Preservation of Bone Tissue Integrity with Temperature Control for In Situ SR-MicroCT Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. SR-MicroCT Imaging
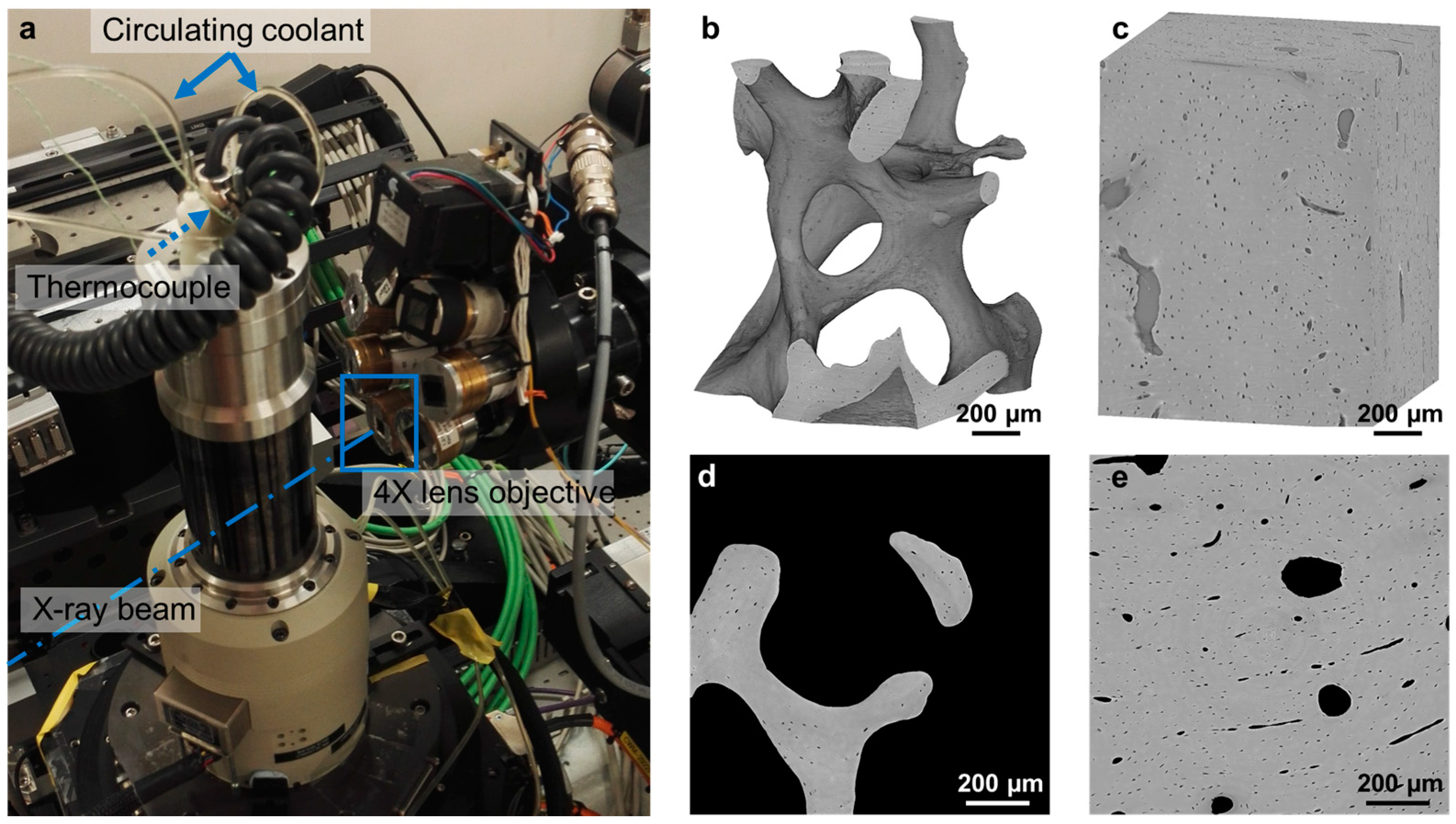
2.3. In Situ Testing and Temperature Control
2.4. Image Post-Processing
2.5. Digital Volume Correlation
3. Results
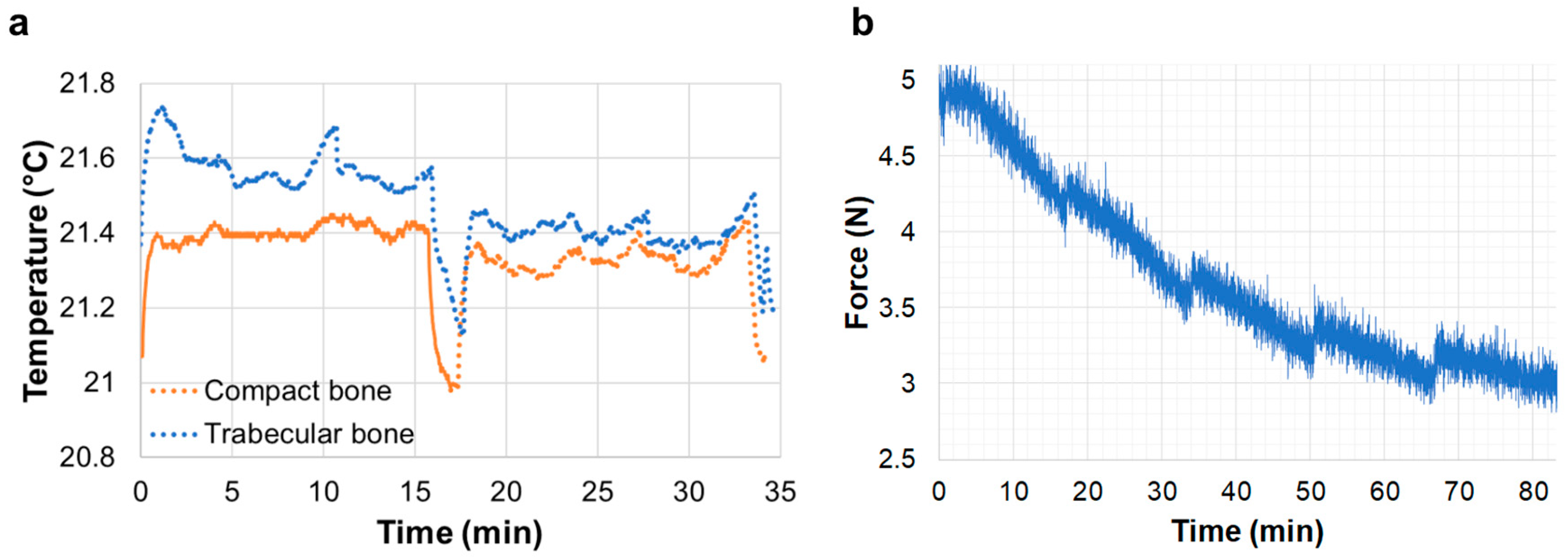
3.1. In Situ Testing and Temperature Control
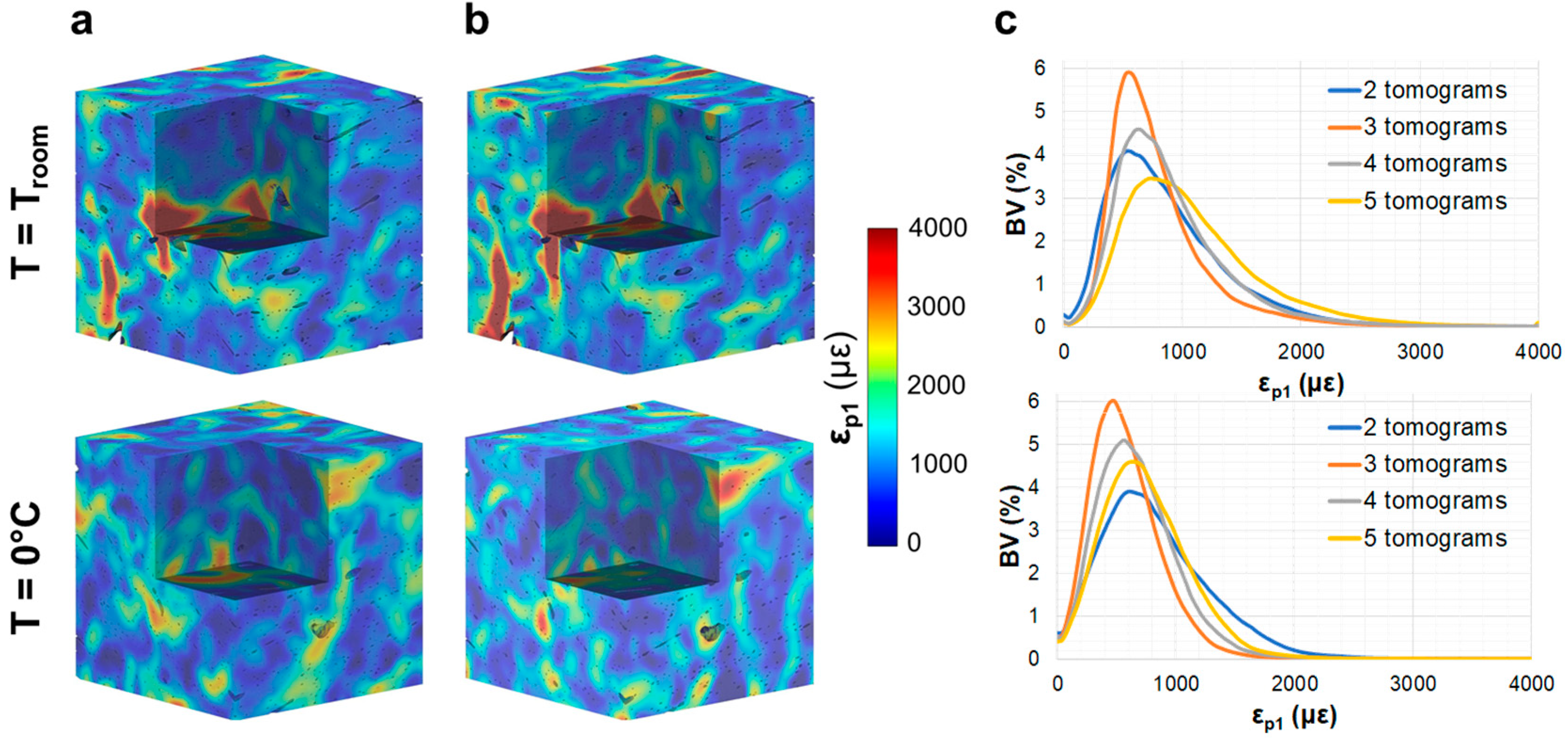
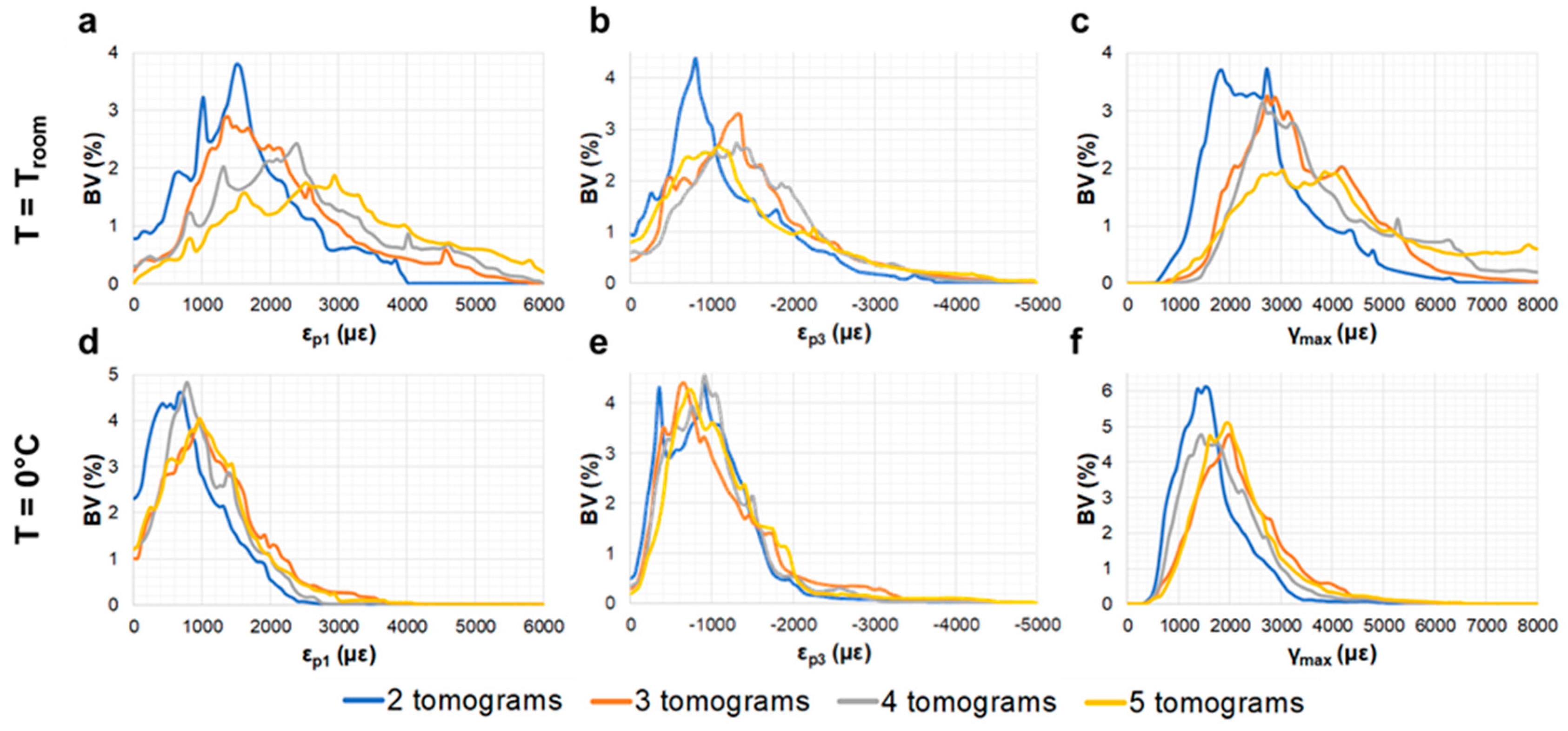
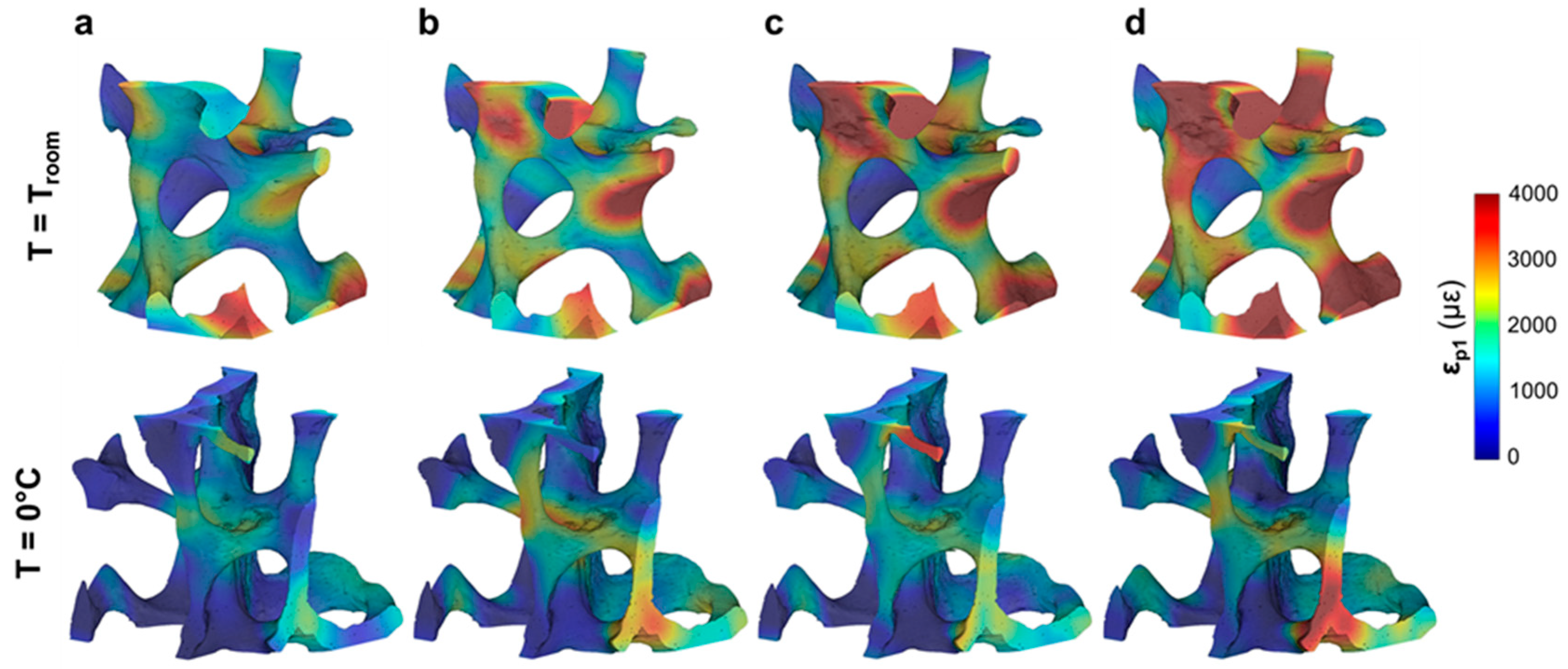
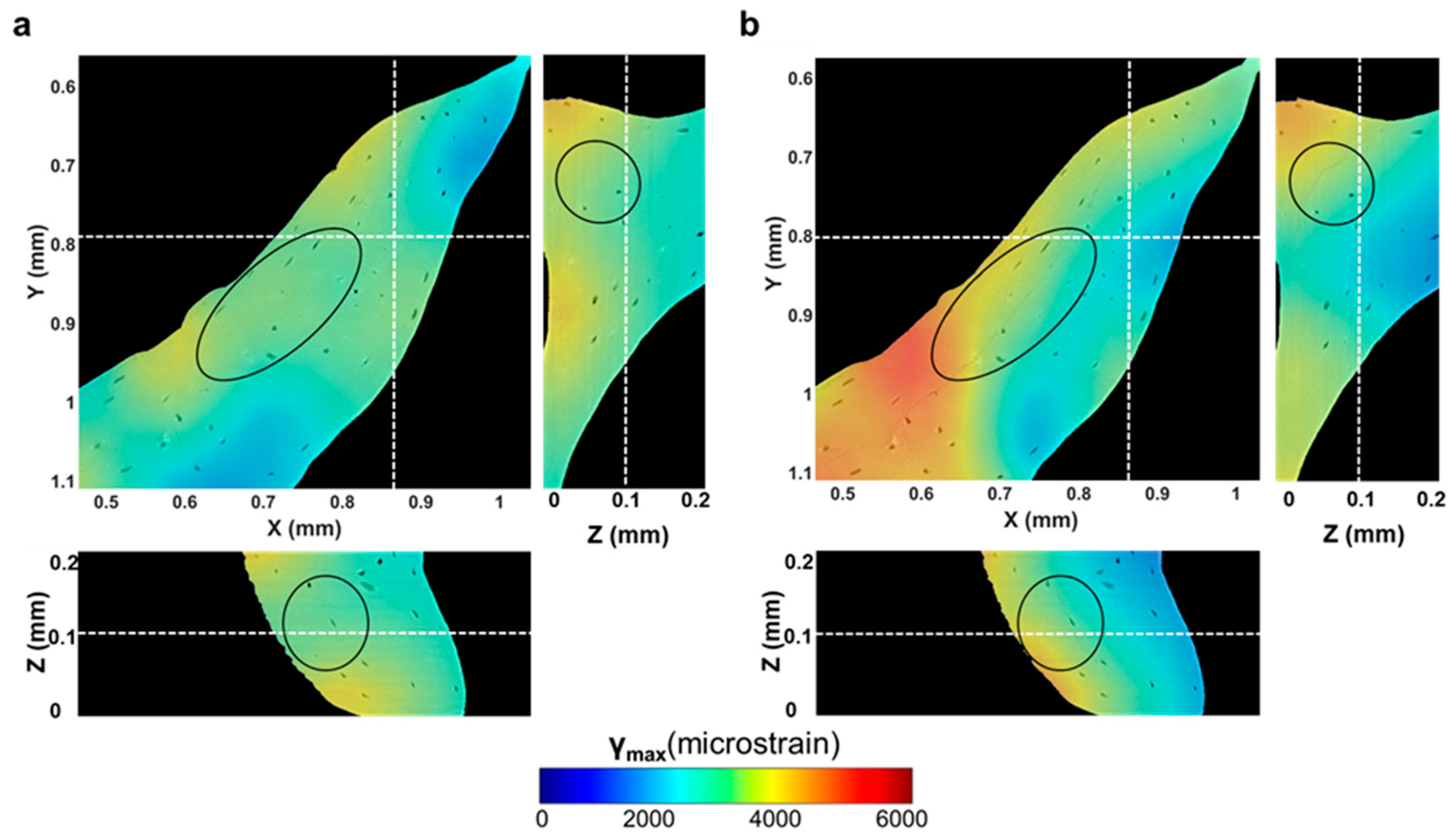
3.2. Compact Bone
3.3. Trabecular Bone
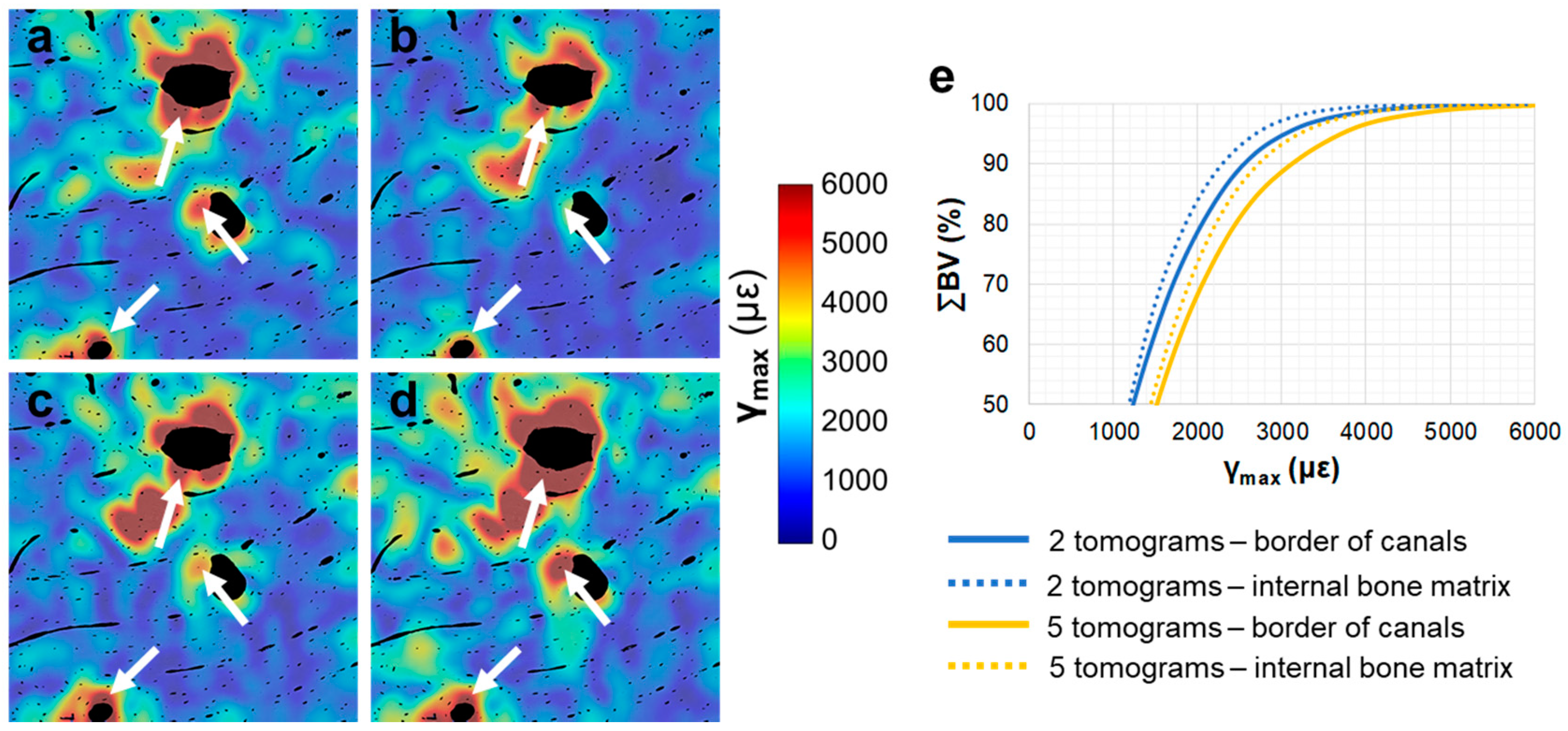
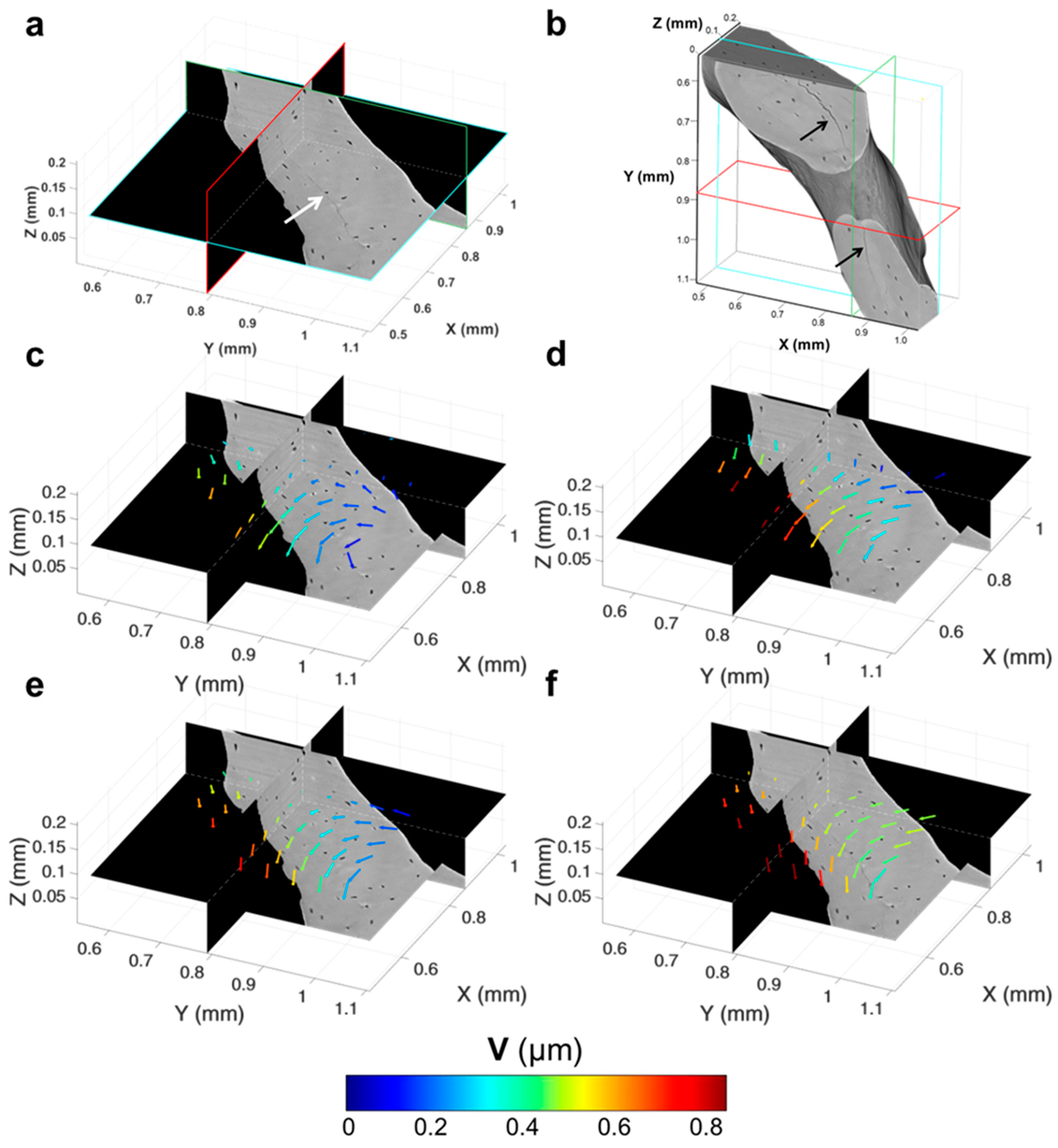
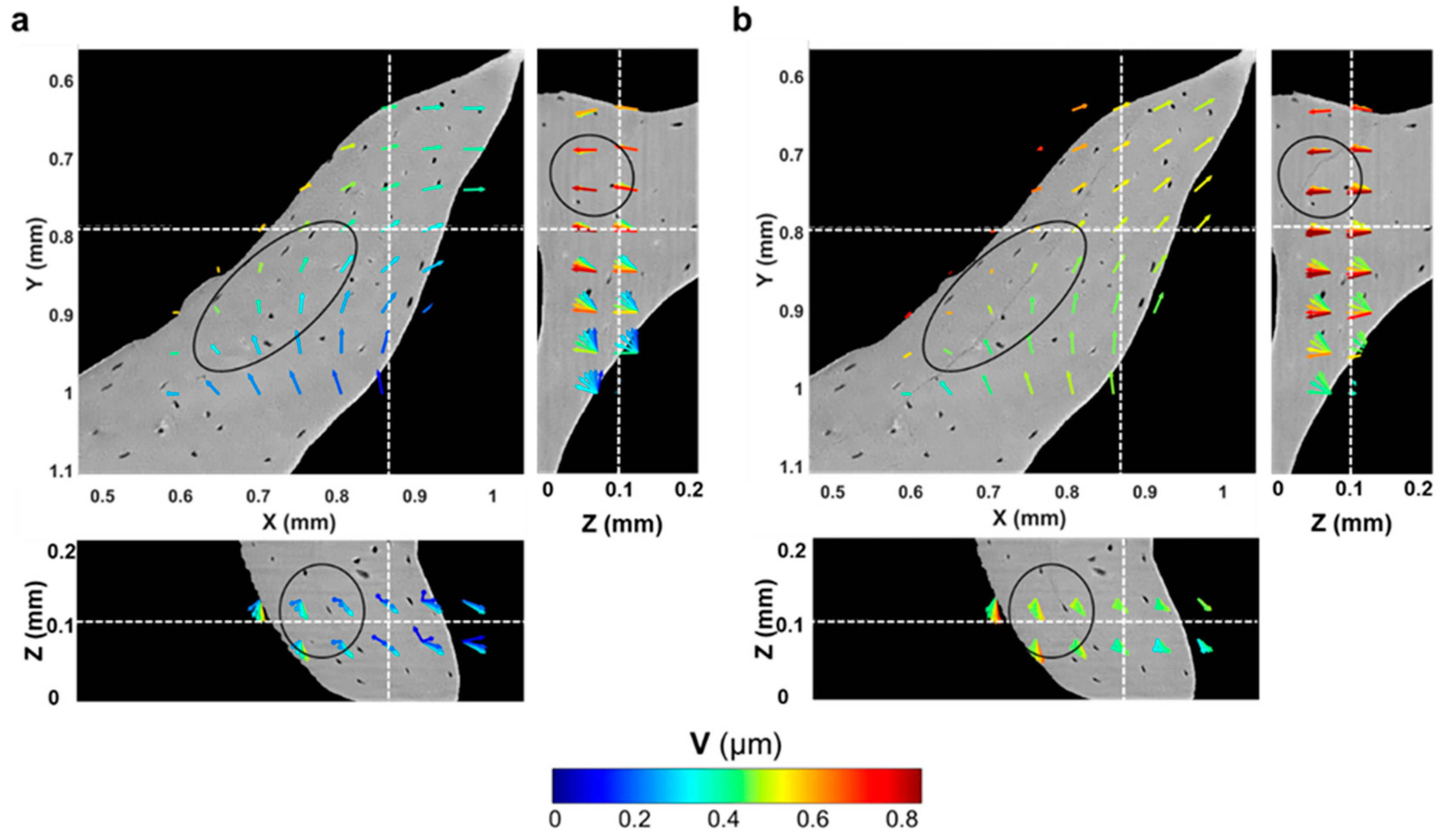
3.4. Tracking of Crack Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Fratzl, P.; Gupta, H.S.; Paschalis, E.P.; Roschger, P. Structure and mechanical quality of the collagen–mineral nano-composite in bone. J. Mater. Chem. 2004, 14, 2115–2123. [Google Scholar] [CrossRef]
- Currey, J.D. How well are bones designed to resist fracture? J. Bone Miner. Res. 2003, 18, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Traub, W.; Wagner, H.D. Lamellar bone: Structure–function relations. J. Struct. Biol. 1999, 126, 241–255. [Google Scholar] [CrossRef] [PubMed]
- de Bakker, C.M.J.; Tseng, W.-J.; Li, Y.; Zhao, H.; Liu, X.S. Clinical Evaluation of Bone Strength and Fracture Risk. Curr. Osteoporos. Rep. 2017, 15, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Voide, R.; Schneider, P.; Stauber, M.; Wyss, P.; Stampanoni, M.; Sennhauser, U.; van Lenthe, G.H.; Müller, R. Time-lapsed assessment of microcrack initiation and propagation in murine cortical bone at submicrometer resolution. Bone 2009, 45, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, U.; Wilke, H.J.; Zysset, P.K. Damage accumulation in vertebral trabecular bone depends on loading mode and direction. J. Biomech. 2011, 44, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Demirci, E.; Silberschmidt, V.V. Variability and anisotropy of mechanical behavior of cortical bone in tension and compression. J. Mech. Behav. Biomed. Mater. 2013, 21, 109–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bay, B.K.; Smith, T.S.; Fyhrie, D.P.; Saad, M. Digital volume correlation: Three-dimensional strain mapping using X-ray tomography. Exp. Mech. 1999, 39, 217–226. [Google Scholar] [CrossRef]
