Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 1: Specificity of Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Electrochemical Measurements
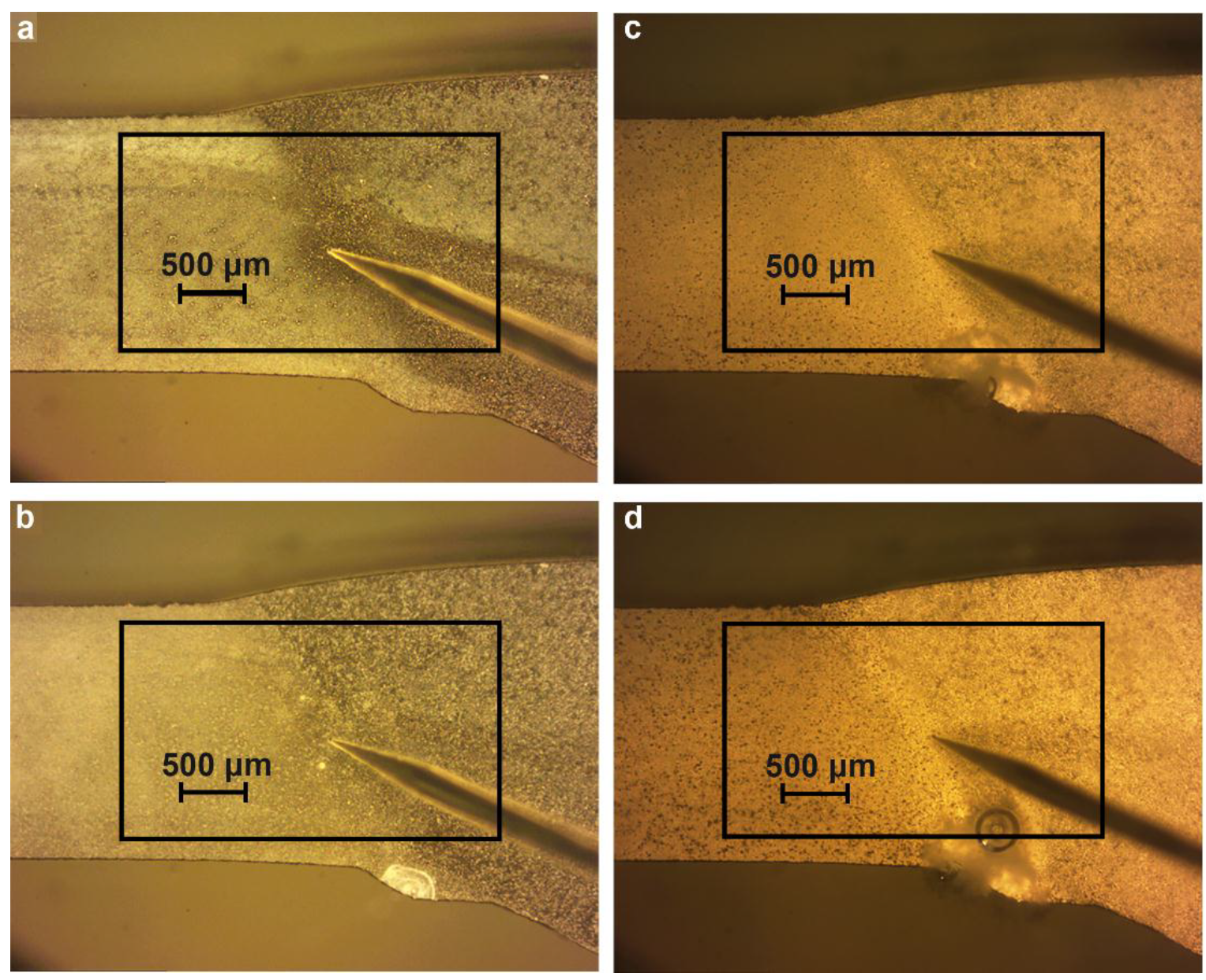
2.3. Cross-Section Preparation
2.4. Immersion Experiments and Corrosion Products Characterization
3. Results and Discussion
