Sorption-Related Characteristics of Surface Charred Spruce Wood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Wettability Measurements
2.3. DVS Sorption Measurements
2.4. FT-IR Analysis
2.5. Microstructural Analysis
2.6. Density Profile
3. Results
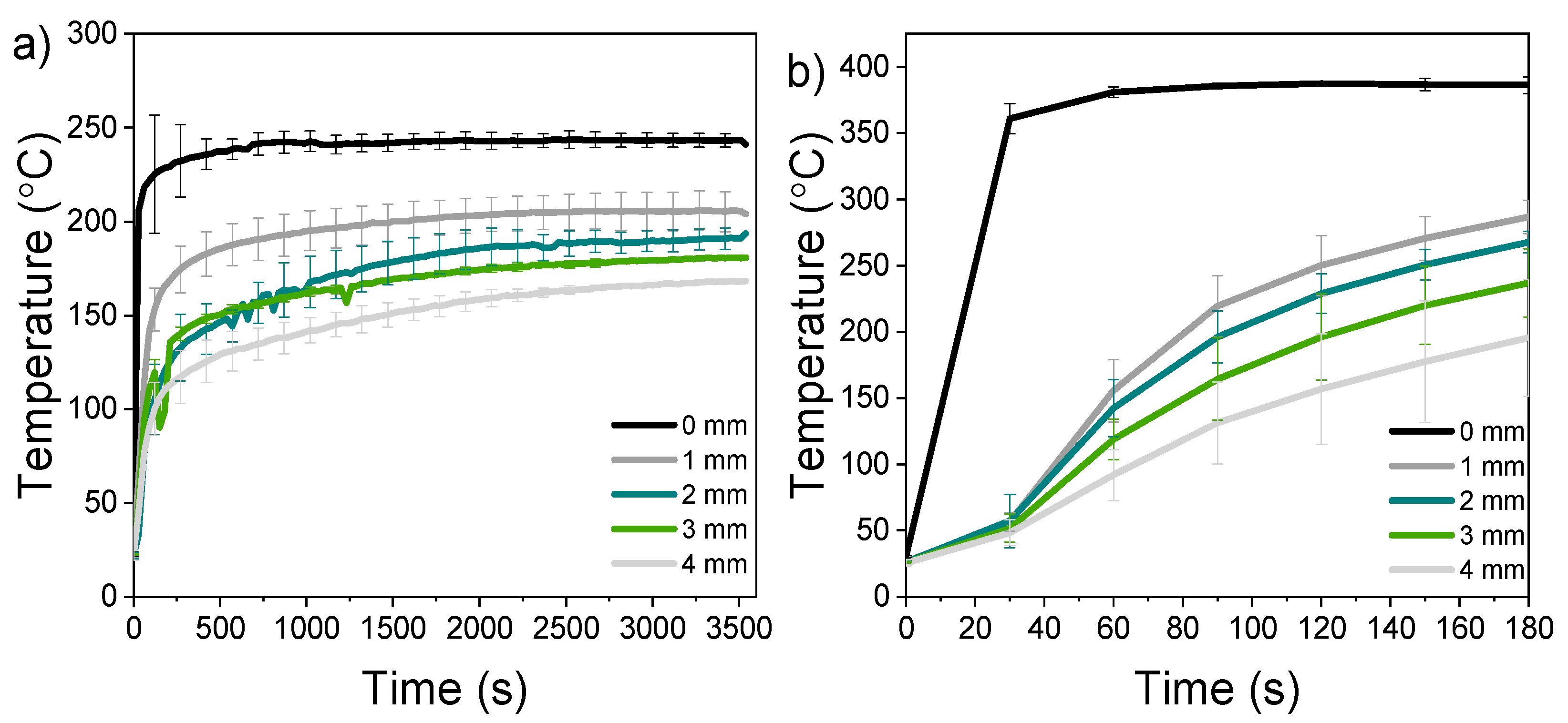
3.1. Temperature Profile
3.2. Microstructural Changes
3.3. Surface Wettability
3.4. Absorption by Water Floating
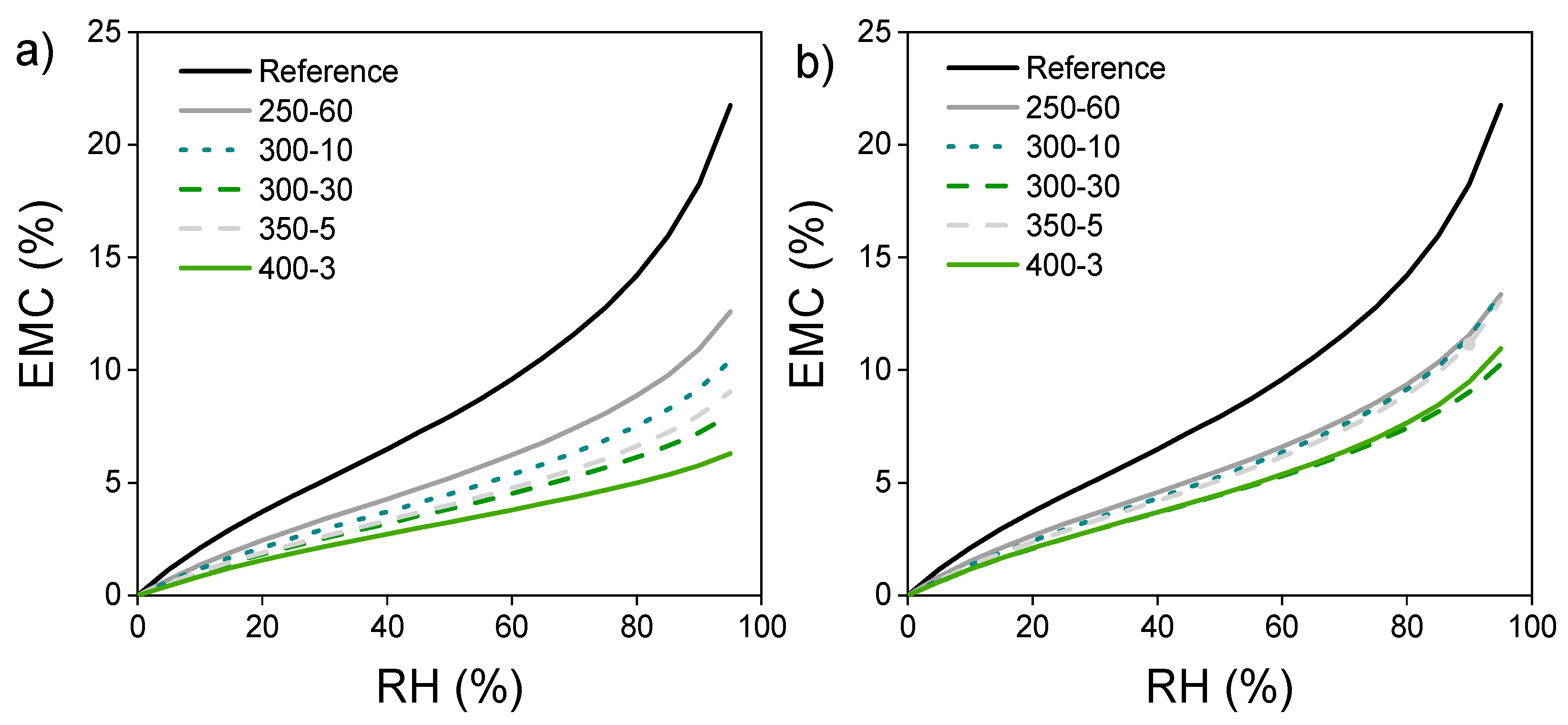
3.5. Sorption Measured by DVS
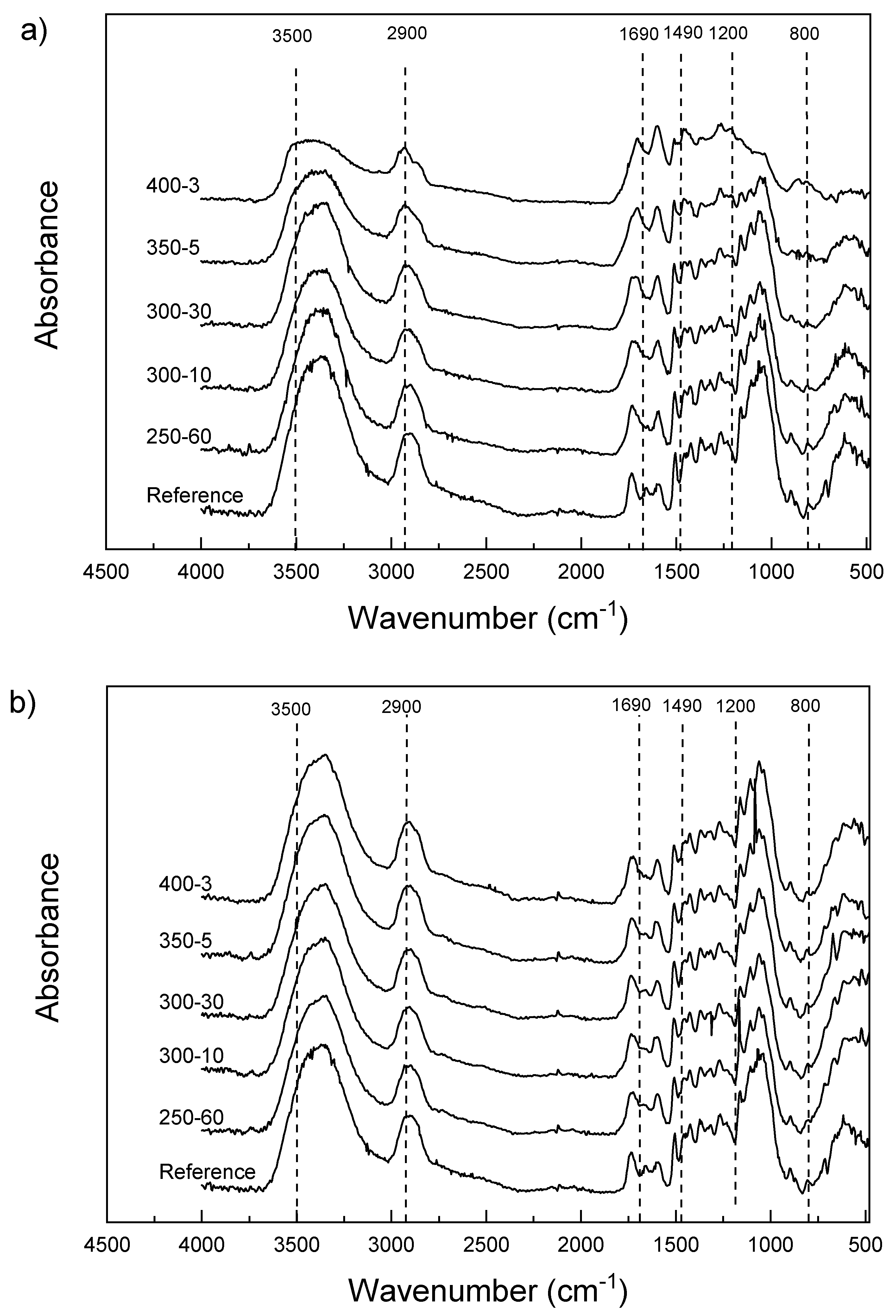
