Preparation of Parabolic Superhydrophobic Material for Oil-Water Separation
Abstract
:1. Introduction
2. Experiment
2.1. Reagents and Materials
2.2. Fabrication of Parabolic Superhydrophobic Material
2.3. Characterization
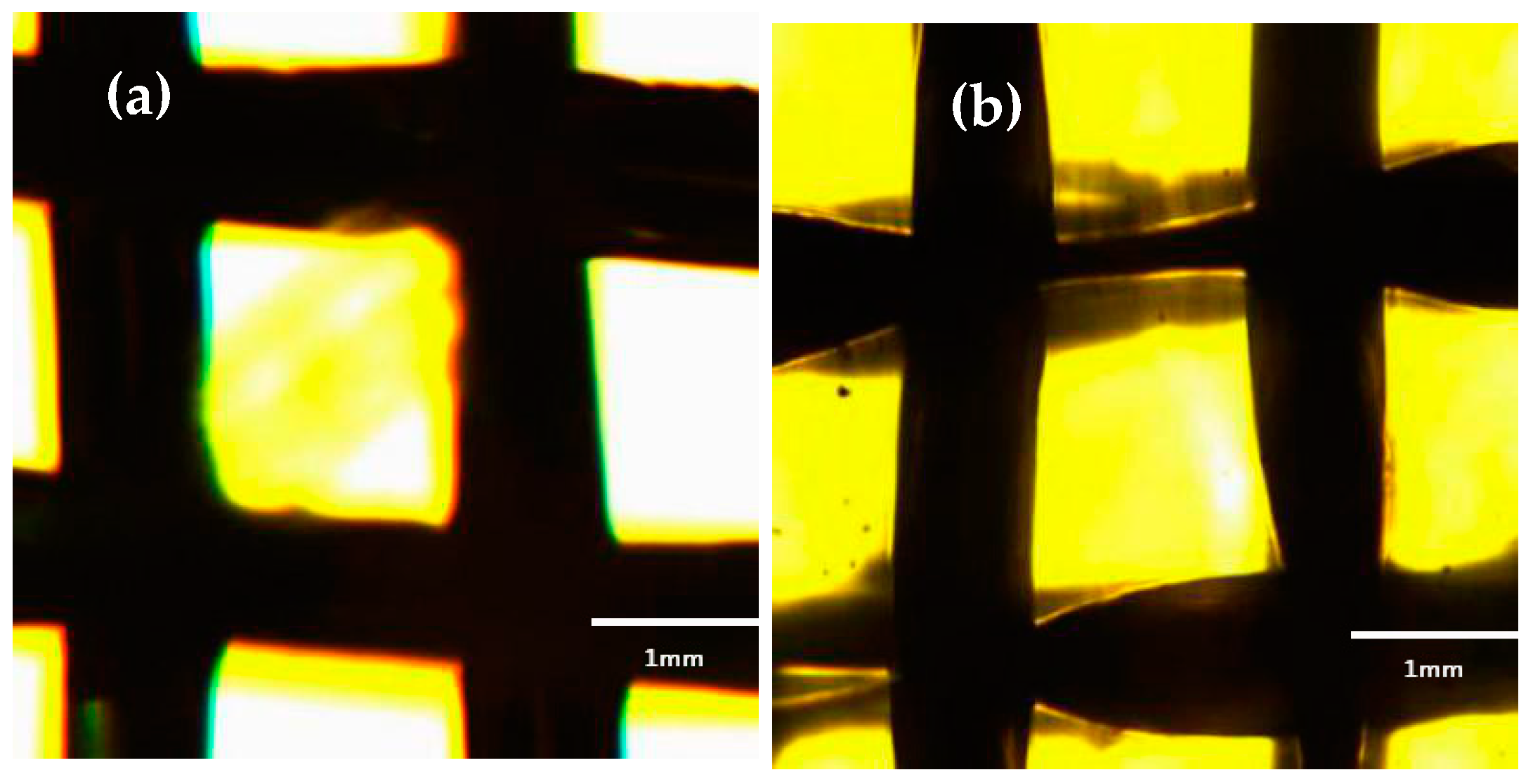
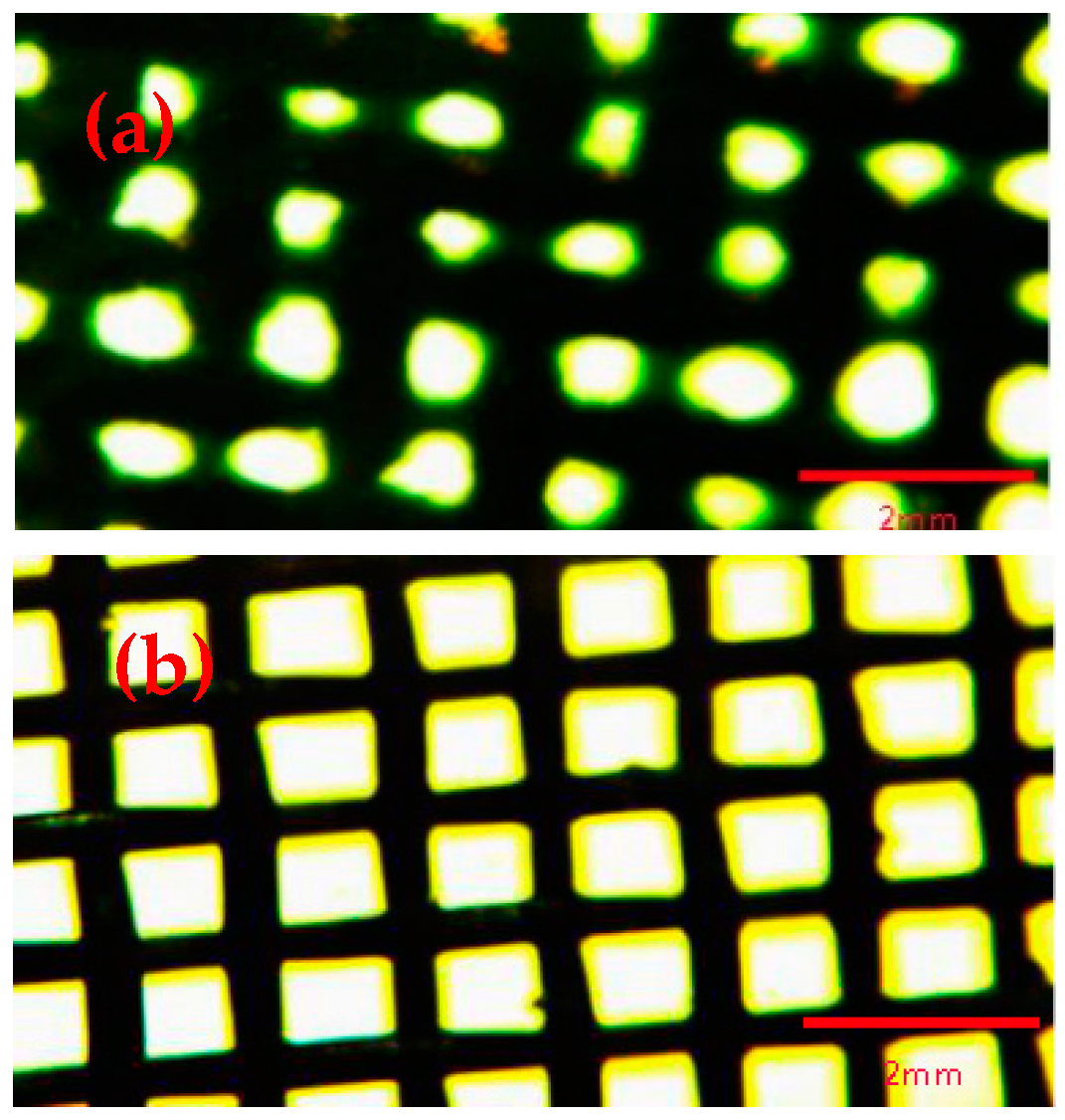
2.3.1. Fluorescence Microscope Characterization
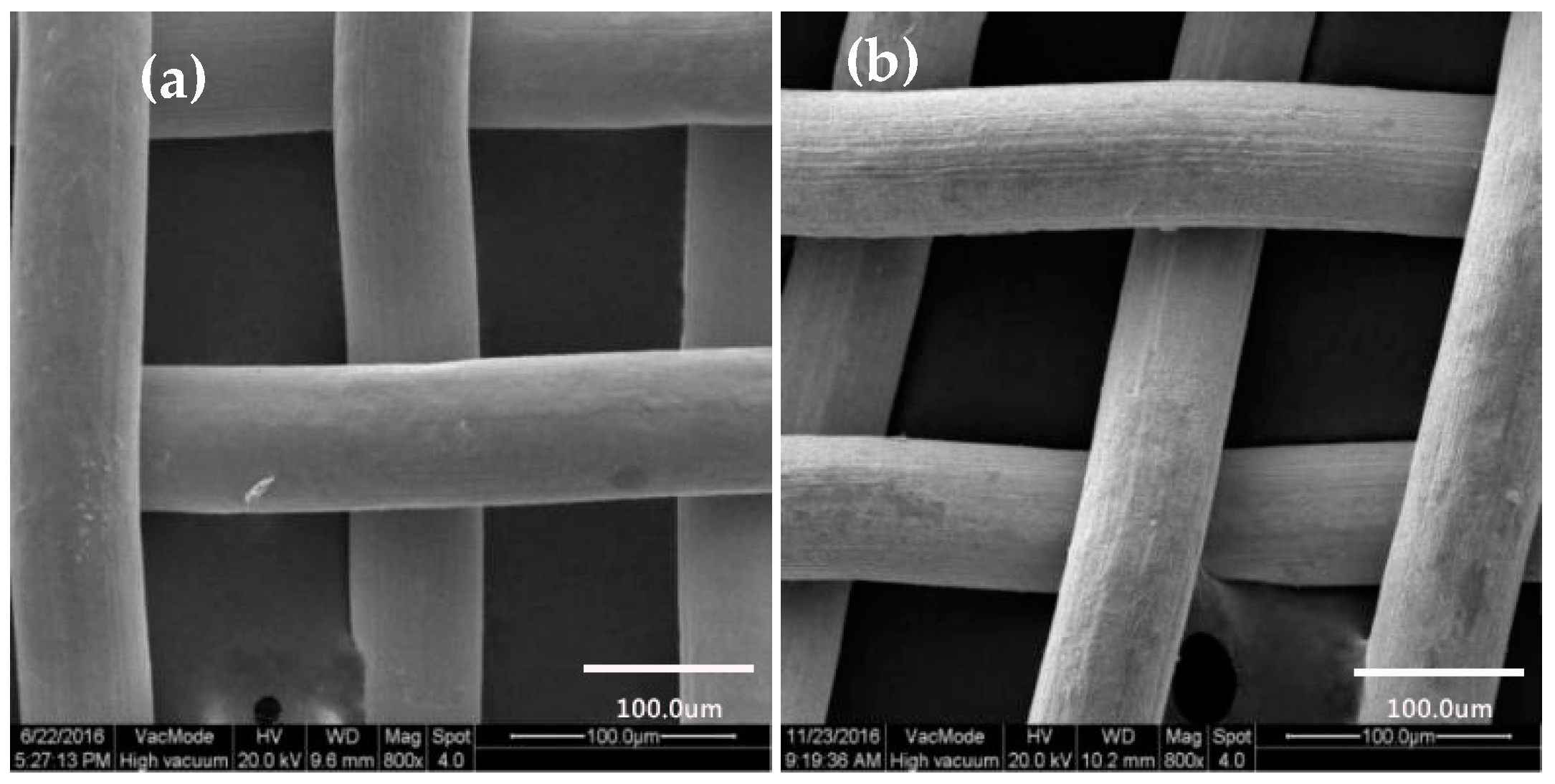
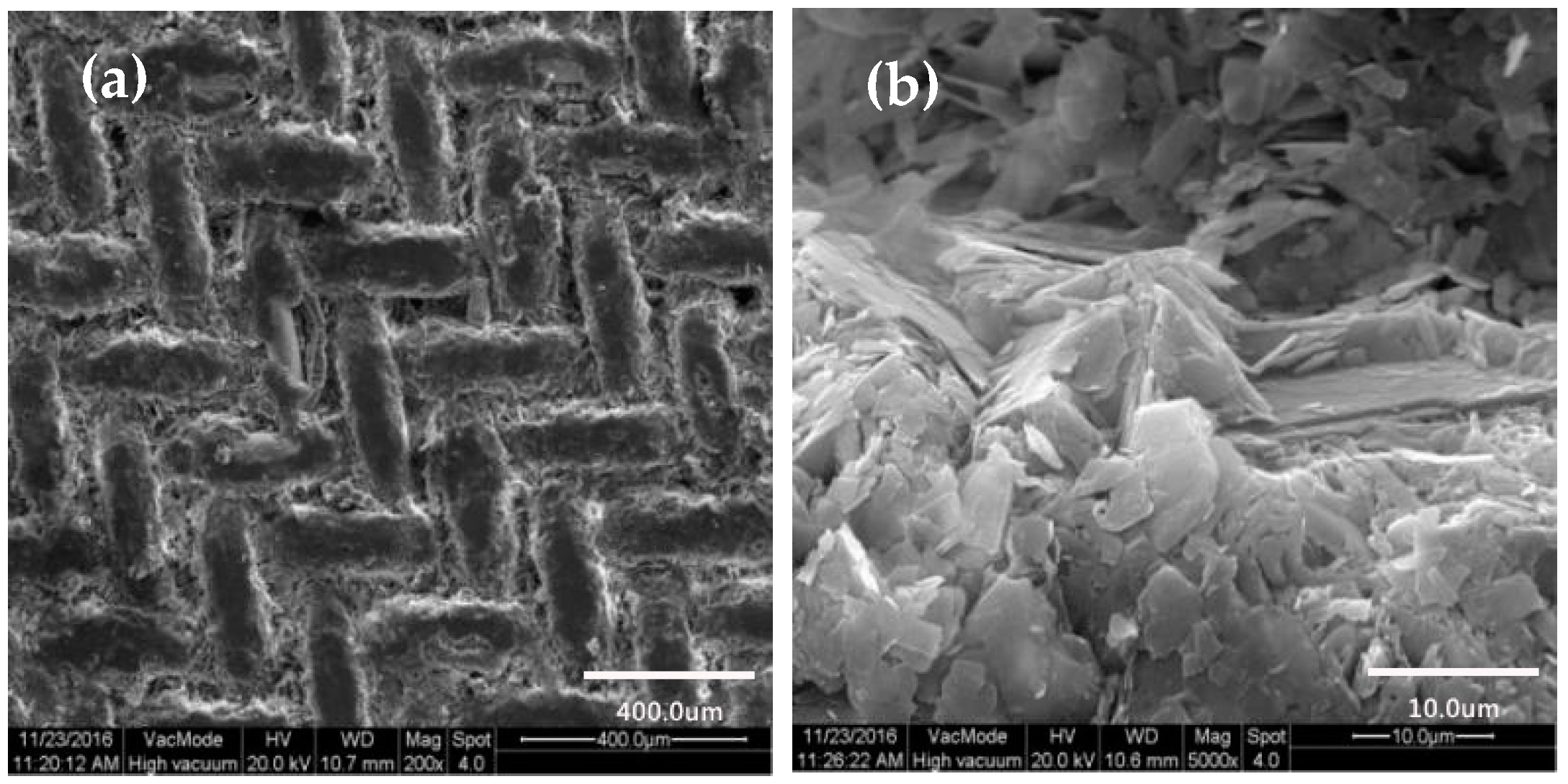
2.3.2. SEM Characterization
2.3.3. Contact Angle Measurements
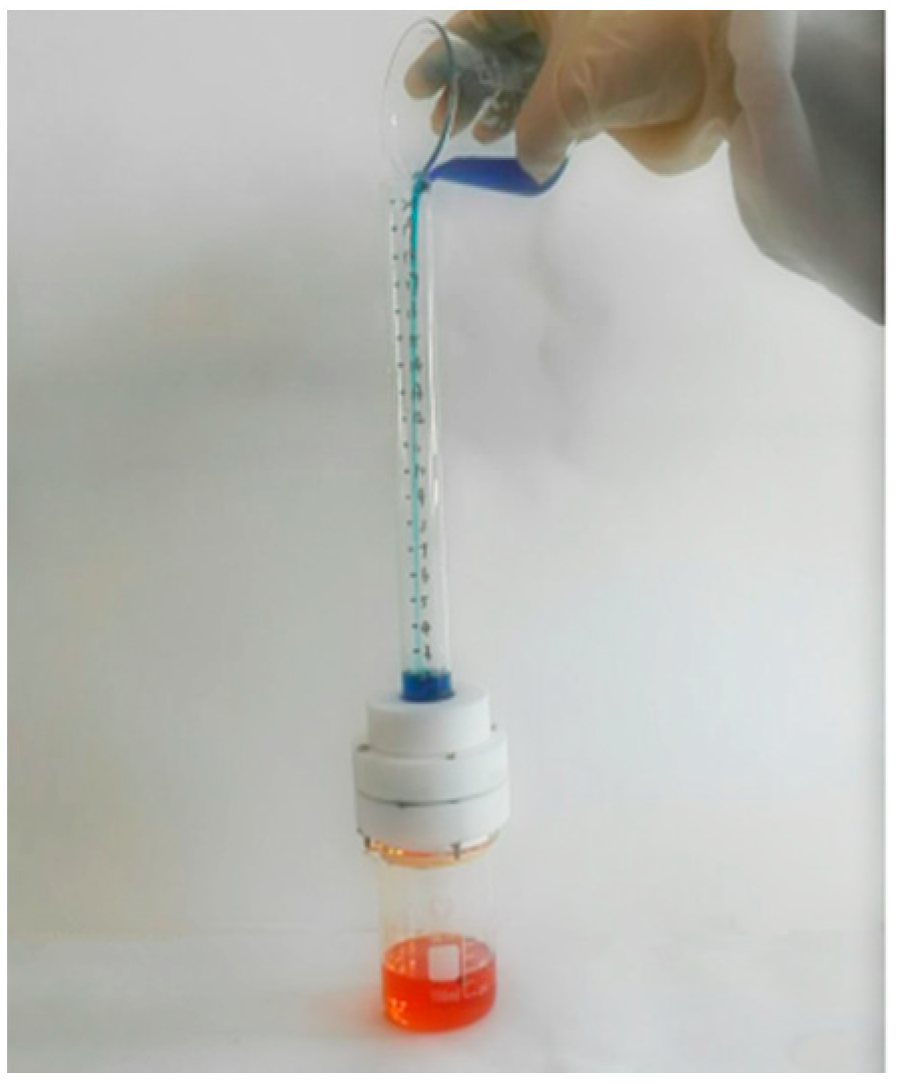
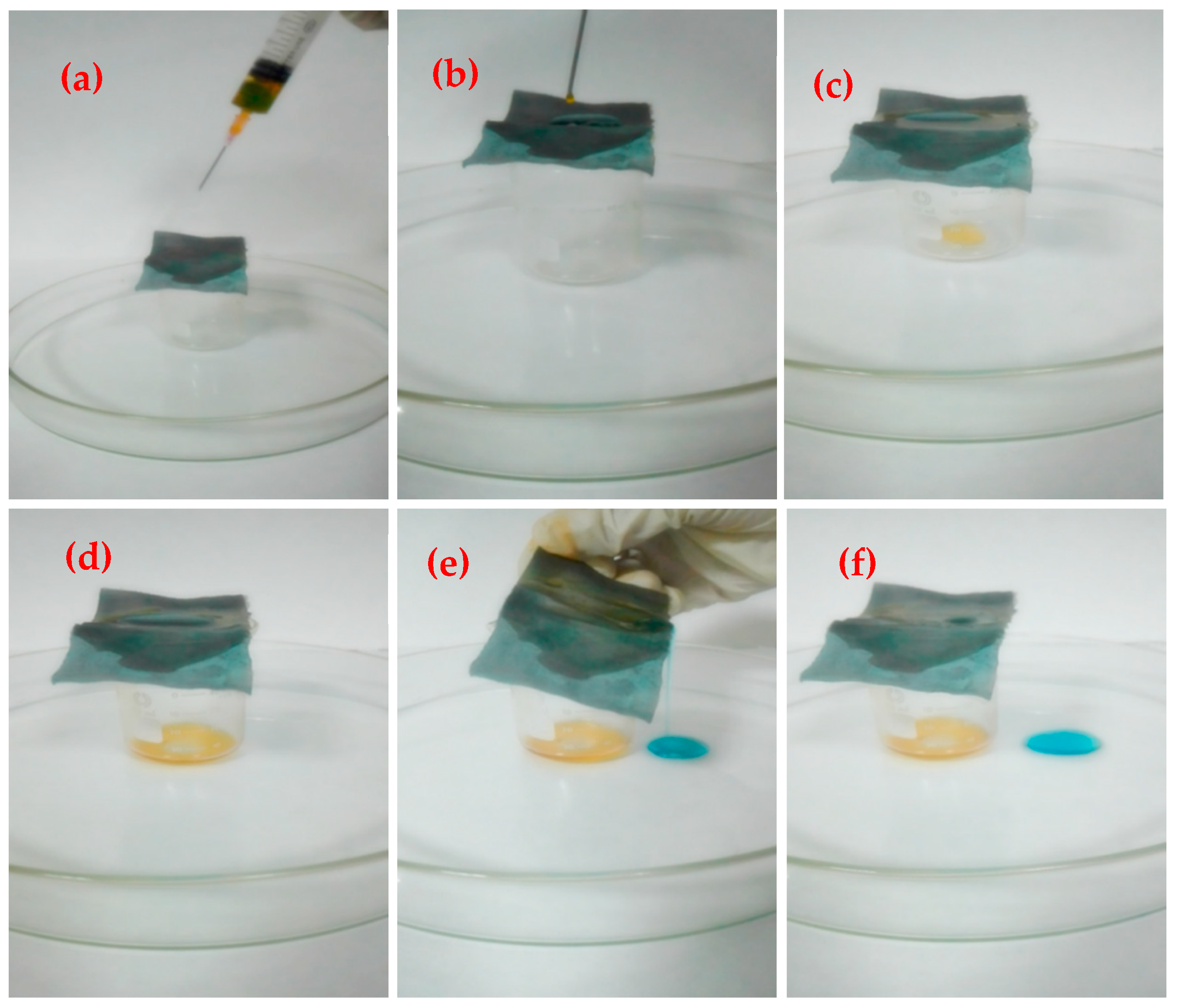
2.4. Oil-Water Separation Test
3. Results and Discussion
3.1. Fluorescence Microscope Characterization
3.2. SEM Characterization
3.2.1. Effect of Oxidation Time of Hydrogen Peroxide
3.2.2. Surface Morphology of Copper Mesh Not Modified by SA
3.2.3. Surface Morphology of Copper Mesh Modified with SA
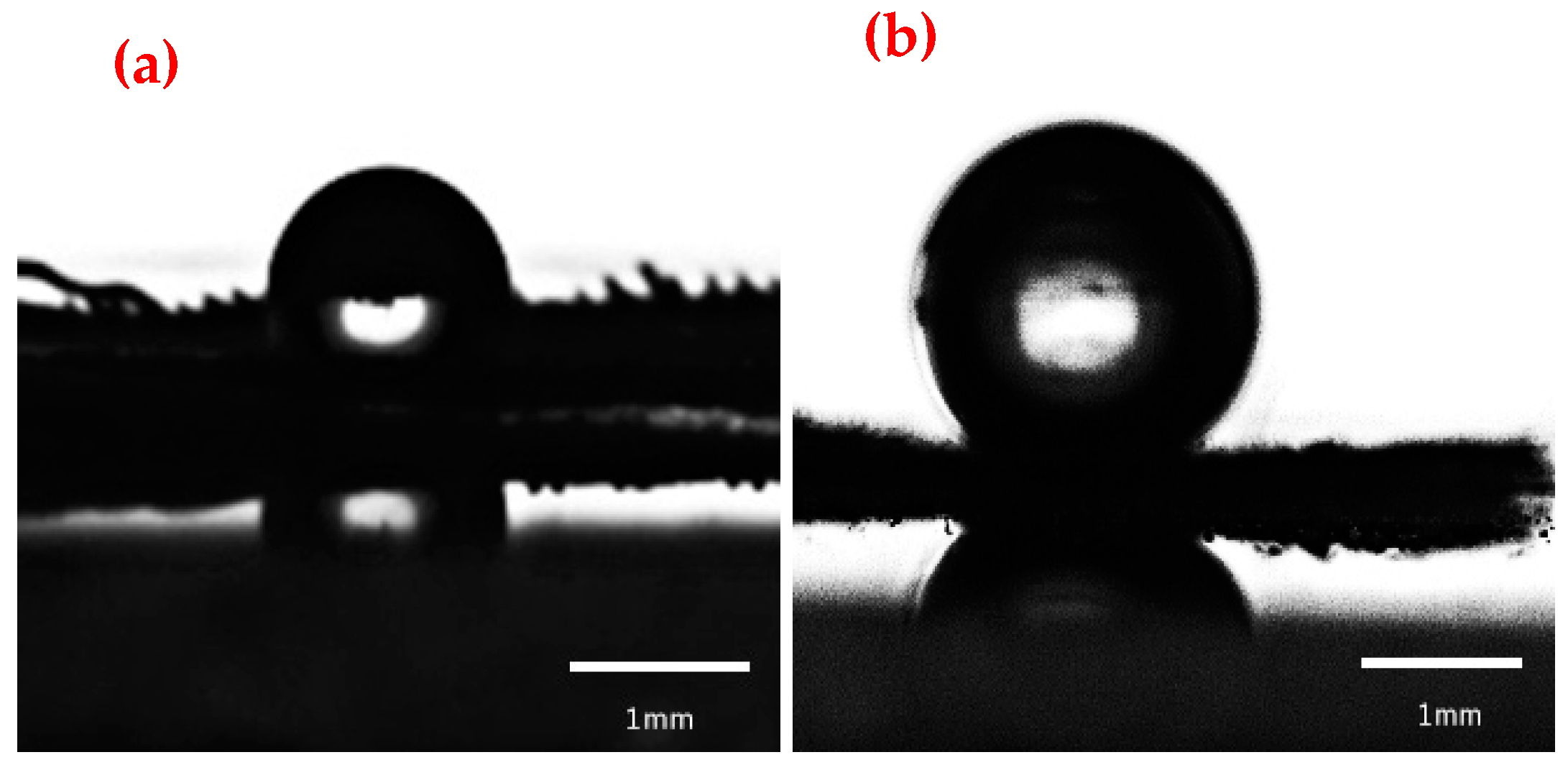
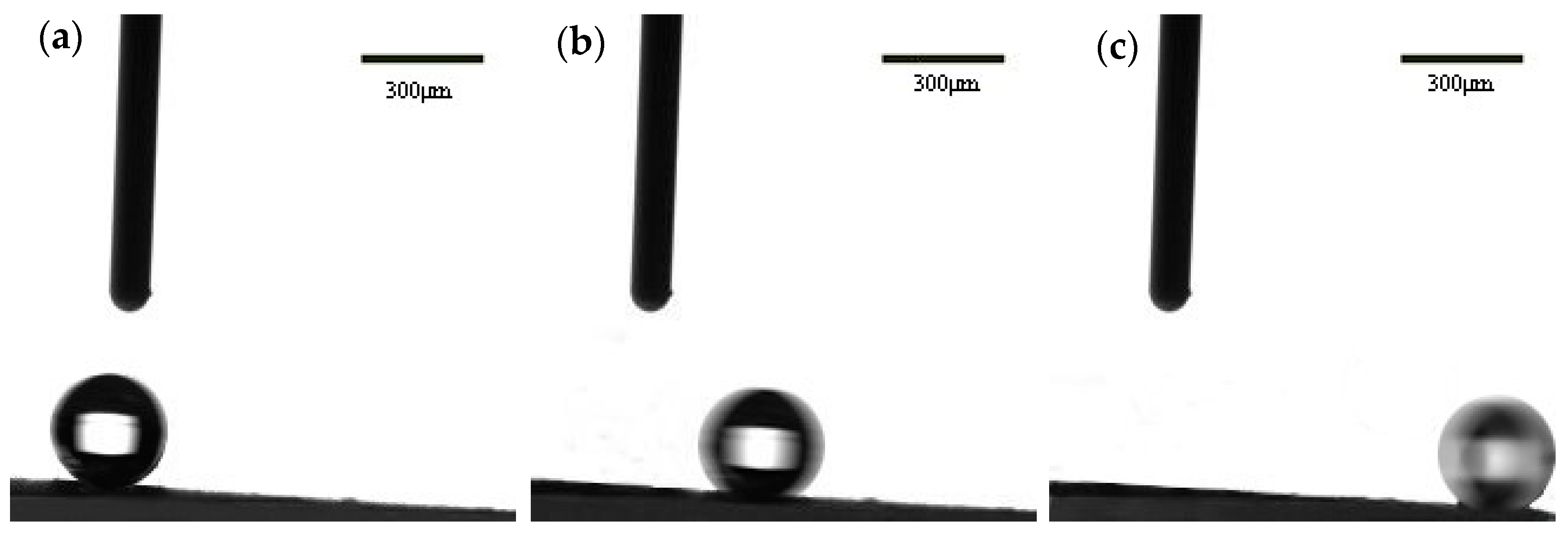
3.3. Wettability Characterization
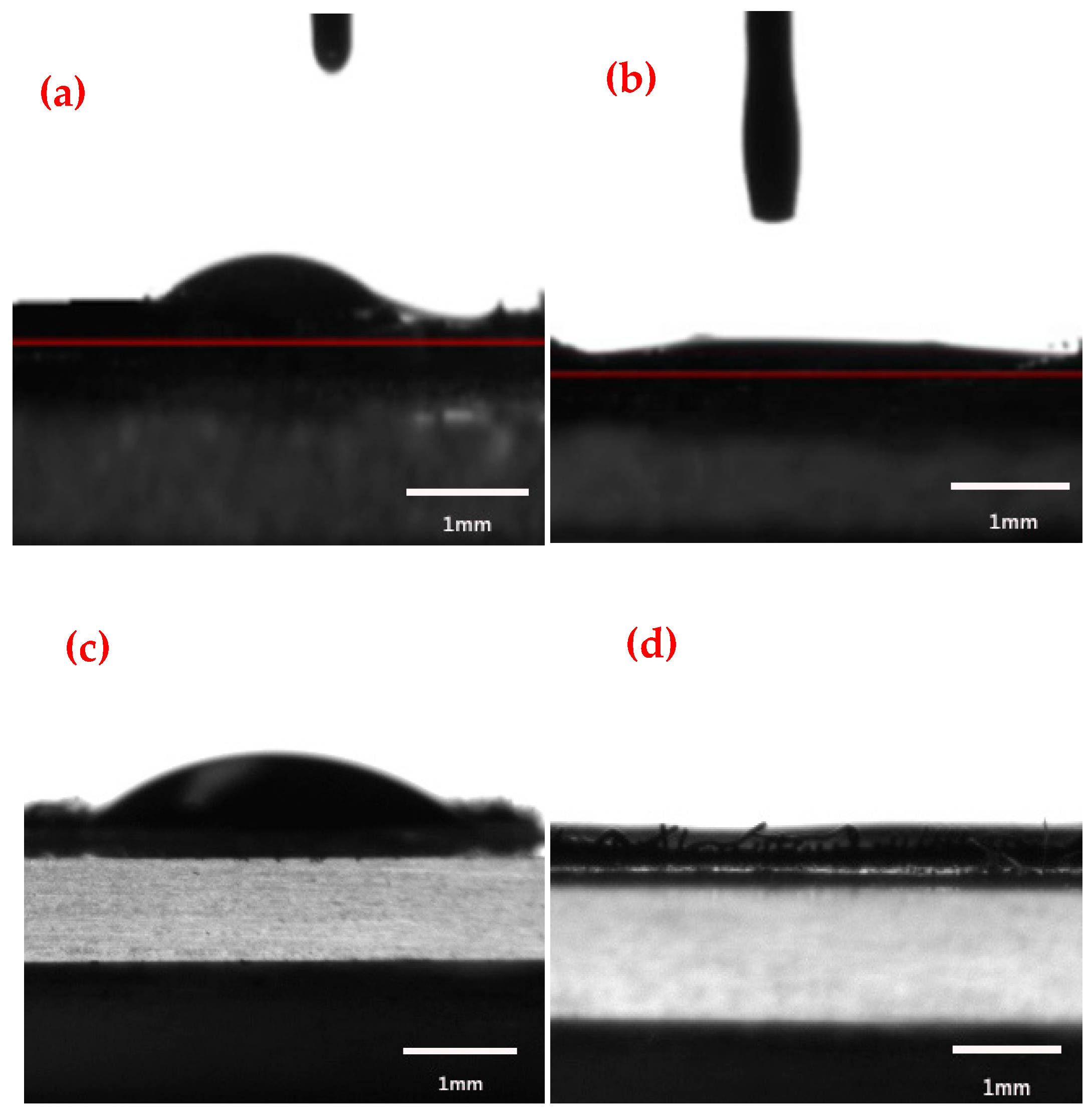
3.4. Determination of Oil/Water Separation Performance
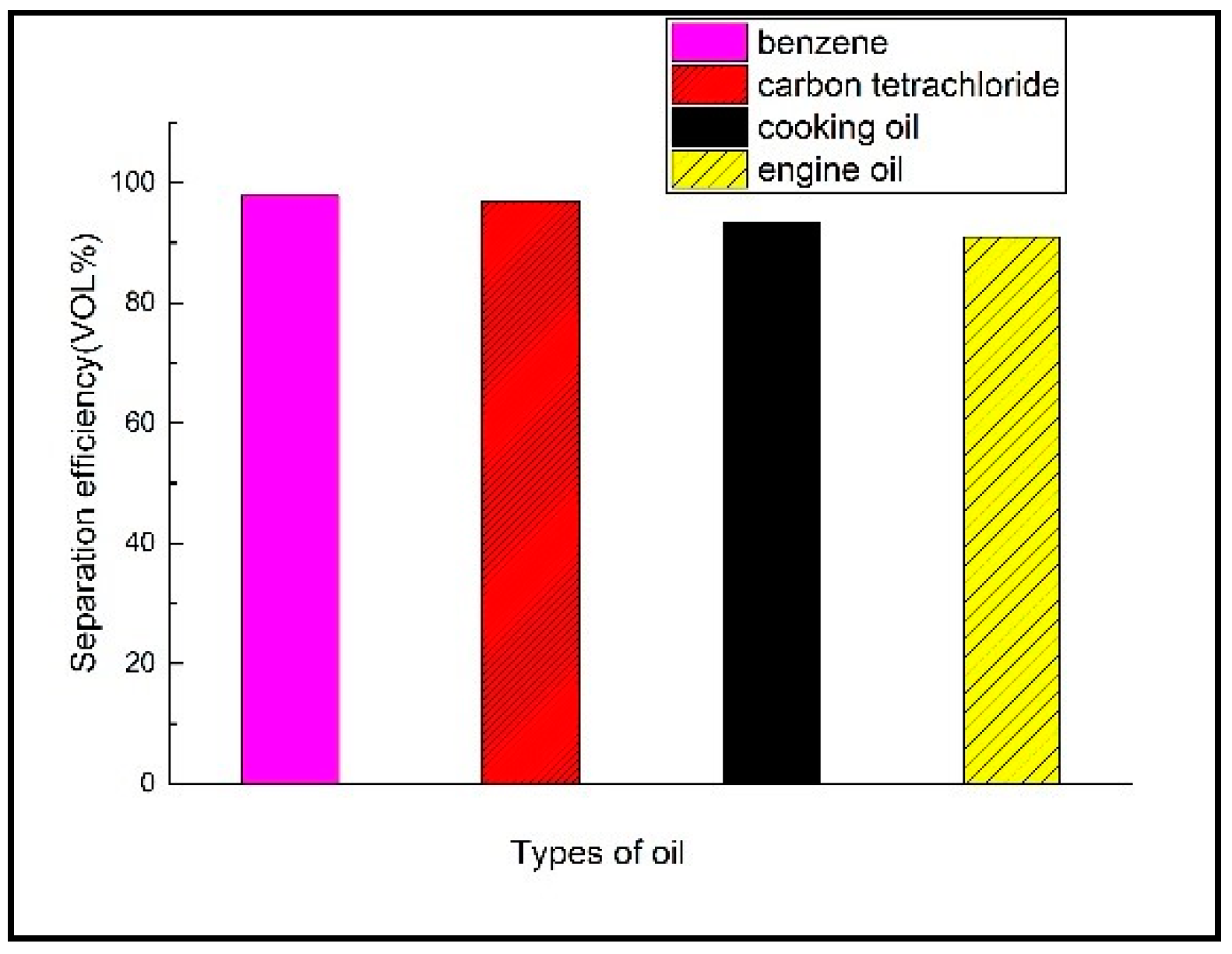
3.4.1. Separation Efficiency of Different Oil-Water Mixtures