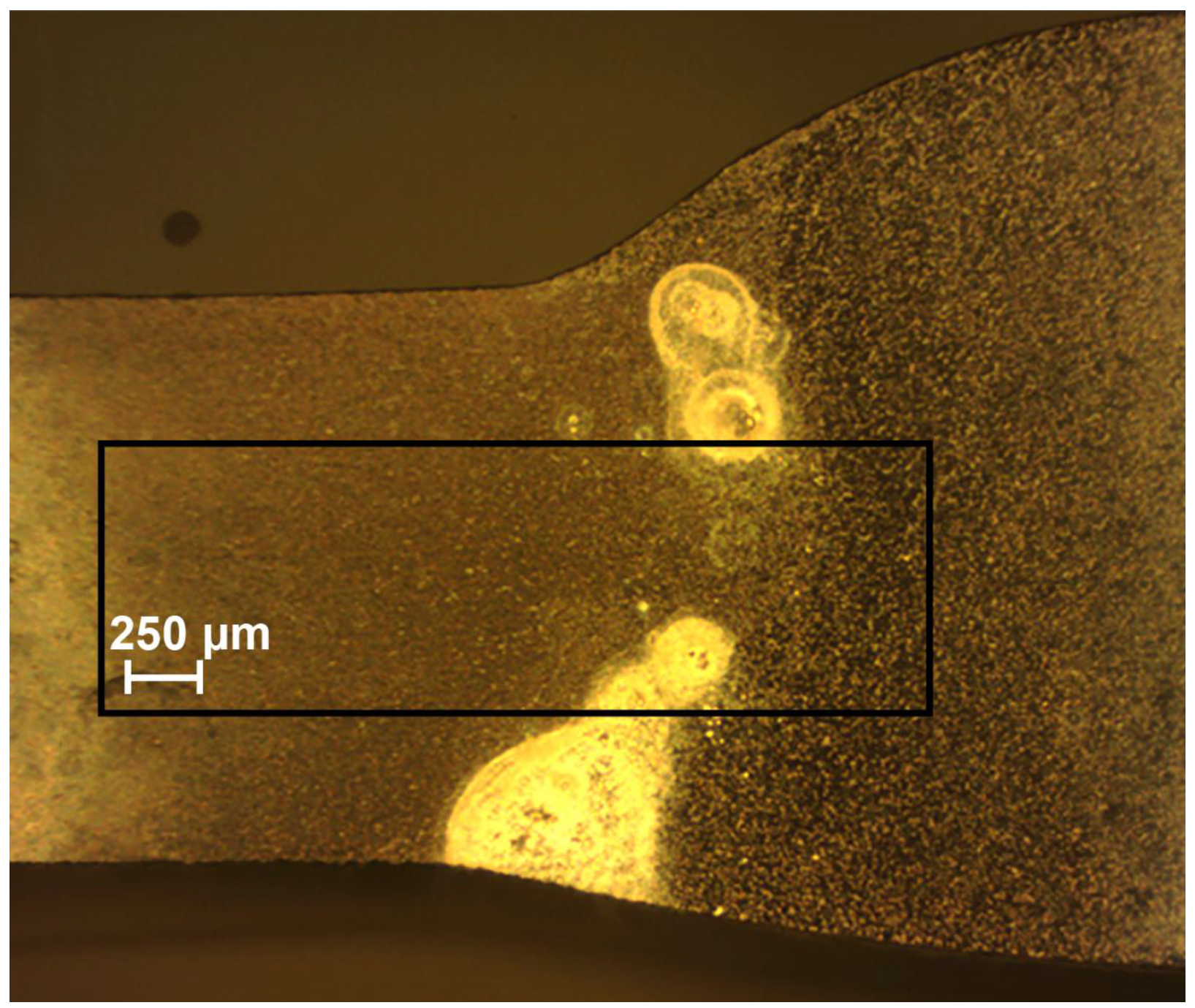
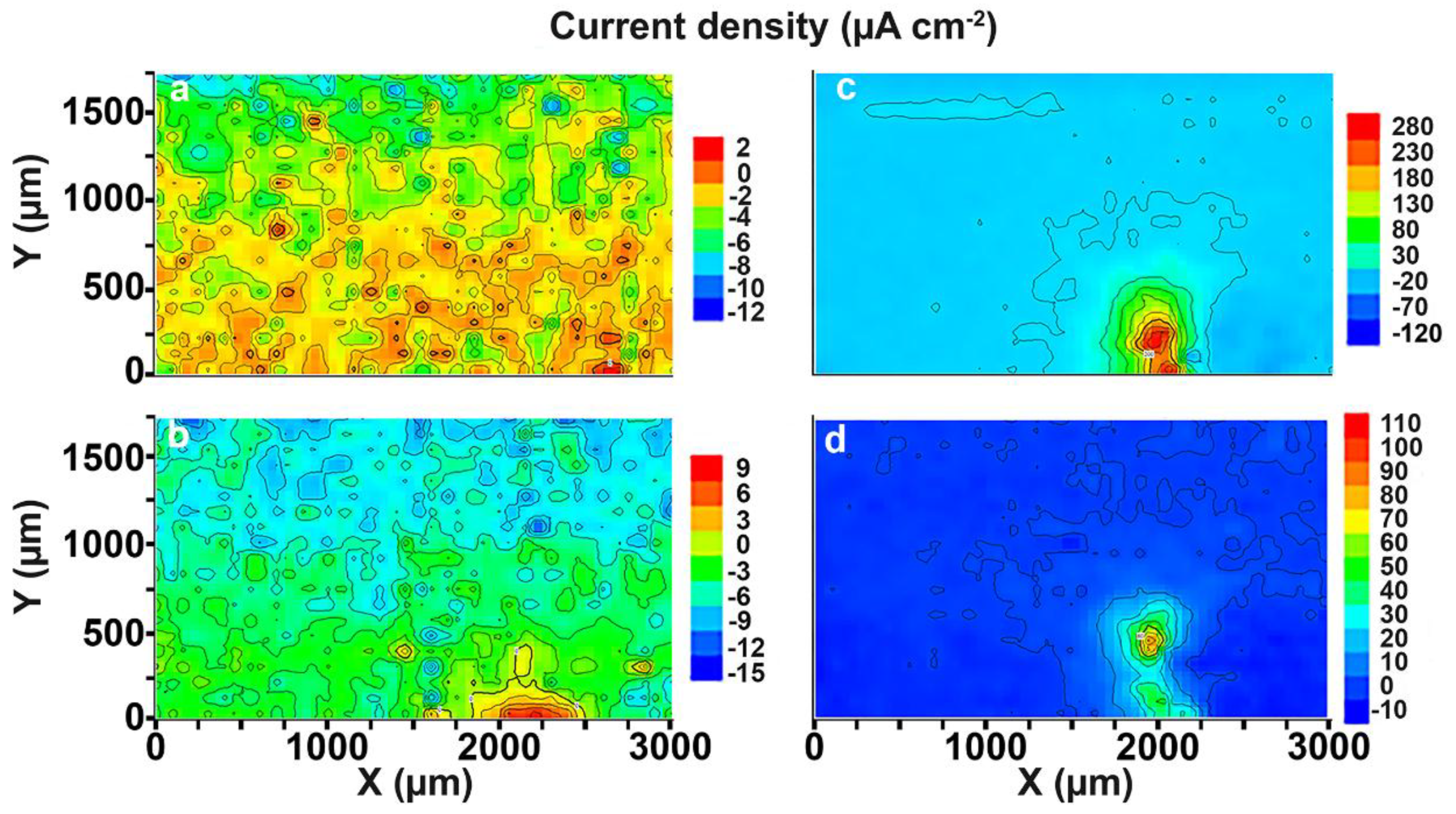
3.1. Study of the Localized Corrosion Process on the 1579 Al Alloy Sample
3.2. Corrosion Performance of the 1579 Al Alloy Specimen
4. Conclusions
- Propagation of the corrosion process within the weld interface has been