3.6. Changes in the Surface Functional Groups
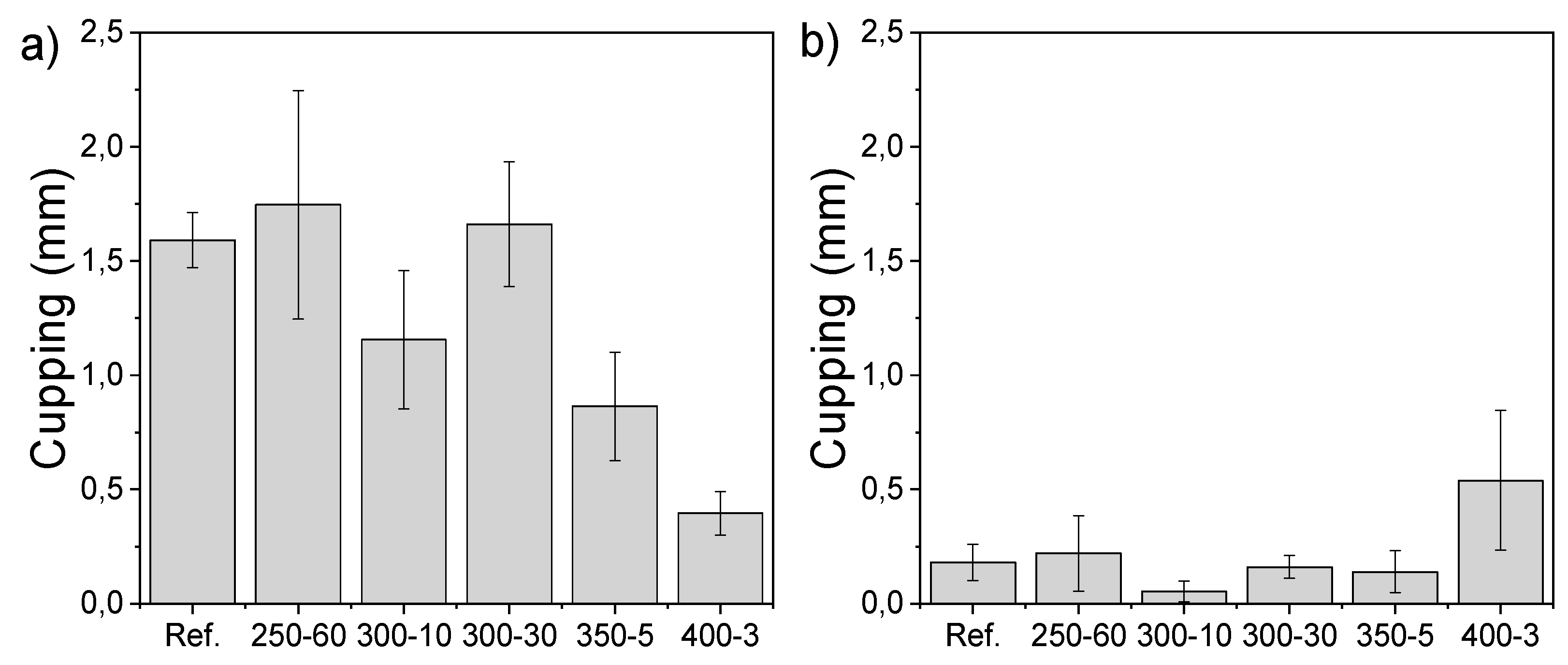
3.7. Cupping
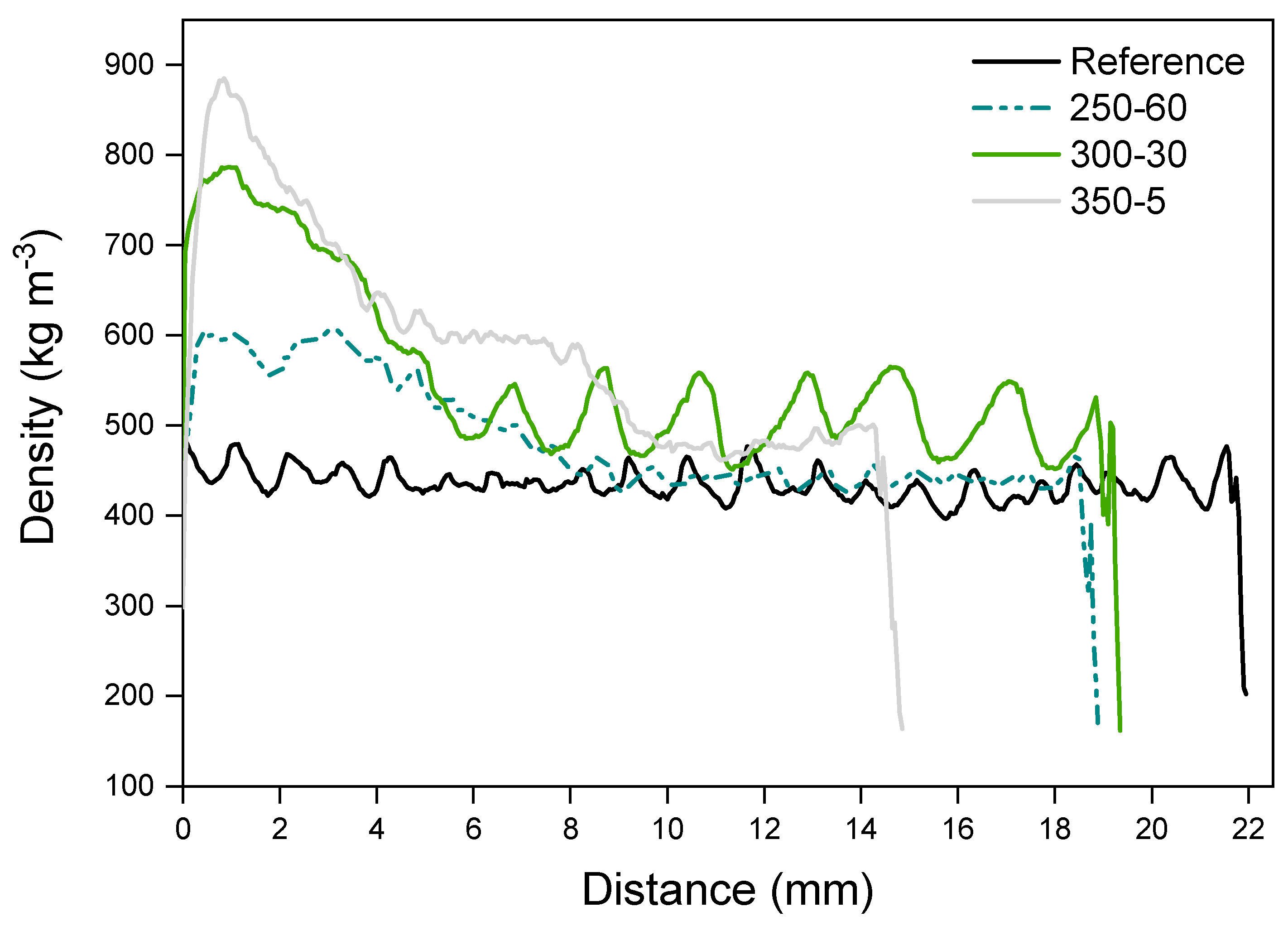
3.8. Density Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marteinsson, B.; Jónsson, B. Overall survey of buildings-performance and maintenance. In Proceedings of the 8th DBMC International conference on durability of building materials and components, Ottawa, ON, Canada, 30 May–3 June 1999. [Google Scholar]
- Ruuska, A.; Häkkinen, T.; Vares, S.; Korhonen, M.-R.; Myllymaa, T. Rakennusmateriaalien Ympäristövaikutukset (Environmental Impacts of Construction Materials); Reports of the Ministry of Environment 8/2013; Ministry of Environment: Helsinki, Finland, 2013; 39p, (In Finnish, English Summary).