3.4.2. Relationship between CAs and Oil-Water Separation Efficiency
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rong, J.; Zhang, T.; Qiu, F.; Xu, J.; Zhu, Y.; Yang, D.; Dai, Y. Design and preparation of efficient, stable and superhydrophobic copper foam membrane for selective oil absorption and consecutive oil–water separation. Mater. Des. 2018, 142, 83–92. [Google Scholar] [CrossRef]
- Kung, C.H.; Zahiri, B.; Sow, P.K.; Mérida, W. On-demand oil-water separation via low-voltage wettability switching of core-shell structures on copper substrates. Appl. Surf. Sci. 2018, 112, 15–27. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Geng, G. Highly efficient oil-in-water emulsion and oil layer/water mixture separation based on durably superhydrophobic sponge prepared via a facile route. Mar. Pollut. Bull. 2018, 127, 108. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Luo, X.; Ren, H.; Feng, J. Hot water-repellent and mechanically durable superhydrophobic mesh for oil/water separation. J. Colloid Interfaces Sci. 2018, 512, 567. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.T.; Lin, B.; Jiang, L.W.; Lin, E.C.; Chen, J.; Zhang, S.J.; Tang, Y.W.; He, F.A.; Li, D.H. Effective preparation of magnetic superhydrophobic Fe3O4/PU sponge for oil-water separation. Appl. Surf. Sci. 2018, 427, 56–64. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.; Pan, Y.; Zhao, X. A Facile and Effective Method to Fabricate Superhydrophobic/Superoeophilic Surface for the Separation of Both Water/Oil Mixtures and Water-in-Oil Emulsions. Polymers 2017, 9, 563. [Google Scholar] [CrossRef]
- Avrămescu, R.E.; Ghica, M.V.; Dinupîrvu, C.; Prisada, R.; Popa, L. Superhydrophobic Natural and Artificial Surfaces-A Structural Approach. Materials 2018, 11, 866. [Google Scholar] [CrossRef] [PubMed]
- Young, T. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1805, 1, 171–172. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Patankar, N.A. Transition between superhydrophobic states on rough surfaces. Langmuir ACS J. Surg. Colloid 2004, 20, 7097–7102. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ogata, S. 3-D thermodynamic analysis of superhydrophobic surfaces. J. Colloid Interfaces Sci. 2008, 326, 471. [Google Scholar] [CrossRef] [PubMed]