registered and studied by SVET and SIET. The welded joint is a zone of corrosion process activation due to the presence of microdefects in the morphological structure.
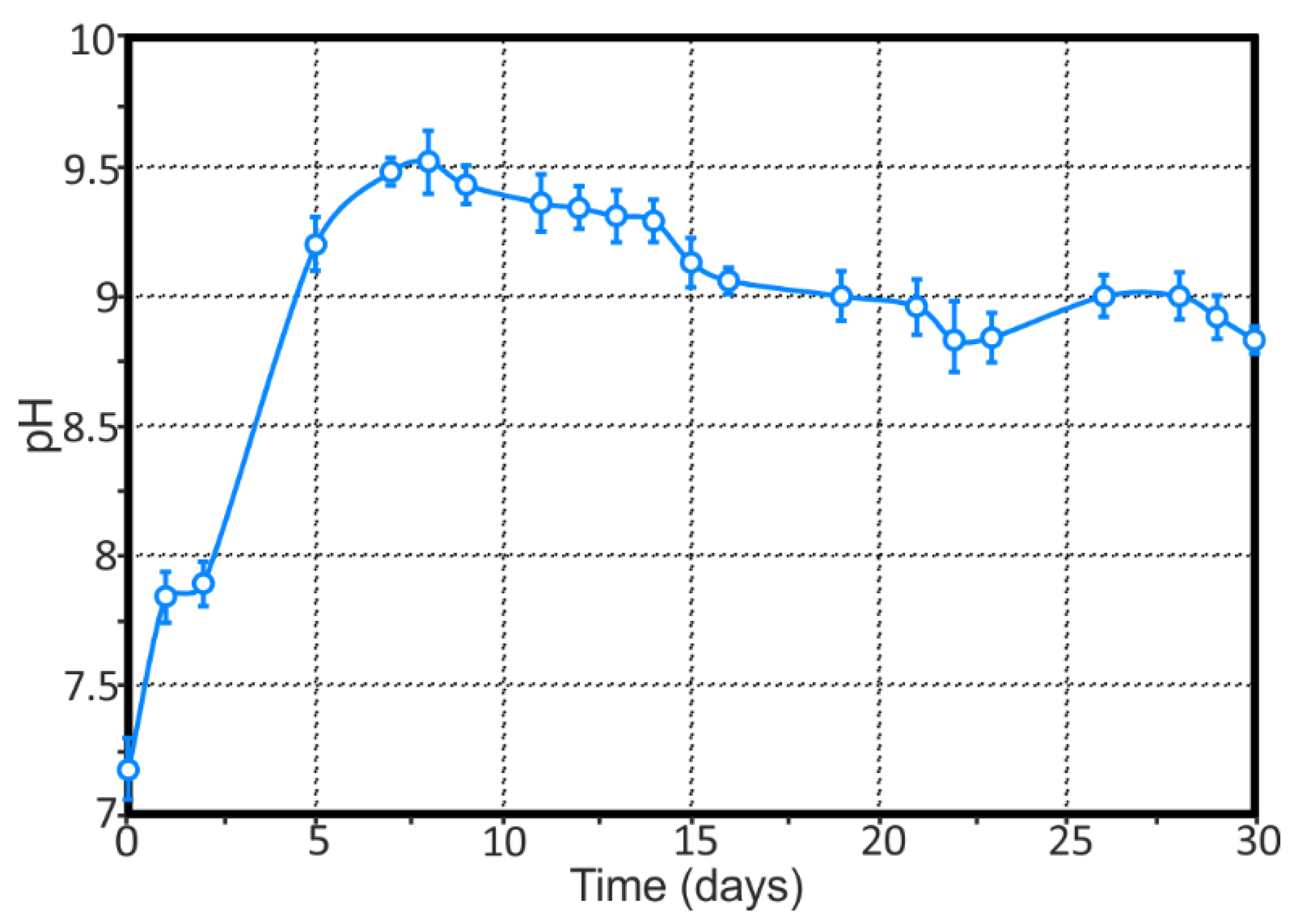
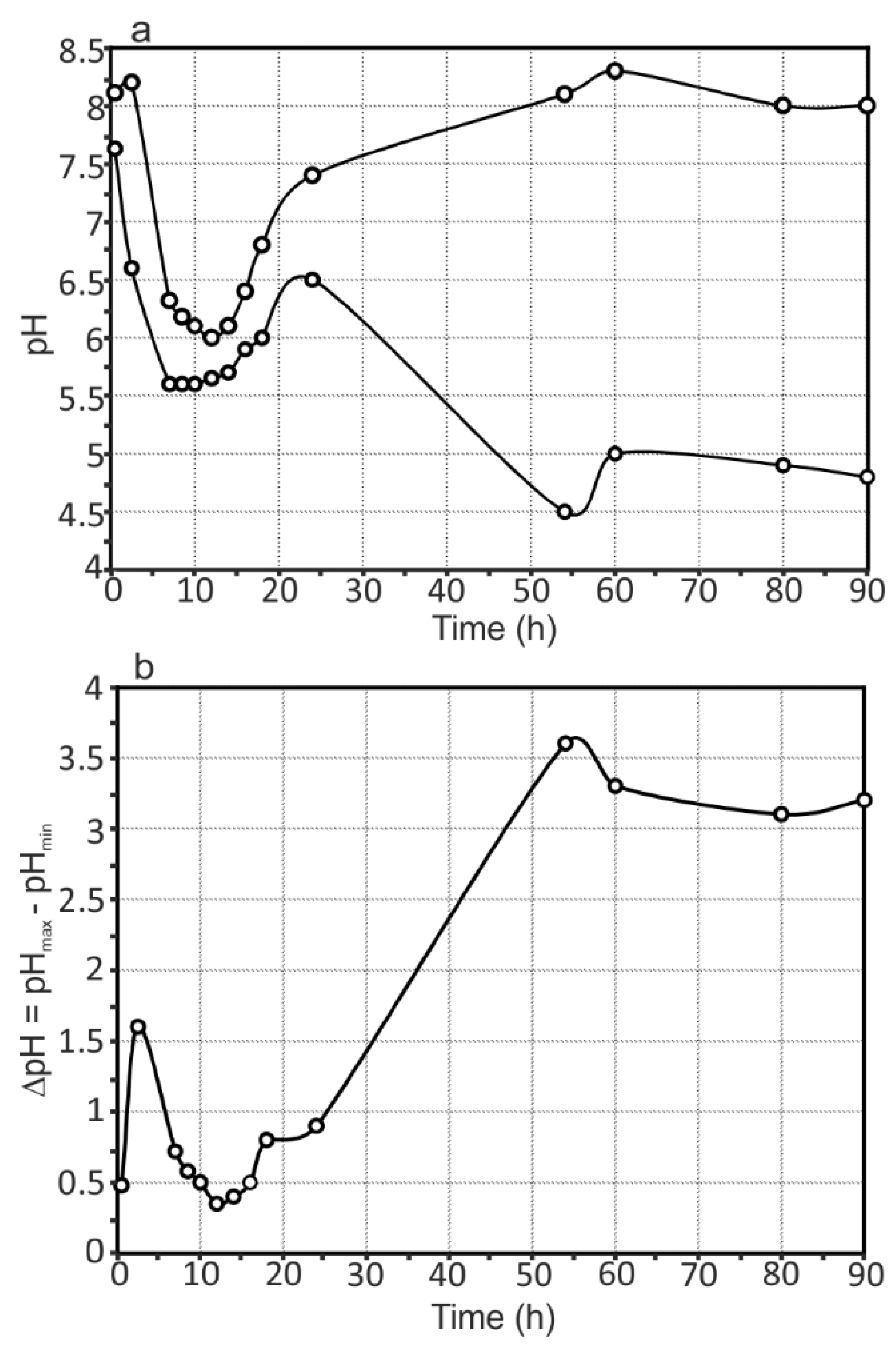
- The presence of Mg as an alloying element in rather a high amount (6.78 wt.%) in the 1579 Al alloy sufficiently shifts the bulk pH values to the alkaline range as a result of the intensive dissolution of Mg, according to the results obtained by means of immersion tests and AAS. This effect accelerates the corrosion activity of the 1579 Al alloy in the aggressive media.