- Sandberg, D.; Kutnar, A. Thermally modified timber: Recent developments in Europe and North America. Wood Fiber Sci. 2016, 48, 28–39. [Google Scholar]
- Bribían, I.Z.; Capilla, A.V.; Usón, A.A. Life cycle assessment of building materials: Comparative analysis of energy and environmental impacts and evaluation of the eco-efficiency improvement potential. Build. Environ. 2011, 46, 1133–1140. [Google Scholar] [CrossRef]
- Häkkinen, T.; Ahola, P.; Vanhatalo, L.; Merra, A. Environmental Impact of Coated Exterior Wooden Cladding; VTT Technical Research Centre of Finland, Building Technology: Espoo, Finland, 1999; 84p, Available online: http://virtual.vtt.fi/virtual/proj6/environ/env_woodclad.pdf (accessed on 30 February 2018).
- Grüll, G.; Tsherne, F.; Spitaler, I.; Forsthuber, B. Comparison of wood coating durability in natural weathering and artificial weathering using fluorescent UV-lamps and water. Eur. J. Wood Wood Prod. 2014, 72, 367–376. [Google Scholar] [CrossRef]
- Čermák, P.; Vahtikari, K.; Rautkari, L.; Laine, K.; Horáček, P.; Baar, J. The effect of wetting cycles on moisture behaviour of thermally modified Scots pine (Pinus sylvestris L.) wood. J. Mater. Sci. 2016, 51, 1504–1511. [Google Scholar]
- Wentzel, M.; Altgen, M.; Militz, H. Analyzing reversible changes in hygroscopicity of thermally modified eucalypt wood from open and closed reactor systems. Wood Sci. Technol. 2018, 52, 889–907. [Google Scholar] [CrossRef]
- Boonstra, M.J.; van Acker, J.; Tjeerdsma, B.F.; Kegel, E.V. Strength properties of thermally modified softwoods and its relation to polymeric structural constituents. Ann. Sci. 2007, 64, 679–690. [Google Scholar] [CrossRef]
- Jun Li, S.; Kocaefe, D.; Zhang, J. Mechanical behaviour of Quebec wood species heat-treated using ThermoWood process. Holz Roh Werkst. 2007, 65, 255–259. [Google Scholar]
- Jämsä, S.; Ahola, P.; Viitaniemi, P. Long-term natural weathering of coated ThermoWood. Pigment Resin Technol. 2000, 29, 68–74. [Google Scholar] [CrossRef]
- Virta, J. Cupping of wooden cladding boards in cyclic conditions—A study of heat-treated and non-heat-treated boards. Build. Environ. 2005, 40, 1395–1399. [Google Scholar]
- Hill, C.A.S. Wood Modification: Chemical, Thermal, and Other Processes; John Wiley & Sons Ltd.: Chichester, UK, 2006; ISBN 978-0-470-02172-9. [Google Scholar]
- Hill, C.A.S. The potential for the use of modified wood products in the built environment. In Proceedings of the 11th International Conference on Non-conventional Materials and Technologies (NOCMAT 2009), Bath, UK, 6–9 September 2009. [Google Scholar]
- Syrjänen, T. Production and Classification of Heat Treated Wood in Finland; Nordic Wood: Helsinki, Finland, 2000; 8p, Available online: http://www.thermallytreatedwood.com/Worldwide/Finland.pdf (accessed on 22 February 2018).
- Esteves, B.M.; Pereira, H.M. Wood modification by heat treatment: A review. Bioresources 2009, 4, 370–404. [Google Scholar]
- Miller, H. Japanese Wood Craftsmanship; Huge Miller Furniture: Liverpool, UK, 2015; 64p, Available online: http://www.hughmillerfurniture.co.uk/blog/japanese-wood-craftsmanship/ (accessed on 20 May 2017).