- Marmur, A. From hygrophilic to superhygrophobic: Theoretical conditions for making high-contact-angle surfaces from low-contact-angle materials. Langmuir ACS J. Surg. Colloid 2008, 24, 7573–7579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Effect of Superhydrophobic Surface Microstructure on Hydrophobic Properties and Its Application; Xiangtan University: Xiangtan, China, 2013. (In Chinese) [Google Scholar]
- Li, J.; Yan, L.; Li, H.; Li, J.; Zha, F.; Lei, Z.Q. A facile one-step spray-coating process for the fabrication of superhydrophobic attapulgite coated mesh used in oil/water separation. RSC Adv. 2015, 5, 53802–53808. [Google Scholar] [CrossRef]
- Rong, J.; Zhang, T.; Qiu, F.; Zhu, Y. Preparation of efficient, stable and reusable laccase-Cu3(PO4)2 hybrid microspheres based on copper foil for decolouration of Congo red. ACS Sustain. Chem. Eng. 2017, 5, 4468–4477. [Google Scholar] [CrossRef]
- Ji, K.; Zhang, J.; Jia, C.; Meng, G.; Ding, Y.; Dai, Z. Centrifugation-Assisted Fog-Collecting Abilities of Metal-Foam Structures with Different Surface Wettabilities. ACS Appl. Mater. Interfaces 2016, 8, 10005. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gu, W.; Fu, J.; Liu, Y.; Chen, S. Preparation of superhydrophobic/oleophilic copper mesh for oil-water separation. Appl. Surf. Sci. 2017, 412, 599–605. [Google Scholar] [CrossRef]
