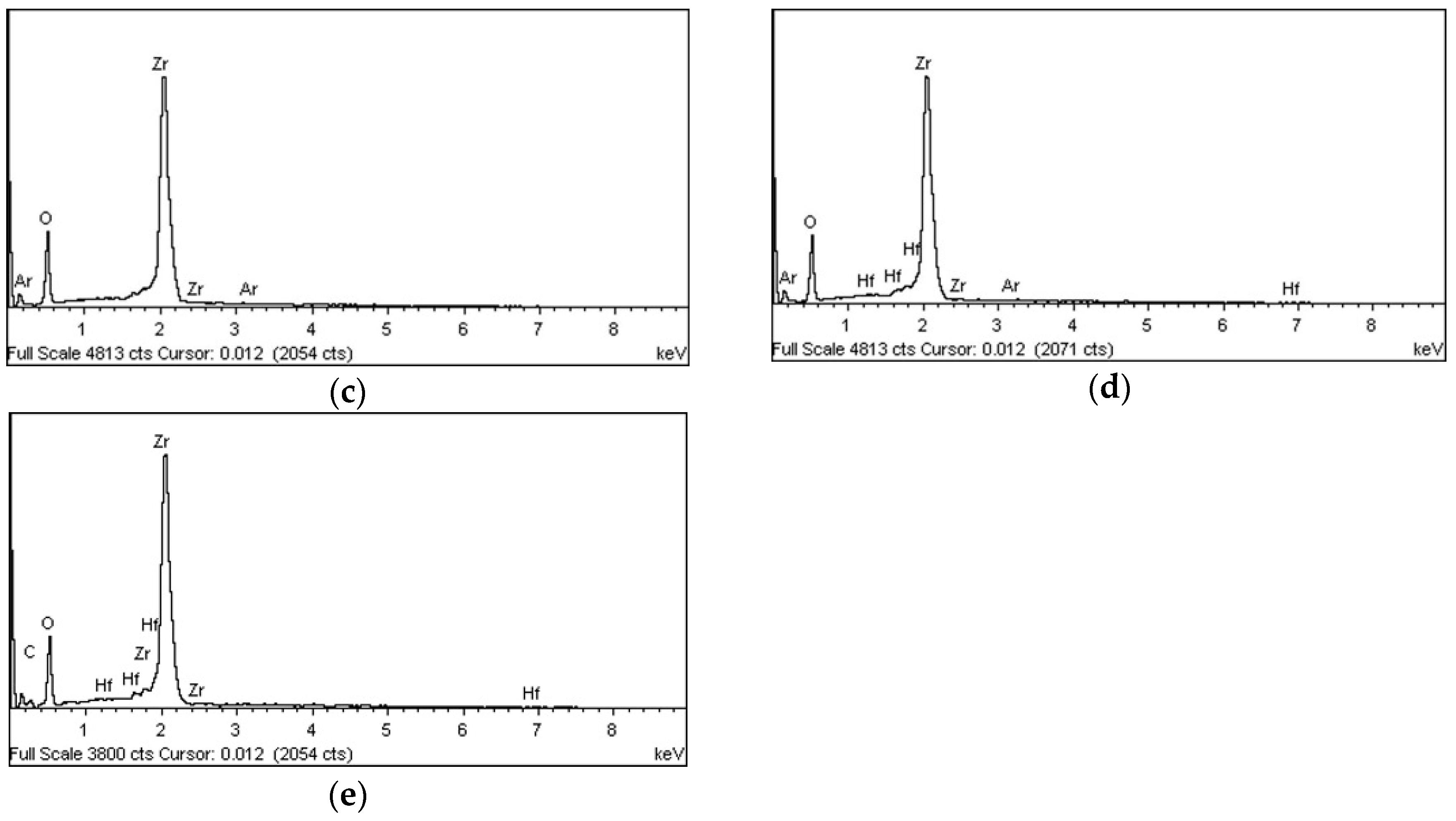



| Group | Plasma Treatment | Storage Conditions |
|---|---|---|
| Control | No | - |
| P0 | Yes | Immediate |
| P1 | Yes | In atmosphere for 24 h |
| P2 | Yes | In atmosphere for 48 h |
| P3 | Yes | In atmosphere for 72 h |
| Resin Cement | Type and Primary Composition | Manufacturer |
|---|---|---|
| RelyX U200TM | Self-cured (vitreous powder, methacrylate monomers, silanated fillers, initiator components) | 3M ESPE |
| Panavia F2.0 | Dual-cured (BPEDMA, 10-MDP, DMA, silica, initiators) | Kuraray Medical Inc. |
| Group Element | Control | P0 | P1 | P2 | P3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Element | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) |
| O | 28.01 | 50.54 | 50.56 | 71.40 | 37.40 | 77.30 | 30.39 | 70.61 | 32.09 | 60.49 |
| Zr | 58.18 | 18.09 | 69.15 | 28.33 | 62.58 | 22.68 | 69.16 | 28.19 | 58.46 | 19.33 |
| C | 12.93 | 31.08 | - | - | - | - | 0.36 | 1.12 | 7.98 | 20.03 |
| Ar | - | - | 0.29 | 0.27 | 0.02 | 0.02 | 0.09 | 0.08 | - | - |
| Element | Control | P0 | P1 | P2 | P3 |
|---|---|---|---|---|---|
| Carbon (%) | 61.57 | 23.94 | 30.46 | 28.62 | 37.30 |
| Oxygen (%) | 38.43 | 76.06 | 69.54 | 71.38 | 62.70 |
| C/O proportion | 1.60 | 0.31 | 0.44 | 0.40 | 0.59 |
| Group | SBS (MPa) | Failure Pattern |
|---|---|---|
| Panavia F2.0, Control | 6.53 (0.81) a | 100% mixed |
| Panavia F2.0, P0 | 4.22 (0.99) b | 100% mixed |
| Panavia F2.0, P1 | 5.19 (0.92) a | 100% mixed |
| Panavia F2.0, P2 | 6.05 (1.07) a | 100% mixed |
| Panavia F2.0, P3 | 6.98 (1.69) a | 100% mixed |
| RelyXTM U200, Control | 3.08 (1.54) c | 10% mixed, 90% adhesive |
| RelyXTM U200, P0 | 5.26 (0.83) d | 100% mixed |
| RelyXTM U200, P1 | 5.08 (1.15) d | 100% mixed |
| RelyXTM U200, P2 | 4.24 (0.76) d | 100% mixed |
| RelyXTM U200, P3 | 2.71 (1.19) c | 100% adhesive |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Park, S.-W.; Yun, K.-D.; Ji, M.-K.; Kim, S.; Yang, Y.P.; Lim, H.-P. Effect of Plasma Treatment and Its Post Process Duration on Shear Bonding Strength and Antibacterial Effect of Dental Zirconia. Materials 2018, 11, 2233. https://doi.org/10.3390/ma11112233
Park C, Park S-W, Yun K-D, Ji M-K, Kim S, Yang YP, Lim H-P. Effect of Plasma Treatment and Its Post Process Duration on Shear Bonding Strength and Antibacterial Effect of Dental Zirconia. Materials. 2018; 11(11):2233. https://doi.org/10.3390/ma11112233
Chicago/Turabian StylePark, Chan, Sang-Won Park, Kwi-Dug Yun, Min-Kyung Ji, Sungwoo Kim, Yunzhi Peter Yang, and Hyun-Pil Lim. 2018. "Effect of Plasma Treatment and Its Post Process Duration on Shear Bonding Strength and Antibacterial Effect of Dental Zirconia" Materials 11, no. 11: 2233. https://doi.org/10.3390/ma11112233





