The Structural Evolution of Al86Ni9La5 Glassy Ribbons during Milling at Room and Cryogenic Temperatures
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Crystallization and Crush in Milling
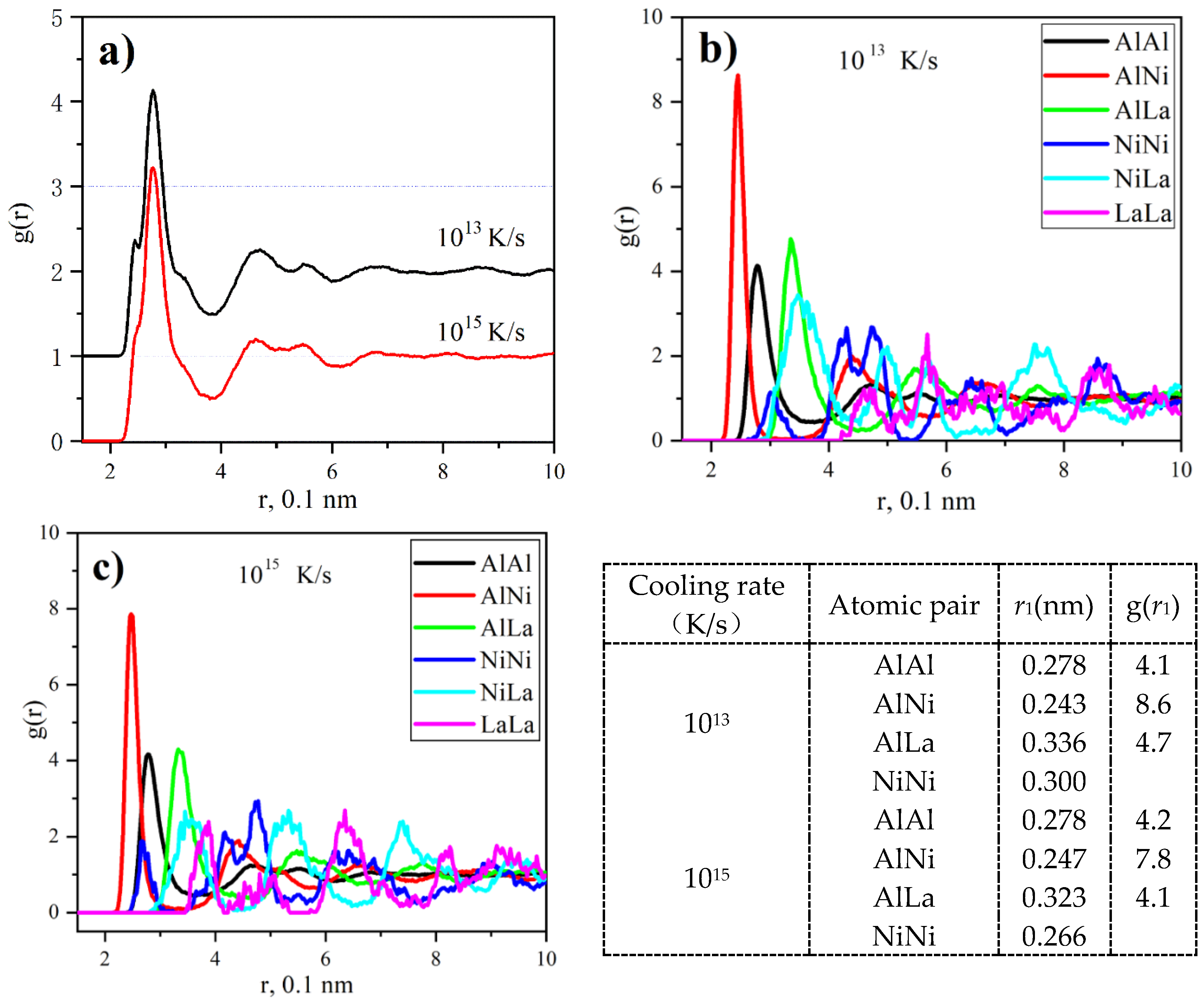
3.2. Atomic Structural Simulation
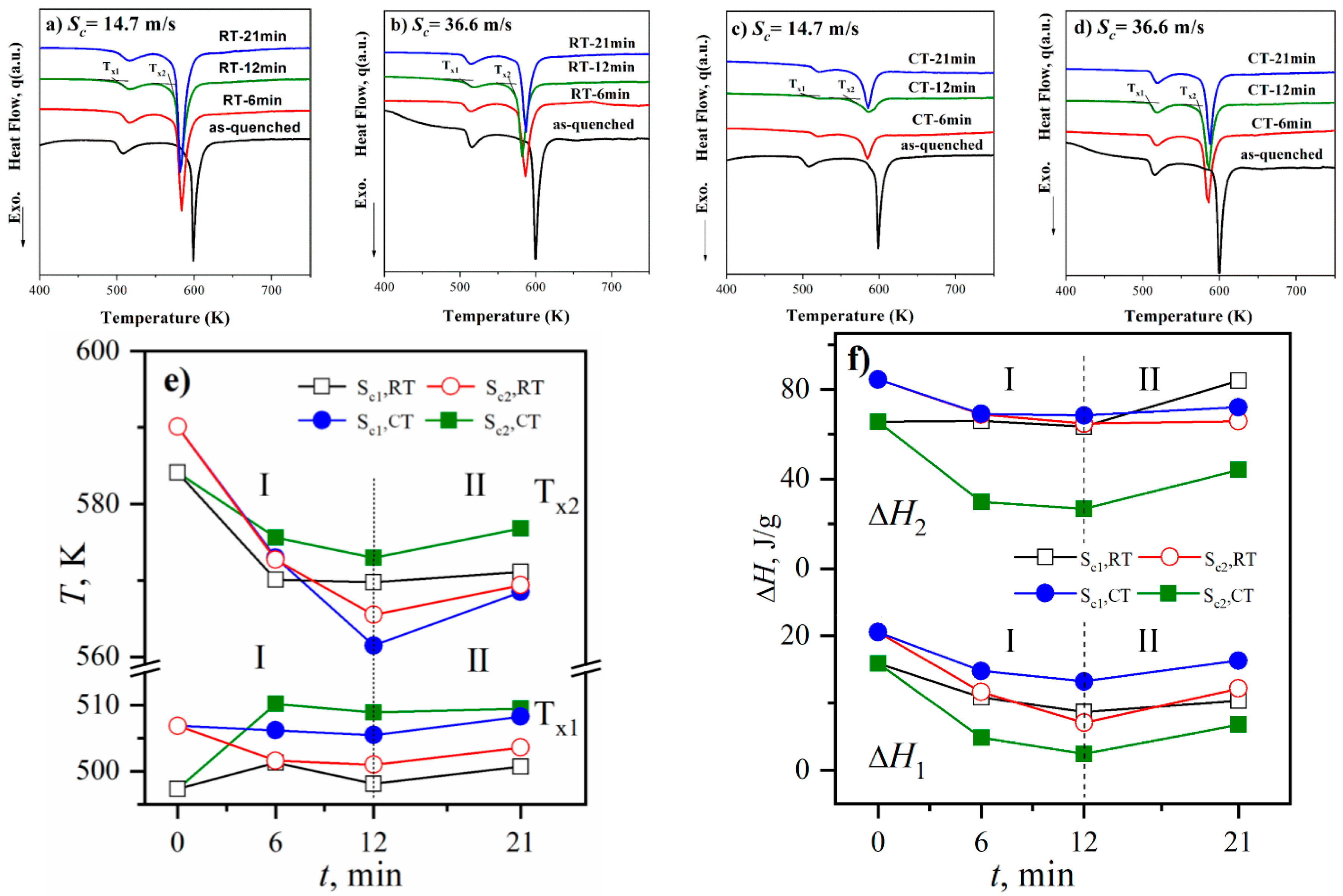
3.3. DSC Analysis and Structural Rejuvenation in Milling
4. Conclusions
- Crystallization occurs in the Al86Ni9La5 metallic glass with Sc = 14.7 m/s during milling at cryogenic temperature, which is weakened at room temperature. As a contrast, no crystallization has been checked in the milled or cryomilled samples with Sc = 33.6 m/s. The Al86Ni9La5 metallic glass with Sc = 14.7 m/s bears more shear bands than that with Sc = 36.6 m/s during milling and the crystallization in former is closely related with the shear bands.