- Li, J.; Xu, C.; Tian, H.; Zha, F.; Qi, W.; Wang, Q. Blend-electrospun poly(vinylidene fluoride)/stearic acid membranes for efficient separation of water-in-oil emulsions. Colloids Surf. A 2018, 538, 494–499. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, Z.; Liang, J.; Wang, Y.; Chen, H. Preparation of superhydrophobic films on copper substrate for corrosion protection. Surf. Coat. Technol. 2014, 244, 1–8. [Google Scholar] [CrossRef]
- Khosravi, M.; Azizian, S. Preparation of superhydrophobic and superoleophilic nanostructured layer on steel mesh for oil-water separation. Sep. Purif. Technol. 2017, 172, 366–373. [Google Scholar] [CrossRef]
- Hardman, S.J.; Muhamadsarih, N.; Riggs, H.J.; Thompson, R.L.; Rigby, J.; Bergius, W.N.A.; Lian, R.H. Electrospinning Superhydrophobic Fibers Using Surface Segregating End-Functionalized Polymer Additives. Macromolecules 2011, 44, 6461–6470. [Google Scholar] [CrossRef] [Green Version]
- Ding, B.; Ogawa, T.; Kim, J.; Fujimoto, K.; Shiratori, S. Fabrication of a super-hydrophobic nanofibrous zinc oxide film surface by electrospinning. Thin Solid Films 2008, 516, 2495–2501. [Google Scholar] [CrossRef]