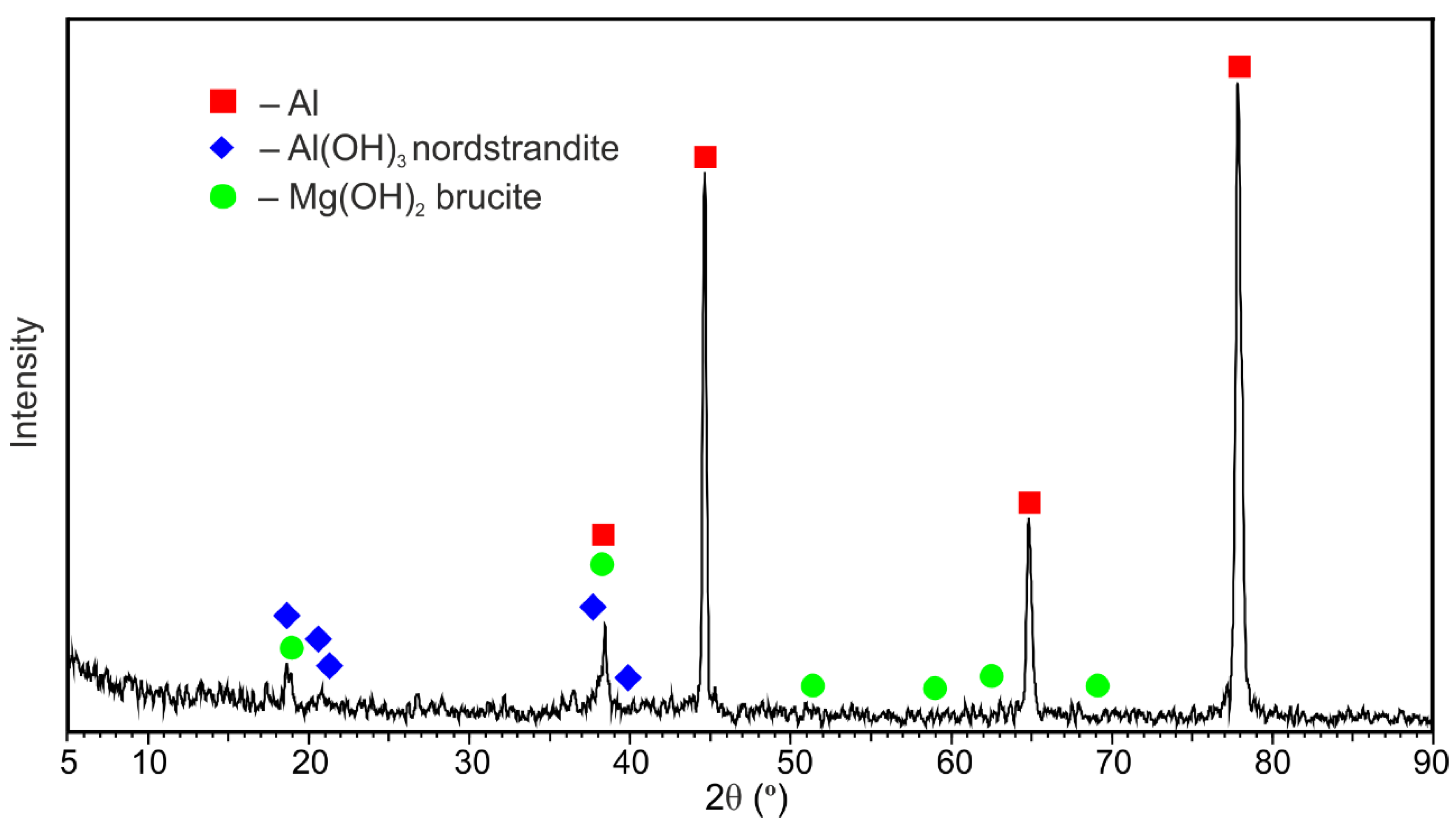
- Al(OH)3 and Mg(OH)2 are the main corrosion products of the 1579 Al alloy, according to XRD.
- Analysis of the evolution of the sum of total cathodic and anodic currents, as well as the evolution of pH distribution for the 1579 Al alloy sample, indicated the necessity of the coating formation to protect this Al alloy against corrosion and to increase the stability of this system in a corrosive environment.