- Besides icosahedral Al-centered clusters, the regular and distorted tricapped trigonal prism (TTP) Ni-centered clusters are dominant in Al86Ni9La5 glass. With decreasing the cooling rate, the frequency of Al-centered cluster keeps almost unchanged, while the frequency of Ni-centered TTP cluster increases drastically. The higher frequency of regular Ni-centered TTP clusters makes them easier to link into MROs and affects the initiation and propagation of shear bands as well as the possible crystallization.
- The changing trend of the onset temperatures (Tx1, Tx2) and crystallization enthalpies (ΔH1, ΔH2) reflects the existence of structural rejuvenation during milling. At the beginning of the milling, the samples are crushed into pieces and the defects in the glass are consequently relaxed; after further milling, the size of samples is small enough compared with the milling ball and the stress state of the samples shifts from heterogeneous to homogeneous, which makes the structure more disordered and results in the structural rejuvenation.
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, Y.; Fu, Z.Q.; Li, D.; Wu, M. Effects of ball milling processes on the microstructure and rheological properties of microcrystalline cellulose as a sustainable polymer additive. Materials 2018, 11, 1057. [Google Scholar] [CrossRef] [PubMed]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Bucella, D.P. Effect of hot mill scale on hydrogen embrittlement of high strength steels for pre-stressed concrete structures. Metals 2018, 8, 158. [Google Scholar] [CrossRef]
- Tomolya, K.; Sycheva, A.; Sveda, M.; Arki, P.; Miko, T.; Roosz, A.; Janovszky, D. Synthesis and characterization of copper-based composites reinforced by CuZrAlNiTi amorphous particles with enhanced mechanical properties. Metals 2017, 7, 92. [Google Scholar] [CrossRef]
- Trudeau, M.L.; Schulz, R.; Dussault, D.; Van Neste, A. Structural changes during high-energy ball milling of iron-based amorphous alloys: Is high-energy ball milling equivalent to a thermal process? Phys. Rev. Lett. 1990, 64, 99–102. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Shiflet, G.J.; Poon, S.J. Ball milling-induced nanocrystal formation in aluminum-based metallic glasses. Acta Metall. Mater. 1995, 43, 83–91. [Google Scholar] [CrossRef]
- Fan, G.J.; Quan, M.X.; Hu, Z.Q. Mechanically induced structural relaxation in an amorphous metallic Fe80B20 alloy. Appl. Phys. Lett. 1996, 68, 319–321. [Google Scholar] [CrossRef]
- Fan, G.J.; Quan, M.X.; Hu, Z.Q. Deformation-enhanced thermal stability of an amorphous Fe80B20 alloy. J. Appl. Phys. 1996, 80, 6055–6057. [Google Scholar] [CrossRef]
- Li, H.; Fan, C.; Tao, K.X.; Choo, H.; Liaw, P.K. Compressive Behavior of a Zr-Based Metallic Glass at Cryogenic Temperatures. Adv. Mater. 2006, 18, 752–754. [Google Scholar] [CrossRef]
- Kawashima, A.; Zeng, Y.Q.; Fukuhara, M.; Kurishita, H.; Nishiyama, N.; Miki, H.; Inoue, A. Mechanical properties of a Ni60Pd20P17B3 bulk glassy alloy at cryogenic temperatures. Mater. Sci. Eng. A 2008, 498, 475–481. [Google Scholar] [CrossRef]
- Pampillo, C.A.; Chen, H.S. Comprehensive plastic deformation of a bulk metallic glass. Mater. Sci. Eng. 1974, 13, 181–188. [Google Scholar] [CrossRef]
- Chen, H.; He, Y.; Shiflet, G.J.; Poon, S.J. Deformation-induced nanocrystal formation in shear bands of amorphous alloys. Nature 1994, 367, 541–543. [Google Scholar] [CrossRef]