- Badge, I.; Sethi, S.; Dhinojwala, A. Carbon nanotube-based robust steamphobic surfaces. Langmuir ACS J. Surg. Colloids 2011, 27, 14726–14731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shi, Z.; Chen, L.; Jiang, Y.; Xu, C.; Wu, Z.; Wang, Y.; Peng, C. Porous superhydrophobic and superoleophilic surfaces prepared by template assisted chemical vapor deposition. Surf. Coat. Technol. 2017, 315, 385–390. [Google Scholar] [CrossRef]
- Li, J.; Wu, R.; Jing, Z.; Yan, L.; Zha, F.; Lei, Z. One-Step Spray-Coating Process for the Fabrication of Colorful Superhydrophobic Coatings with Excellent Corrosion Resistance. Langmuir ACS J. Surg. Colloids 2015, 31, 10702–10707. [Google Scholar] [CrossRef] [PubMed]
- Belsanti, L.; Ogihara, H.; Mahanty, S.; Luciano, G. Electrochemical behaviour of superhydrophobic coating fabricated by spraying a carbon nanotube suspension. Bull. Mater. Sci. 2015, 38, 579–582. [Google Scholar] [CrossRef]
- Liang, Y.; Peng, J.; Li, X.; Huang, J.; Qiu, R.; Zhang, Z.; Ren, L. Wettability and Contact Time on a Biomimetic Superhydrophobic Surface. Materials 