- Grassi, L.; Isaksson, H. Extracting accurate strain measurements in bone mechanics: A critical review of current methods. J. Mech. Behav. Biomed. Mater. 2015, 50, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall’Ara, E.; Peña-Fernández, M.; Palanca, M.; Giorgi, M.; Cristofolini, L.; Tozzi, G. Precision of DVC approaches for strain analysis in bone imaged with µCT at different dimensional levels. Front. Mater. 2017, 4, 31. [Google Scholar] [CrossRef]
- Christen, D.; Levchuk, A.; Schori, S.; Schneider, P.; Boyd, S.K.; Müller, R. Deformable image registration and 3D strain mapping for the quantitative assessment of cortical bone microdamage. J. Mech. Behav. Biomed. Mater. 2012, 8, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Gillard, F.; Boardman, R.; Mavrogordato, M.; Hollis, D.; Sinclair, I.; Pierron, F.; Browne, M. The application of digital volume correlation (DVC) to study the microstructural behaviour of trabecular bone during compression. J. Mech. Behav. Biomed. Mater. 2014, 29, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Peña Fernández, M.; Cipiccia, S.; Bodey, A.J.; Parwani, R.; Dall’Ara, E.; Blunn, G.; Pani, M.; Barber, A.H.; Tozzi, G. Effect of SR-microCT exposure time on the mechanical integrity of trabecular bone using in situ mechanical testing and digital volume correlation. J. Mech. Behav. Biomed. Mater. 2018, 88, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Palanca, M.; Bodey, A.J.; Giorgi, M.; Viceconti, M.; Lacroix, D.; Cristofolini, L.; Dall’Ara, E. Local displacement and strain uncertainties in different bone types by digital volume correlation of synchrotron microtomograms. J. Biomech. 2017, 58, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Peña Fernández, M.; Barber, A.H.; Blunn, G.W.; Tozzi, G. Optimisation of digital volume correlation computation in SR-microCT images of trabecular bone and bone-biomaterial systems. J. Microsc. 2018, 00, 1–16. [Google Scholar] [CrossRef]