- Janovszky, D.; Kristaly, F.; Miko, T.; Racz, A.; Sveda, M.; Sycheva, A.; Koziel, T. Phase Transformation and Morphology Evolution of Ti50Cu25Ni20Sn5 during Mechanical Milling. Materials 2018, 11, 1769. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, T.C.; Fan, C.; Ott, R.T.; Li, J.; Brennan, S. Controlling shear band behavior in metallic glasses through microstructural design. Intermetallics 2002, 10, 1163–1166. [Google Scholar] [CrossRef]
- Dmowski, W.; Yokoyama, Y.; Chuang, A.; Ren, Y.; Umemoto, M.; Tsuchiya, K.; Inoue, A.; Egami, T. Structural rejuvenation in a bulk metallic glass induced by severe plastic deformation. Acta Mater. 2010, 58, 429–438. [Google Scholar] [CrossRef]
- Toozandehjani, M.; Matori, K.; Ostovan, F.; Aziz, S.A.; Mamat, M. Effect of Milling Time on the Microstructure, Physical and Mechanical Properties of Al-Al2O3 Nanocomposite Synthesized by Ball Milling and Powder Metallurgy. Materials 2017, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.X.; Geng, H.R.; Yang, Z.X. Amorphization and crystallization of Zr66.7−xCu33.3Nbx (x = 0, 2, 4) alloys during mechanical alloying. J. Alloys Comp. 2009, 474, 152–157. [Google Scholar] [CrossRef]
- Zhang, J.T.; Wang, W.M.; Ma, H.J.; Li, G.H.; Li, R.; Zhang, Z.H. Isochronal and isothermal crystallization kinetics of amorphous Fe-based alloys. Thermochim. Acta 2010, 505, 41–46. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Sweitzer, J.E.; Shiflet, G.J.; Scully, J.R. Localized corrosion of Al90Fe5Gd5 and Al87Ni8.7Y4.3 alloys in the amorphous, nanocrystalline and crystalline states: Resistance to micrometer-scale pit formation. Electrochim. Acta 2003, 48, 1223–1234. [Google Scholar] [CrossRef]
- Li, W.; Cong, H.R.; Zhang, J.X.; Bian, X.F.; Li, H.; Qin, J.Y. Medium-range order structure in Al80Fe20 alloy during rapid solidification. Phys. Lett. A 2002, 301, 477–483. [Google Scholar] [CrossRef]
- Liu, R.; Wang, W.M.; Ma, H.J.; Li, G.H.; Qin, J.Y.; Zhang, Z.H.; Tang, X.W. Correlation between pre-peak in structure factor and physical properties in Al-based amorphous alloys. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2011, 21, 80–87. [Google Scholar] [CrossRef]
- Wang, C.; Cao, Q.P.; Wang, X.D.; Zhang, D.X.; Ramamurty, U.; Narayan, R.L.; Jiang, J.Z. Intermediate Temperature Brittleness in Metallic Glasses. Adv. Mater. 2017, 29, 1605537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, X.K.; Zhao, D.Q.; Pan, M.X.; Wang, W.H.; Wu, Y.; Lewandowski, J.J. Fracture of brittle metallic glasses: Brittleness or plasticity. Phys. Rev. Lett. 2005, 94, 125510. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Shan, G.B.; Gao, K.W.; Qiao, L.J.; Chu, W.Y. In situ SEM study of formation and growth of shear bands and microcracks in bulk metallic glasses. Mater. Sci. Eng. A 2003, 354, 337–343. [Google Scholar] [CrossRef]
- Ott, R.T.; Sansoz, F.; Molinari, J.F.; Almer, J.; Ramesh, K.T.; Hufnagel, T.C. Micromechanics of deformation of metallic-glass-matrix composites from in situ synchrotron strain measurements and finite element modeling. Acta Mater. 2005, 53, 1883–1893. [Google Scholar] [CrossRef]
- Pan, S.P.; Qin, J.Y.; Wang, W.M.; Gu, T.K. Origin of splitting of the second peak in the pair-distribution function for metallic glasses. Phys. Rev. B 2011, 84, 92201. [Google Scholar] [CrossRef]