2017, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jia, S.; Wang, S.; Yan, Q.; Yan, N.; Wang, Q.; Meng, T. A facile and novel emulsion for efficient and convenient fabrication of durable superhydrophobic materials. Chem. Eng. J. 2017, 328, 186–196. [Google Scholar] [CrossRef]
- Wei, C.; Dai, F.; Lin, L.; An, Z.; He, Y.; Chen, X.; Chen, L.; Zhao, Y. Simplified and robust adhesive-free superhydrophobic SiO2-decorated PVDF membranes for efficient oil/water separation. J. Membr. Sci. 2018, 555, 220–228. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Wang, H.; Wang, C.; Li, H.; Zhang, X.; Yuan, R.; Zhao, Y. Facile preparation of superhydrophobic metal foam for durable and high efficient continuous oil-water separation. Chem. Eng. J. 2017, 322, 157–166. [Google Scholar] [CrossRef]
- Cao, C.; Cheng, J. Fabrication of robust surfaces with special wettability on porous copper substrates for various oil/water separations. Chem. Eng. J. 2018, 347, 585–594. [Google Scholar] [CrossRef]
- Wu, R.; Liang, S.; Pan, A.; Yuan, Z.; Tang, Y. Fabrication of nano-structured super-hydrophobic film on aluminum by controllable immersing method. Appl. Surf. Sci. 2012, 258, 5933–5937. [Google Scholar] [CrossRef]