- Thurner, P.J.; Wyss, P.; Voide, R.; Stauber, M.; Stampanoni, M.; Sennhauser, U.; Müller, R. Time-lapsed investigation of three-dimensional failure and damage accumulation in trabecular bone using synchrotron light. Bone 2006, 39, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Larrue, A.; Rattner, A.; Laroche, N.; Vico, L.; Peyrin, F. Feasibility of micro-crack detection in human trabecular bone images from 3D synchrotron microtomography. Annu. Int. Conf. IEEE Eng. Med. Biol. Proc. 2007, 3918–3921. [Google Scholar] [CrossRef]
- Tozzi, G.; Peña Fernández, M.; Parwani, R.; Bodey, A.J.; Dall’Ara, E.; Blunn, G.W.; Barber, A.H. Micromechanics and DVC of bone-biomaterial systems produced by osteorgenerative biomaterials in vivo. In Proceedings of the 23rd Congress of the European Society of Biomechanics (ESB 2017), Seville, Spain, 2–5 July 2017. [Google Scholar]
- Barth, H.D.; Launey, M.E.; MacDowell, A.A.; Ager, J.W.; Ritchie, R.O. On the effect of X-ray irradiation on the deformation and fracture behavior of human cortical bone. Bone 2010, 46, 1475–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, H.D.; Zimmermann, E.A.; Schaible, E.; Tang, S.Y.; Alliston, T.; Ritchie, R.O. Characterization of the effects of x-ray irradiation on the hierarchical structure and mechanical properties of human cortical bone. Biomaterials 2011, 32, 8892–8904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Singh, D.; Singh, A. Radiation sterilization of tissue allografts: A review. World J. Radiol. 2016, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Hamer, A.J.; Strachan, J.R.; Black, M.M.; Ibbotson, C.J.; Stockley, I.; Elson, R.A. Biomechanical Properties of Cortical Allograft Bone Using a New Method of Bone Strength Measurement: A Comparison of Fresh, Fresh-Frozen and Irradiated Bone. J Bone Jt. Surg. Br 1996, 78, 363–368. [Google Scholar] [CrossRef]
