1. Introduction
In recent years, there has been a shift from amputation to treatment with endoprosthetics, which is driven by the desire to improve surgical techniques. Thus, the number of patients with surgically implanted artificial devices in bone and joint tissues is currently increasing [
1,
2]. These implants allow people to lead more normal lives without feeling restricted in movement. In the medical field, ceramics are used to construct prostheses or their parts to manufacture medical instruments, as membranes for the separation and purification of biological fluids, and to produce porous elements for dosed drug administration [
3,
4,
5]. As biologically inert and electrolytically passive materials, ceramics do not conduct electric current. Ceramics also show good integration ability with bone tissue, making them advantageous in comparison with metal implants [
6,
7,
8,
9,
10,
11].
In addition to the above-mentioned advantages, ceramics have some significant disadvantages when used as implant materials [
3]. For example, the passivity of ceramics with respect to living tissue means that the bone may not grow into the implant, and the contact site will be filled with a fibrous tissue that covers the foreign body. The high strength of ceramic materials results in considerable rigidity. This can lead to the local redistribution of load on the bone at the contact area between the implant and bone, often resulting in the destruction of the bone. In addition, as a result of the brittle nature of ceramics, the ceramic itself can be susceptible to fractures under certain conditions. Despite these disadvantages, ceramic implants have no alternatives in some areas of traumatology and orthopaedics, including the endoprostheses of small joints (finger, foot and wrist joints), ankle bone, intervertebral discs, femoral head, knee joint and teeth [
12,
13,
14,
15]. Ceramic steel made from alumina (Al
2O
3) or zirconia (ZrO
2) is the most widely used ceramic implant material [
16,
17,
18,
19,
20].
Biomedical-grade zirconia is usually stabilised with 3 mol% yttria (Y
2O
3) because yttria decreases the driving force for the tetragonal-to-monoclinic transformation. Zirconia ceramics stabilised with yttrium oxide provide several advantages over other ceramics, including excellent mechanical and tribological properties along with biocompatibility. Therefore, yttrium-oxide-stabilised zirconia ceramics are widely applied as ball heads in hip-joint implants. Furthermore, zirconia ceramics are aesthetically more appealing as dental implants compared to grey titanium implants [
21,
22,
23,
24].
The implant-bone system only functions properly when the bone tissue is fully integrated with the implant. In order to improve this integration between the bone tissue and the implant, various bioactive coatings are commonly deposited on implant surfaces [
25,
26]. Oxidising treatment [
27,
28], electrolytic deposition [
29], plasma spraying [
30] and vacuum-ion plasma deposition [
31,
32] are effective methods for producing bioinert and bioactive coatings on metal implant surfaces. Recent research efforts have focused on reducing the thickness of implant coatings to the nano level [
33,
34,
35,
36,
37]. Numerous studies have shown that the micro- and nanotopography of the implant surface strongly affects the biological properties of the implant (e.g., cell attachment, spreading and proliferation) [
38,
39,
40]. Thus, greatly reducing the film thickness can significantly change the chemistry of the implant surface without affecting its morphology [
37]. The use of ultrathin coatings on dental implant materials prevents the implant from loosening, which is caused by the gap between the implant and the healthy bone in the case of rapid dissolution of the thick coating and its incomplete replacement by bone tissue [
41]. In addition, thin coatings adhere much more strongly to the substrate than thicker coatings, especially in the case of screw-shaped implants.
Few studies have focused on the modification of ceramic implants. The promising methods to produce the thick ceramic coatings on ceramic substrates are electrophoretic deposition and airbrush spraying [
42,
43]. The available studies used various techniques for thin-film deposition. Because the deposition method significantly affects the physical, chemical and, consequently, medical and biological properties of the implant, it is difficult to determine the optimal coating for a ceramic implant and assess its clinical effectiveness. We are unaware of any studies that comparatively analysed coatings with different compositions deposited on ceramic substrates using the same deposition method.
Calcium phosphate materials, such as hydroxyapatite, tricalcium phosphate (α,β), dicalcium phosphate dihydrate and octacalcium phosphate, possess great potential as materials for bioactive coatings [
26,
44,
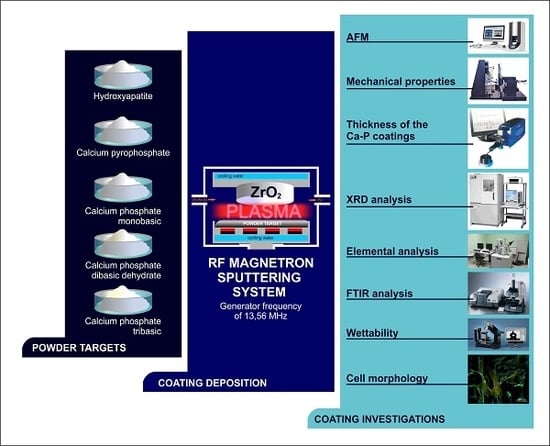
45]. This paper provides a comparative study of coatings deposited by the radiofrequency (RF) magnetron sputtering of different Ca-P powders on zirconia substrates. Unlike other methods, RF magnetron sputtering allows the deposition of bioactive coatings on the surfaces of metal implants along with ceramic and polymer substrates [
46,
47,
48]. RF-sputtered coatings provide strong adhesion to various substrates, and they also provide high elasticity, spatial uniformity and the ability to coat implants with complex shapes. Generally, coatings formed using plasma-chemical deposition methods are amorphous and can be irreversibly transformed into the crystalline state [
49]. This property can be used to control the biomedical properties of the coatings.