- Rath, J.; Wolfinger, M.G.; Steiner, G.; Krammer, G.; Barontini, F.; Cozzani, V. Heat of wood pyrolysis. Fuel 2003, 82, 81–91. [Google Scholar] [CrossRef]
- Chow, S.Z.; Pickles, K.J. Thermal softening and degradation of wood and bark. Wood Fiber Sci. 2007, 3, 166–178. [Google Scholar]
- Byrne, C.E.; Nagle, D.C. Carbonization of wood for advanced materials applications. Carbon 1997, 35, 259–266. [Google Scholar] [CrossRef]
- Tjeerdsma, B.F.; Boonstra, M.; Pizzi, A.; Tekely, P.; Militz, H. Characterisation of thermally modified wood: Molecular reasons for wood performance improvement. Holz Roh Werkst. 1998, 56, 149–153. [Google Scholar] [CrossRef]
- Sivonen, H.; Maunu, S.L.; Sundholm, F.; Jämsä, S.; Viitaniemi, P. Magnetic resonance studies of thermally modified wood. Holzforschung 2002, 56, 648–654. [Google Scholar] [CrossRef]
- Browne, F.L. Theories of the Combustion of Wood and Its Control; Report No. 2136; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 1958.
- Schaffer, E.L. Charring Rate of Selected Woods—Transverse to Grain; U.S. Forest Service Research Paper FPL 69; U.S. Department of Agriculture Forest Service, Forest Products Laboratory: Washington, DC, USA, 1967.
- Frangi, A.; Fontana, M. Charring rates and temperature profiles of wood sections. Fire Mater. 2003, 27, 91–102. [Google Scholar] [CrossRef]
- White, R.H. Charring rate of composite timber products. In Proceedings of the Wood and Fire Safety 4th International Conference, The High Tatras, Slovakia, 14–19 May 2000. [Google Scholar]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gomez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Blankenhorn, P.R.; Kline, D.E.; Beall, F.C. Dynamic mechanical behavior of carbonized black cherry wood. Carbon 1973, 11, 603–611. [Google Scholar] [CrossRef]
- Bergman, P.C.A. Combined Torrefaction and Pelletisation: The TOP Process; ECN-C--05-073; ECN: Petten, The Netherlands, 2005; 29p. [Google Scholar]
- Kymäläinen, M.; Hautamäki, S.; Lillqvist, K.; Segerholm, K.; Rautkari, L. Surface modification of solid wood by charring. J. Mater. Sci. 2017, 52, 6111–6119. [Google Scholar] [CrossRef]
- SFS-EN 927-5. Paints and Varnishes. Coating Materials and Coating Systems for Exterior Wood. Part 5: Assessment of The Liquid Water Permeability; Finnish Standards Association: Helsinki, Finland, 2007; 20p. [Google Scholar]
- Faix, O.; Böttcher, J.H. The influence of particle size and concentration in transmission and diffuse reflectance spectroscopy of wood. Holz Roh Werkst. 1992, 50, 221–226. [Google Scholar] [CrossRef]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–27. [Google Scholar] [CrossRef]
- Rutherford, D.W.; Wershaw, R.L.; Cox, L.G. Changes in Composition and Porosity Occurring during the Thermal Degradation of Wood and Wood Components; Scientific Investigations Report 2004-5292; U.S. Department of the interior, U.S. Geological Survey: Reston, VA, USA, 2004.