- Currey, J.D.; Foreman, J.; Laketić, I.; Mitchell, J.; Pegg, D.E.; Reilly, G.C. Effects of ionizing radiation on the mechanical properties of human bone. J. Orthop. Res. 1997, 15, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Vastel, L.; Meunier, A.; Siney, H.; Sedel, L.; Courpied, J.P. Effect of different sterilization processing methods on the mechanical properties of human cancellous bone allografts. Biomaterials 2004, 25, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Deymier-Black, A.C.; Almer, J.D.; Dunand, D.C. Effect of high-energy X-ray doses on bone elastic properties and residual strains. J. Mech. Behav. Biomed. Mater. 2011, 4, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, C.D.; Unal, M.; Akkus, O.; Rimnac, C.M. Raman spectral markers of collagen denaturation and hydration in human cortical bone tissue are affected by radiation sterilization and high cycle fatigue damage. J. Mech. Behav. Biomed. Mater. 2017, 75, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Gouk, S.-S.; Kocherginsky, N.M.; Kostetski, Y.Y.; Moser, M.O.; Yang, P.; Lim, T.-M.; Sun, W.Q.; Moser, H.O.; Yang, P.; Lim, T.-M.; et al. Synchrotron radiation-induced formation and reaction of free radicals in the human acellular dermal matrix. Radiat. Res. 2005, 163, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Akkus, O.; Belaney, R.M.; Das, P. Free radical scavenging alleviates the biomechanical impairment of gamma radiation sterilized bone tissue. J. Orthop. Res. 2005, 23, 838–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.; Morgan, D.A.F.; Forwood, M.R. Sterilization of allograft bone: Effects of gamma irradiation on allograft biology and biomechanics. Cell Tissue Bank. 2007, 8, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Hamer, A.J.; Stockley, I.; Elson, R.A. Changes in allograft bone irradiated at different temperatures. J. Bone Joint Surg. Br. 1999, 81, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Cornu, O.; Boquet, J.; Nonclercq, O.; Docquier, P.L.; Van Tomme, J.; Delloye, C.; Banse, X. Synergetic effect of freeze-drying and gamma irradiation on the mechanical properties of human cancellous bone. Cell Tissue Bank. 2011, 12, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, H.; Tersmette, M.; Vos, A.H.V.; Over, J.; Berkel, M.P.; Bree, H. Inactivation of human immunodeficiency virus by gamma radiation and its effect on plasma and coagulation factors. Transfusion 1991, 31, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Grieb, T.A.; Forng, R.Y.; Stafford, R.E.; Lin, J.; Almeida, J.; Bogdansky, S.; Ronholdt, C.; Drohan, W.N.; Burgess, W.H. Effective use of optimized, high-dose (50 kGy) gamma irradiation for pathogen inactivation of human bone allografts. Biomaterials 2005, 26, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- Carzaniga, R.; Domart, M.C.; Collinson, L.M.; Duke, E. Cryo-soft X-ray tomography: A journey into the world of the native-state cell. Protoplasma 2014, 251, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Duke, E.; Dent, K.; Razi, M.; Collinson, L.M. Biological applications of cryo-soft X-ray tomography. J. Microsc. 2014, 255, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Carzaniga, R.; Domart, M.-C.; Duke, E.; Collinson, L.M. Correlative Cryo-Fluorescence and Cryo-Soft X-Ray Tomography of Adherent Cells at European Synchrotrons. Methods Cell Biol. 2014, 124, 151–178. [Google Scholar] [CrossRef] [PubMed]
- Voggenreiter, G.; Ascherl, R.; Blumel, G.; Schmit-Neuerburg, K.P. Effects of preservation and sterilization on cortical bone grafts. Arch. Orthop. Trauma Surg. 1994, 113, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Cornu, O.; Banse, X.; Docquier, P.L.; Luyckx, S.; Delloye, C. Effect of freeze-drying and gamma irradiation on the mechanical properties of human cancellous bone. J. Orthop. Res. 2005, 18, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Venkatesan, S.; Webb, D.; Kalyanasundaram, S.; Qin, Q.H. Effect of cryo-induced microcracks on microindentation of hydrated cortical bone tissue. Mater. Charact. 2009, 60, 783–791. [Google Scholar] [CrossRef]