- Liu, X.J.; Xu, Y.; Hui, X.; Lu, Z.P.; Li, F.; Chen, G.L.; Lu, J.; Liu, C.T. Metallic liquids and glasses: Atomic order and global packing. Phys. Rev. Lett. 2010, 105, 155501. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.P.; Gheribi, A.E.; Chartrand, P. On the determination of the glass forming ability of AlxZr1-x alloys using molecular dynamics, Monte Carlo simulations, and classical thermodynamics. J. Appl. Phys. 2012, 112, 073508. [Google Scholar] [CrossRef]
- Egami, T. The atomic structure of aluminum based metallic glasses and universal criterion for glass formation. J. Non-Cryst. Solids 1996, 205, 575–582. [Google Scholar] [CrossRef]
- Poon, S.J.; Shiflet, G.J.; Guo, F.Q.; Ponnambalam, V. Glass formability of ferrous- and aluminum-based structural metallic alloys. J. Non-Cryst. Solids 2003, 317, 1–9. [Google Scholar] [CrossRef]
- Inoue, A.; Takeuchi, A. Recent progress in bulk glassy, nanoquasicrystalline and nanocrystalline alloys. Mater. Sci. Eng. A 2004, 375–377, 16–30. [Google Scholar] [CrossRef]
- Guan, P.F.; Fujita, T.; Hirata, A.; Liu, Y.H.; Chen, M.W. Structural origins of the excellent glass forming ability of Pd40Ni40P20. Phys. Rev. Lett. 2012, 108, 175501. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, S.L.; An, B.; Wang, Y.G.; Li, Y.J.; Zhang, L.C.; Wang, W.M. Effects of mechanical compression and autoclave treatment on the backbone clusters in the Al86Ni9La5 amorphous alloy. J. Alloys Comp. 2014, 587, 59–65. [Google Scholar] [CrossRef]
- Kano, J.; Saito, F. Correlation of powder characteristics of talc during Planetary Ball Milling with the impact energy of the balls simulated by the Particle Element Method. Powder Technol. 1998, 98, 166–170. [Google Scholar] [CrossRef]
- Kim, Y.H.; Inoue, A.; Masumoto, T. Ultrahigh Mechanical Strengths of Al88Y2Ni10−xMx (M = Mn, Fe or Co) Amorphous Alloys Containing Nanoscale fcc-Al Particles. Mater. Trans. JIM 1991, 32, 599–608. [Google Scholar] [CrossRef]
- Egami, T.; Maeda, K.; Vitek, V. Structural defects in amorphous solids A computer simulation study. Philos. Mag. A 1980, 41, 883–901. [Google Scholar] [CrossRef]
- Heggen, M.; Spaepen, F.; Feuerbacher, M. Creation and annihilation of free volume during homogeneous flow of a metallic glass. J. Appl. Phys. 2005, 97, 033506. [Google Scholar] [CrossRef] [Green Version]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Liu, Y.; Pan, S.; Hu, Y.; Pang, J.; Wang, W. The Structural Evolution of Al86Ni9La5 Glassy Ribbons during Milling at Room and Cryogenic Temperatures. Materials 2018, 11, 1956. https://doi.org/10.3390/ma11101956
Yan Z, Liu Y, Pan S, Hu Y, Pang J, Wang W. The Structural Evolution of Al86Ni9La5 Glassy Ribbons during Milling at Room and Cryogenic Temperatures. Materials. 2018; 11(10):1956. https://doi.org/10.3390/ma11101956
Chicago/Turabian StyleYan, Zhicheng, Yan Liu, Shaopeng Pan, Yihua Hu, Jing Pang, and Weimin Wang. 2018. "The Structural Evolution of Al86Ni9La5 Glassy Ribbons during Milling at Room and Cryogenic Temperatures" Materials 11, no. 10: 1956. https://doi.org/10.3390/ma11101956
APA StyleYan, Z., Liu, Y., Pan, S., Hu, Y., Pang, J., & Wang, W. (2018). The Structural Evolution of Al86Ni9La5 Glassy Ribbons during Milling at Room and Cryogenic Temperatures. Materials, 11(10), 1956. https://doi.org/10.3390/ma11101956