3. Results and Discussion
Based on the AFM data, the zirconia substrate was characterised by relatively equiaxed grains with an average size of 130 nm and an Sq of 30 nm (
Figure 1a). Coating the zirconia substrate with Ca-P powders using RF magnetron sputtering led to changes in its morphology. The surface of the CPT coating was wavy as a result of alternating hillocks and valleys (
Figure 1b). Higher magnification revealed a plate-like structure composed of thin plates with lengths of 80 nm and widths of 30 nm. Sq of the CPT coating was 7.00 nm. The surface of the HA powder coating consisted of spherical globules with a mean size of ~20 nm and an Sq of 9.10 nm (
Figure 1c). The calcium phosphate monobasic, DCPD and CPP coatings had more homogeneous structures with average grain sizes of 13.50, 10.60 and 7.60 nm, respectively (
Figure 1d–f) and similar Sq values of 3.60, 3.80 and 3.60 nm, respectively.
It is necessary to consider the differences in the mechanical properties between the coating and the substrate as significant differences in mechanical properties can cause stress at the coating–substrate interface, resulting in delamination [
52,
53]. The mechanical properties of the Ca-P coatings in this study are presented in
Table 2.
According to the international standard ISO 14577, the nanoindentation method allows the measurement of mechanical characteristics in three size ranges: nano (h
max ≤ 200 nm), micro (h
max > 200 nm and F
max < 2 N) and macro (2 N ≤ F
max ≤ 30 kN) [
54]. In this study, in order to exclude the influence of the substrate when measuring the mechanical properties of the coatings, the load on the indenter did not exceed 0.5 mN, and the maximum indenter penetration depth was 90 nm.
Based on the nanoindentation data, the nanohardness of the CPT coating was comparable to that of the zirconia substrate. In contrast, the calcium phosphate monobasic coating exhibited the lowest nanohardness and elastic modulus among all samples, and its nanohardness was significantly different from that of the substrate. The nanohardness and elastic modulus values of the HA, DCPD and CPP coatings were practically the same.
The good elastic properties of the zirconia substrate coated with CPT powder were confirmed by its higher elastic recovery value (R) compared to the other samples. The value of R depends on the modulus of elasticity of the material. In noncrystalline materials, the elastic component arises from the elastic displacement of the points of the structural grid under external load; its value is proportional to the mechanical compliance of the material.
XRD analysis indicated that all the deposited Ca-P coatings were amorphous. The elemental compositions of the coatings are presented in
Table 3. Here, we primarily consider the elements Ca and P, which are important for cell activity [
53] and contribute to the formation of bone tissue [
55]. The largest content of Ca was observed in the HA coating, followed by the DCPD coating. The trend in P content in the Ca-P coatings was similar to the trend observed for Ca content. The largest P contents were observed in the HA and DCPD coatings. The presence of Y, Zr and O elements was attributed to the substrate composition, whereas the presence of C was associated with the deposition process. All samples are characterized by practically the same Ca/P content ratio that varies in the range 1.84–2.33. Such composition is usual for RF-sputtered Ca-P coatings on metallic substrates [
56]. Highly likely, a variation of Ca/P ratio had no significant effect on diverse cell adhesion described in
Table 4.
The FTIR spectra of all the investigated coatings exhibited weak absorption bands at 560 and 600 cm
−1 along with an absorption band between 1000 and 1200 cm
−1 (
Figure 2). The intensities of the latter bands were greatest in the spectrum of the CPT coating. The bands mentioned above correspond to the valence vibrations of the PO
43− group [
57,
58]. No other characteristic absorption bands were observed in the FTIR spectra of the Ca-P coatings in this study.
The deposition of Ca-P coatings on the zirconia substrates led to changes in the surface free energy (
Table 5). The HA coating resulted in the largest surface free energy, whereas CPP produced the smallest value (
Table 2).
The surface free energy data are in good agreement with the roughness data; that is, the greatest roughness value corresponds to the largest surface free energy. Micro-relief and surface roughness are known to be closely related to surface free surface [
59] and, consequently, to surface wettability [
60,
61]. Compared to a smooth surface, surface roughness increases the contact area between the liquid and the solid. In turn, this results in a proportional increase in the specific surface free energy of the rough surface.