- Atwood, R.C.; Bodey, A.J.; Price, S.W.T.; Basham, M.; Drakopoulos, M. A high-throughput system for high-quality tomographic reconstruction of large datasets at Diamond Light Source. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140398. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.T.; Atwood, R.C.; Drakopoulos, M. Radial lens distortion correction with sub-pixel accuracy for X-ray micro-tomography. Opt. Express 2015, 23, 32859–32868. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Buades, A.; Coll, B.; Morel, J.-M. Non-Local Means Denoising. Image Process. Line 2011, 1, 490–530. [Google Scholar] [CrossRef]
- Immerkær, J. Fast noise variance estimation. Comput. Vis. Image Underst. 1996, 64, 300–302. [Google Scholar] [CrossRef]
- Tozzi, G.; Danesi, V.; Palanca, M.; Cristofolini, L. Elastic Full-Field Strain Analysis and Microdamage Progression in the Vertebral Body from Digital Volume Correlation. Strain 2016, 52, 446–455. [Google Scholar] [CrossRef] [Green Version]
- Palanca, M.; Tozzi, G.; Cristofolini, L.; Viceconti, M.; Dall’Ara, E. 3D Local Measurements of Bone Strain and Displacement: Comparison of Three Digital Volume Correlation Approaches. J. Biomech. Eng. 2015, 137, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deymier-Black, A.C.; Singhal, A.; Almer, J.D.; Dunand, D.C. Effect of X-ray irradiation on the elastic strain evolution in the mineral phase of bovine bone under creep and load-free conditions. Acta Biomater. 2013, 9, 5305–5312. [Google Scholar] [CrossRef] [PubMed]
- Witala, M.; Han, J.; Menzel, A.; Nygård, K. In situ small-angle X-ray scattering characterization of X-ray-induced local heating. J. Appl. Crystallogr. 2014, 47, 2078–2080. [Google Scholar] [CrossRef] [Green Version]
- Bras, W.; Stanley, H. Unexpected effects in non crystalline materials exposed to X-ray radiation. J. Non-Cryst. Solids 2016, 451, 153–160. [Google Scholar] [CrossRef]
- Wallander, H.; Wallentin, J. Simulated sample heating from a nanofocused X-ray beam. J. Synchrotron Radiat. 2017, 24, 925–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfram, U.; Schwiedrzik, J.; Bürki, A.; Rack, A.; Olivier, C.; Peyrin, F.; Best, J.; Michler, J.; Zysset, P.K. Microcrack evolution in microindentation of ovine cortical bone investigated with lapsed SRmicroCT. In Proceedings of the 23rd Congress of the European Society of Biomechanics (ESB 2017), Seville, Spain, 2–5 July 2017. [Google Scholar]