Author Contributions
Funding
Conflicts of Interest
References
- Moreto, J.A.; Marino, C.E.B.; Bose Filho, W.W.; Rocha, L.A.; Fernandes, J.C.S. SVET, SKP and EIS study of the corrosion behaviour of high strength Al and Al-Li alloys used in aircraft fabrication. Corros. Sci. 2014, 84, 30–41. [Google Scholar] [CrossRef]
- Grilli, R.; Baker, M.A.; Castle, J.E.; Dunn, B.; Watts, J.F. Localized corrosion of a 2219 aluminium alloy exposed to a 3.5% NaCl solution. Corros. Sci. 2010, 52, 2855–2866. [Google Scholar] [CrossRef] [Green Version]
- Metikoš-Huković, M.; Babić, R.; Grubač, Z.; Brinć, S. Impedance spectroscopic study of aluminium and Al-alloys in acid solution: Inhibitory action of nitrogen containing compounds. J. Appl. Electrochem. 1994, 24, 772–778. [Google Scholar] [CrossRef]
- Akid, R.; Gobara, M.; Wang, H. Hybrid sol-gel/conducting polymer coatings: Self-healing coatings for the corrosion protection of aerospace alloys. In Self-Healing Properties of New Surface Treatments; Fedrizzi, L., Furbeth, W., Montemor, F., Eds.; Maney Publishing: Leed, UK, 2011; pp. 119–133. [Google Scholar]
- Lv, J.; Luo, H. Investigation of microstructure and corrosion behavior of burnished aluminum alloy by TEM, EWF, XPS and EIS techniques. Mater. Res. Bull. 2016, 83, 148–154. [Google Scholar]
- Zhang, X.; Liu, B.; Zhou, X.; Wang, J.; Hashimoto, T.; Luo, C.; Sun, Z.; Tang, Z.; Lu, F. Laser welding introduced segregation and its influence on the corrosion behaviour of Al-Cu-Li alloy. Corros. Sci. 2018, 135, 177–191. [Google Scholar] [CrossRef]
- Bocchi, S.; Cabrini, M.; D’Urso, G.; Giardini, C.; Lorenzi, S.; Pastore, T. The influence of process parameters on mechanical properties and corrosion behavior of friction stir welded aluminum joints. J. Manuf. Process. 2018, 35, 1–15. [Google Scholar] [CrossRef]
- Sinhmar, S.; Dwivedi, D.K. A study on corrosion behavior of friction stir welded and tungsten inert gas welded AA2014 aluminium alloy. Corros. Sci. 2018, 133, 25–35. [Google Scholar] [CrossRef]
- Hou, L.; Yu, J.; Zhang, D.; Zhuang, L.; Zhou, L.; Zhang, J. Corrosion behavior of friction stir welded Al-Mg-(Zn) alloys. Rare Met. Mater. Eng. 2017, 46, 2437–2444. [Google Scholar] [CrossRef]
- Won, S.; Seo, B.; Park, J.M.; Kim, H.K.; Song, K.H.; Min, S.-H.; Ha, T.K.; Park, K. Corrosion behaviors of friction welded dissimilar aluminum alloys. Mater. Charact. 2018, 144, 652–660. [Google Scholar] [CrossRef]
- Li, S.; Dong, H.; Shi, L.; Li, P.; Ye, F. Corrosion behavior and mechanical properties of Al-Zn-Mg aluminum alloy weld. Corros. Sci. 2017, 123, 243–255. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Localized corrosion of the Mg alloys with inhibitor-containing coatings: SVET and SIET studies. Corros. Sci. 2016, 102, 269–278. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Inhibitor-containing composite coatings on Mg alloys: Corrosion mechanism and self-healing protection. Solid State Phenom. 2016, 245, 89–96. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Features of the corrosion processes development at the magnesium alloys surface. Surf. Coat. Technol. 2013, 225, 112–118. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Features of the magnesium alloys corrosion in the chloride-containing media. Solid State Phenom. 2014, 213, 143–148. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Microscale morphology and properties of the PEO-coating surface. Phys. Procedia 2012, 23, 98–101. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N.; Grace, R. Inhibition of magnesium localised corrosion in chloride containing electrolyte. Electrochim. Acta 2010, 55, 7824–7833. [Google Scholar] [CrossRef]
- Simões, A.M.; Bastos, A.C.; Ferreira, M.G.; González-García, Y.; González, S.; Souto, R.M. Use of SVET and SECM to study the galvanic corrosion of an iron–zinc cell. Corros. Sci. 2007, 49, 726–739. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Williams, G.; Birbilis, N. Observations of the galvanostatic dissolution of pure magnesium. Corros. Sci. 2012, 65, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Pampliega, A.; Lamaka, S.V.; Taryba, M.G.; Madani, M.; De Strycker, J.; Tourwé, E.; Ferreira, M.G.S.; Terryn, H. Cut-edge corrosion study on painted aluminum rich metallic coated steel by scanning vibrating electrode and micro-potentiometric techniques. Electrochim. Acta 2012, 61, 107–117. [Google Scholar] [CrossRef]