Accordingly, Fan and Lee [
59] observed a linear increase in the surface free energy of WN
x films with increasing Sq. Barshilia et al. [
62] demonstrated that substrate roughness affected the superhydrophobic behaviour of polytetrafluoroethylene thin films. The authors showed that the large water contact angle and low hysteresis of the contact angle corresponded to low surface free energy values. Moreover, they established an optimum Sq value at which the polytetrafluoroethylene films exhibited superhydrophobic properties. These results are in good agreement with the data obtained for the Ca-P coatings in this study.
The wettability results indicated that the DCPD, CPT and CPP coatings had hydrophobic properties, whereas the HA and calcium phosphate monobasic coatings exhibited strong and weak hydrophilic properties, respectively. For these coatings, the polar component of the free surface energy is maximal, which indicates a high wettability of these coatings by polar liquids.
Surface free energy and surface wettability have important effects on the interaction between the implant surface and the biological environment [
62,
63,
64]. Numerous studies have shown that high surface free energy and good wettability (hydrophilicity) facilitate cell adhesion, whereas hydrophobic surfaces with low surface free energies are unfavorable for cell attachment and spreading [
65,
66,
67].
However, greater part of results was received on metal [
68], glass and plastic substrata [
64]. Some studies have found that a contact angle of 60–70° is optimal for cell adherence and direct proliferation [
65,
66]; at this time, other studies have failed to confirm this conclusion [
63,
65,
67,
68]. Therefore, the wettability and surface energy (
SE) are recognized as the fundamental physicochemical properties of a material surface, which strongly but contradictorily influences the attachment of cells [
69,
70,
71,
72].
The present article focuses on the widely exploited biomaterial Ca-P coatings prepared by magnetron sputtering a set of methods, such as sol-gel, plasma spraying, microarc oxidation, detonation gas spraying methods, etc. A wide range of cells (fibroblasts, osteoblasts, osteoclasts, nerve cells, and embryonic cells, among others) respond to artificial surfaces as described, in particular in [
73]. However, the connection between the multiple extracellular physicochemical events that control and trigger MSCs is still unclear.
One of the main distinguishing features of MSC cultures is their ability to adhere to and spread on material surfaces; thus, the area occupied by a single cell is an important characteristic of its functional activity. Furthermore, a larger number of cells and their surface area corresponds to a greater functional activity [
74,
75] and biocompatibility of artificial materials tested.
Table 4 presents the number of adhered MSCs, the overall cell area and the area of a single cell for the Ca-P coatings in this study. Only the sample coated with CPT showed a significantly higher cell adhesion rate, as confirmed by a higher number of cells and higher overall area covered by cells. The samples coated with other calcium phosphates demonstrated similar levels of cell adhesion,
Table 4). In all samples, including the zirconia substrate, the cells were well spread uniformly over the surface, and the cell density did not differ significantly between different areas on the surface. The fluorescence microscopy indicated that the adhered MSCs formed continuous cell layers on the substrates (
Figure 3). Besides the diffuse staining of the cytoplasm, the numerous focal adhesions between the cells and with the material were performed after vinculin staining. Actin was observed in the trabecular meshwork in almost all cells (
Figure 3). Vinculin was predominantly found in the cytoplasm, although focal adhesion was not obvious.
It is should be noted that significantly higher adhesion of the adipose-derived MSCs was observed on the hydrophobic surface with the weak polar component of the free surface energy (coating from the CPT powder;
Table 4 and
Table 5). RF-magnetron coating prepared from HA target had reverse properties as compared with CPT (
Table 5), and the number of adherent MSCs was minimal (
Table 4). The combined effect of wettability and the positive charge on the HA surface has been known to enable differential in vitro binding of inorganic ions and organic cell adhesive proteins (e.g., fibronectin, vitronectin etc.) with different surface properties, which results in accelerated or decelerated cell adhesion on HA surface [
76]. Because of uniform roughness, charge and Ca/P ratio of RF-magnetron HA coatings, a single cell-mediated effect of their wettability and surface energy may be found.
The surface chemistry of materials has an interactive influence on optimal adhesion of mammalian cells, which is critical in determining the cell viability on substrate surfaces. There is the opinion that the cells prefer to adhere to hydrophilic surfaces (see above). In turn, MSCs and adipose-derived MSCs are lipophilic cells; they transform easily into fat cells [
77,
78] and adhere to the hydrophobic surface of polymers [
79]. Thus, our results showed that adipose-derived MSCs are capable of adhering in vitro to both types of RF-magnetron CP coating, but obviously prefer hydrophobic cites. The in vitro study showed no sign of the samples’ toxic effect on MSC culture (
Table 6). In this connection, diverse MSC adhesion on CPT or HA coatings did not affect their possible cytotoxicity.