- Zioupos, P.; Currey, J.D.; Hamer, A.J. The role of collagen in the declining mechanical properties of aging human cortical bone. J. Biomed. Mater. Res. 1999, 45, 108–116. [Google Scholar] [CrossRef]
- Mitchell, T.W.; Rigby, B.J. In vivo and in vitro aging of collagen examined using an isometric melting technique. Biochim. Biophys. Acta 1975, 393, 531–541. [Google Scholar] [CrossRef]
- Lee, J.M.; Pereira, C.A.; Abdulla, D.; Naimark, W.A.; Crawford, I. A multi-sample denaturation temperature tester for collagenous biomaterials. Med. Eng. Phys. 1995, 17, 115–121. [Google Scholar] [CrossRef]
- Unal, M.; Creecy, A.; Nyman, J.S. The Role of Matrix Composition in the Mechanical Behavior of Bone. Curr. Osteoporos. Rep. 2018, 16, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Ginoza, W. Radiosensitive Molecular Weight of Single-Stranded Virus Nucleic Acids. Nature 1963, 199, 453. [Google Scholar] [CrossRef] [PubMed]
- Kempner, E.S.; Haigler, H.T. The influence of low temperature on the radiation sensitivity of enzymes. J. Biol. Chem. 1982, 257, 13297–13299. [Google Scholar] [PubMed]
- Panjabi, M.M.; Krag, M.; Summers, D.; Videman, T. Biomechanical time-tolerance of fresh cadaveric human spine specimens. J. Orthop. Res. 1985, 3, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Linde, F.; Sørensen, H.C.F. The effect of different storage methods on the mechanical properties of trabecular bone. J. Biomech. 1993, 26, 1249–1252. [Google Scholar] [CrossRef]
- Kang, Q.; An, Y.H.; Friedman, R.J. Effects of multiple freezing-thawing cycles on ultimate indentation load and stiffness of bovine cancellous bone. Am. J. Vet. Res. 1997, 58, 1171–1173. [Google Scholar] [PubMed]
- Borchers, R.E.; Gibson, L.J.; Burchardt, H.; Hayes, W.C. Effects of selected thermal variables on the mechanical properties of trabecular bone. Biomaterials 1995, 16, 545–551. [Google Scholar] [CrossRef]