- Raps, D.; Hack, T.; Wehr, J.; Zheludkevich, M.L.; Bastos, A.C.; Ferreira, M.G.S.; Nuyken, O. Electrochemical study of inhibitor-containing organic–inorganic hybrid coatings on AA2024. Corros. Sci. 2009, 51, 1012–1021. [Google Scholar] [CrossRef]
- Karavai, O.V.; Bastos, A.C.; Zheludkevich, M.L.; Taryba, M.G.; Lamaka, S.V.; Ferreira, M.G.S. Localized electrochemical study of corrosion inhibition in microdefects on coated AZ31 magnesium alloy. Electrochim. Acta 2010, 55, 5401–5406. [Google Scholar] [CrossRef]
- Yan, M.; Gelling, V.J.; Hinderliter, B.R.; Battocchi, D.; Tallman, D.E.; Bierwagen, G.P. SVET method for characterizing anti-corrosion performance of metal-rich coatings. Corros. Sci. 2010, 52, 2636–2642. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Knörnschild, G.; Snihirova, D.V.; Taryba, M.G.; Zheludkevich, M.L.; Ferreira, M.G.S. Complex anticorrosion coating for ZK30 magnesium alloy. Electrochim. Acta 2009, 55, 131–141. [Google Scholar] [CrossRef]
- Liu, W.; Cao, F.; Xia, Y.; Chang, L.; Zhang, J. Localized Corrosion of Magnesium Alloys in NaCl Solutions Explored by Scanning Electrochemical Microscopy in Feedback Mode. Electrochim. Acta 2014, 132, 377–388. [Google Scholar] [CrossRef]
- Falcón, J.M.; Otubo, L.M.; Aoki, I.V. Highly ordered mesoporous silica loaded with dodecylamine for smart anticorrosion coatings. Surf. Coat. Technol. 2016, 303, 319–329. [Google Scholar] [CrossRef]
- Montemor, M.F.; Snihirova, D.V.; Taryba, M.G.; Lamaka, S.V.; Kartsonakis, I.A.; Balaskas, A.C.; Kordas, G.C.; Tedim, J.; Kuznetsova, A.; Zheludkevich, M.L.; et al. Evaluation of self-healing ability in protective coatings modified with combinations of layered double hydroxides and cerium molibdate nanocontainers filled with corrosion inhibitors. Electrochim. Acta 2012, 60, 31–40. [Google Scholar] [CrossRef]
- Nazarov, V.A.; Taryba, M.G.; Zdrachek, E.A.; Andronchyk, K.A.; Egorov, V.V.; Lamaka, S.V. Sodium- and chloride-selective microelectrodes optimized for corrosion studies. J. Electroanal. Chem. 2013, 706, 13–24. [Google Scholar] [CrossRef]
- Dauphin-Ducharme, P.; Mauzeroll, J. Surface Analytical Methods Applied to Magnesium Corrosion. Anal. Chem. 2015, 87, 7499–7509. [Google Scholar] [CrossRef] [PubMed]
- Snihirova, D.; Taryba, M.; Lamaka, S.V.; Montemor, M.F. Corrosion inhibition synergies on a model Al-Cu-Mg sample studied by localized scanning electrochemical techniques. Corros. Sci. 2016, 112, 408–417. [Google Scholar] [CrossRef]
- Mohedano, M.; Serdechnova, M.; Starykevich, M.; Karpushenkov, S.; Bouali, A.C.; Ferreira, M.G.S.; Zheludkevich, M.L. Active protective PEO coatings on AA2024: Role of voltage on in-situ LDH growth. Mater. Des. 2017, 120, 36–46. [Google Scholar] [CrossRef]
- Taryba, M.; Lamaka, S.V.; Snihirova, D.; Ferreira, M.G.S.; Montemor, M.F.; Wijting, W.K.; Toews, S.; Grundmeier, G. The combined use of scanning vibrating electrode technique and micro-potentiometry to assess the self-repair processes in defects on “smart” coatings applied to galvanized steel. Electrochim. Acta 2011, 56, 4475–4488. [Google Scholar] [CrossRef]
- Taryba, M.G.; Lamaka, S.V. Plasticizer-free solid-contact pH-selective microelectrode for visualization of local corrosion. J. Electroanal. Chem. 2014, 725, 32–38. [Google Scholar] [CrossRef]
- Skupov, A.A.; Panteleev, M.D.; Ioda, E.N. Microstructure and mechanical properties of V-1579 and V-1481 laser welds. Proc. VIAM 2017, 7. [Google Scholar] [CrossRef]
- Ryabov, D.K.; Vakhromov, R.O.; Ivanova, A.O. Effect of small addition of alloying elements with high solubility in aluminium on microstructure of ingots and cold-rolled sheets of Al-Mg-Sc alloy. Proc. VIAM 2015, 5. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Electrochemical Behaviour of the 1579 Al Alloy with Welded Joint: Corrosion Features and Coating Protection. Available online: https://www.ariel.ac.il/sites/conf/mmt/mmt-2018/service files/papers/chapter2/2-1-8.pdf (accessed on 8 August 2018).