- Derkacheva, O.; Sukhov, D. Investigation of lignins by FTIR spectroscopy. Macromol. Symp. 2008, 265, 61–68. [Google Scholar] [CrossRef]
- Rautkari, L.; Kutnar, A.; Kamke, F.A.; Hughes, M. Wood surface densification using different methods. In Proceedings of the 11th World Conference on Timber Engineering, Riva del Garda, Italy, 20–24 June 2010. [Google Scholar]
- Rautkari, L.; Hänninen, T.; Johansson, L.-S.; Hughes, M. A study by X-ray photoelectron spectroscopy (XPS) of the chemistry of the surface of Scots pine (Pinus sylvestris L.) modified by friction. Holzforschung 2012, 66, 93–96. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Belt, T.; Rautkari, L.; Laine, K.; Hill, C.A.S. Cupping behavior of surface densified Scots pine wood: The effect of process parameters and correlation with density profile characteristics. J. Mater. Sci. 2013, 48, 6426–6430. [Google Scholar] [CrossRef]
- Laine, K.; Antikainen, T.; Rautkari, L.; Hughes, M. Analysing density profile characteristics of surface densified solid wood using computational approach. Int. Wood Prod. J. 2013, 4, 144–149. [Google Scholar] [CrossRef]
- Virta, J.; Koponen, S.; Absetz, I. Modelling moisture distribution in wooden cladding board as a result of short-term single-sided water soaking. Build. Environ. 2006, 41, 1593–1599. [Google Scholar] [CrossRef]
- Wannapeera, J.; Fungtammasan, B.; Worasuwannarak, N. Effects of temperature and holding time during torrefaction on the pyrolysis behaviors of woody biomass. J. Anal. Appl. Pyrol. 2011, 92, 99–105. [Google Scholar] [CrossRef]
- Nuopponen, M.; Vuorinen, T.; Jämsä, S.; Viitaniemi, P. The effects of a heat treatment on the behaviour of extractives in softwood studied by FTIR spectroscopic measurements. Wood Sci. Technol. 2003, 37, 109–115. [Google Scholar] [CrossRef]
- Labbe, N.; Harper, D.; Rials, T. Chemical structure of wood charcoal by infrared spectroscopy and multivariate analysis. J. Agric. Food Chem. 2006, 54, 3492–3497. [Google Scholar] [CrossRef] [PubMed]
- McBeath, A.V.; Smernik, R.J.; Schneider, M.P.W.; Schmidt, M.W.I.; Plant, E.L. Determination of the aromaticity and the degree of aromatic condensation of a thermosequence of wood charcoal using NMR. Org. Chem. 2011, 42, 194–1202. [Google Scholar] [CrossRef]
- Shaaban, A.; Se, S.-M.; Dimin, M.F.; Juoi, J.M.; Husin, M.H.M.; Mitan, N.M.M. Influence of heating temperature and holding time on biochars derived from rubber wood sawdust via slow pyrolysis. J. Anal. Appl. Pyrol. 2014, 107, 31–39. [Google Scholar] [CrossRef]
- Kymäläinen, M.; Mäkelä, M.R.; Hildén, K.; Kukkonen, J. Fungal colonisation and moisture uptake of torrefied wood, charcoal, and thermally treated pellets during storage. Eur. J. Wood Wood Prod. 2015, 73, 709–717. [Google Scholar] [CrossRef]


| Time (min) | Temperature (°C) | Code |
|---|---|---|
| 60 | 250 | 250-60 |
| 10 | 300 | 300-10 |
| 30 | 300 | 300-30 |
| 5 | 350 | 350-5 |
| 3 | 400 | 400-3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kymäläinen, M.; Turunen, H.; Čermák, P.; Hautamäki, S.; Rautkari, L. Sorption-Related Characteristics of Surface Charred Spruce Wood. Materials 2018, 11, 2083. https://doi.org/10.3390/ma11112083
Kymäläinen M, Turunen H, Čermák P, Hautamäki S, Rautkari L. Sorption-Related Characteristics of Surface Charred Spruce Wood. Materials. 2018; 11(11):2083. https://doi.org/10.3390/ma11112083
Chicago/Turabian StyleKymäläinen, Maija, Hannu Turunen, Petr Čermák, Saara Hautamäki, and Lauri Rautkari. 2018. "Sorption-Related Characteristics of Surface Charred Spruce Wood" Materials 11, no. 11: 2083. https://doi.org/10.3390/ma11112083