- Lee, W.; Jasiuk, I. Effects of freeze-thaw and micro-computed tomography irradiation on structure-property relations of porcine trabecular bone. J. Biomech. 2014, 47, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz, A. The effect of trabecular bone storage method on its elastic properties. Acta Bioeng. Biomech. 2018, 20, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Fantner, G.E.; Hassenkam, T.; Kindt, J.H.; Weaver, J.C.; Birkedal, H.; Pechenik, L.; Cutroni, J.A.; Cidade, G.A.G.; Stucky, G.D.; Morse, D.E.; et al. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat. Mater. 2005, 4, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Hedan, S.; Fauchille, A.L.; Valle, V.; Cabrera, J.; Cosenza, P. One-year monitoring of desiccation cracks in Tournemire argillite using digital image correlation. Int. J. Rock Mech. Min. Sci. 2014, 68, 22–35. [Google Scholar] [CrossRef]
- Wei, X.; Hattab, M.; Bompard, P.; Fleureau, J.-M. Highlighting some mechanisms of crack formation and propagation in clays on drying path. Géotechnique 2016, 66, 287–300. [Google Scholar] [CrossRef]
- Bodey, A.J.; Rau, C. Launch of the I13-2 data beamline at the Diamond Light Source synchrotron. J. Phys. Conf. Ser. 2017, 849, 012038. [Google Scholar] [CrossRef] [Green Version]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña Fernández, M.; Dall’Ara, E.; Kao, A.P.; Bodey, A.J.; Karali, A.; Blunn, G.W.; Barber, A.H.; Tozzi, G. Preservation of Bone Tissue Integrity with Temperature Control for In Situ SR-MicroCT Experiments. Materials 2018, 11, 2155. https://doi.org/10.3390/ma11112155
Peña Fernández M, Dall’Ara E, Kao AP, Bodey AJ, Karali A, Blunn GW, Barber AH, Tozzi G. Preservation of Bone Tissue Integrity with Temperature Control for In Situ SR-MicroCT Experiments. Materials. 2018; 11(11):2155. https://doi.org/10.3390/ma11112155
Chicago/Turabian StylePeña Fernández, Marta, Enrico Dall’Ara, Alexander P. Kao, Andrew J. Bodey, Aikaterina Karali, Gordon W. Blunn, Asa H. Barber, and Gianluca Tozzi. 2018. "Preservation of Bone Tissue Integrity with Temperature Control for In Situ SR-MicroCT Experiments" Materials 11, no. 11: 2155. https://doi.org/10.3390/ma11112155