- Gnedenkov, S.V.; Khrisanfova, O.A.; Sinebryukhov, S.L.; Puz, A.V.; Gnedenkov, A.S. Composite Protective Coatings on Nitinol Surface. Mater. Manuf. Process. 2008, 23, 879–883. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Nadaraia, K.V.; Gnedenkov, A.S.; Bouznik, V.M. Composite fluoropolymer coatings on the MA8 magnesium alloy surface. Corros. Sci. 2016, 111, 175–185. [Google Scholar] [CrossRef]
- Egorkin, V.S.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Vyaliy, I.E.; Gnedenkov, A.S.; Chizhikov, R.G. Increasing thickness and protective properties of PEO-coatings on aluminum alloy. Surf. Coatings Technol. 2018, 334, 29–42. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Puz, A.V.; Gnedenkov, A.S.; Vyaliy, I.E.; Mashtalyar, D.V.; Egorkin, V.S. Plasma electrolytic oxidation coatings on titanium formed with microsecond current pulses. Solid State Phenom. 2014, 213, 149–153. [Google Scholar] [CrossRef]
- ISO 15614-2:2005 Specification and Qualification of Welding Procedures for Metallic Materials—Welding Procedure Test—Part 2: Arc Welding of Aluminium and Its Alloys; BSI: London, UK, 2005; pp. 1–29.
- Dolgikh, O.; Demeter, A.; Lamaka, S.V.; Taryba, M.; Bastos, A.C.; Quevedo, M.C.; Deconinck, J. Simulation of the role of vibration on Scanning Vibrating Electrode Technique measurements close to a disc in plane. Electrochim. Acta 2016, 203, 379–387. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Taryba, M.; Salak, A.N.; Kallip, S.; Zheludkevich, M.L.; Ferreira, M.G.S.; Montemor, M.F. Hydroxyapatite Microparticles as Feedback-Active Reservoirs of Corrosion Inhibitors. ACS Appl. Mater. Interfaces 2010, 2, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Lamaka, S.V.; Taryba, M.; Montemor, M.F.; Isaacs, H.S.; Ferreira, M.G.S. Quasi-simultaneous measurements of ionic currents by vibrating probe and pH distribution by ion-selective microelectrode. Electrochem. Commun. 2011, 13, 20–23. [Google Scholar] [CrossRef]
- McMurray, H.N.; Williams, D.; Worsley, D.A. Artifacts Induced by Large-Amplitude Probe Vibrations in Localized Corrosion Measured by SVET. J. Electrochem. Soc. 2003, 150, B567. [Google Scholar] [CrossRef]
- Worsley, D.A.; McMurray, H.N.; Sullivan, J.H.; Williams, I.P. Quantitative Assessment of Localized Corrosion Occurring on Galvanized Steel Samples Using the Scanning Vibrating Electrode Technique. Corrosion 2004, 60, 437–447. [Google Scholar] [CrossRef]
- Souto, R.M.; González-García, Y.; Bastos, A.C.; Simões, A.M. Investigating corrosion processes in the micrometric range: A SVET study of the galvanic corrosion of zinc coupled with iron. Corros. Sci. 2007, 49, 4568–4580. [Google Scholar] [CrossRef]
- Shi, H.; Tian, Z.; Hu, T.; Liu, F.; Han, E.-H.; Taryba, M.; Lamaka, S.V. Simulating corrosion of Al2CuMg phase by measuring ionic currents, chloride concentration and pH. Corros. Sci. 2014, 88, 178–186. [Google Scholar] [CrossRef]
- Nardeli, J.V.; Snihirova, D.V.; Fugivara, C.S.; Montemor, M.F.; Pinto, E.R.P.; Messaddecq, Y.; Benedetti, A.V. Localised corrosion assessement of crambe-oil-based polyurethane coatings applied on the ASTM 1200 aluminum alloy. Corros. Sci. 2016, 111, 422–435. [Google Scholar] [CrossRef]
- Guseva, O.; Schmutz, P.; Suter, T.; von Trzebiatowski, O. Modelling of anodic dissolution of pure aluminium in sodium chloride. Electrochim. Acta 2009, 54, 4514–4524. [Google Scholar] [CrossRef]
- Yan, S.; Chen, H.; Ma, C.; Nie, Y.; Wang, X.; Qin, Q.H. Local corrosion behaviour of hybrid laser-MIG welded Al–Zn–Mg alloy joints. Mater. Des. 2015, 88, 1353–1365. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Vyaliy, I.E.; Egorkin, V.S.; Gnedenkov, S.V. Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 1: Specificity of Development. Materials 2018, 11, 2053. https://doi.org/10.3390/ma11102053
Gnedenkov AS, Sinebryukhov SL, Mashtalyar DV, Vyaliy IE, Egorkin VS, Gnedenkov SV. Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 1: Specificity of Development. Materials. 2018; 11(10):2053. https://doi.org/10.3390/ma11102053
Chicago/Turabian StyleGnedenkov, Andrey S., Sergey L. Sinebryukhov, Dmitry V. Mashtalyar, Igor E. Vyaliy, Vladimir S. Egorkin, and Sergey V. Gnedenkov. 2018. "Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 1: Specificity of Development" Materials 11, no. 10: 2053. https://doi.org/10.3390/ma11102053