- Pan, L.; Dong, H.; Bi, P. Facile preparation of superhydrophobic copper surface by HNO3 etching technique with the assistance of CTAB and ultrasonication. Appl. Surf. Sci. 2010, 257, 1707–1711. [Google Scholar] [CrossRef]
- Cao, H.; Fu, J.; Liu, Y.; Chen, S.; Cao, H.; Fu, J.; Liu, Y.; Chen, S.; Cao, H.; Fu, J. Facile Design of Superhydrophobic and Superoleophilic Copper Mesh Assisted by Candle Soot for Oil Water Separation. Colloids Surf. A 2017, 537, 294–302. [Google Scholar] [CrossRef]
- Beshkar, F.; Khojasteh, H.; Salavatiniasari, M. Flower-Like CuO/ZnO Hybrid Hierarchical Nanostructures Grown on Copper Substrate: Glycothermal Synthesis, Characterization, Hydrophobic and Anticorrosion Properties. Materials 2017, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Marmur, A.; Bittoun, E. When Wenzel and Cassie are right: Reconciling local and global considerations. Langmuir ACS J. Surg. Colloids 2009, 25, 1277. [Google Scholar] [CrossRef] [PubMed]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic surfaces developed by mimicking hierarchical surface morphology of lotus leaf. Molecules 2014, 19, 4256–4283. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sarkar, D.K.; Chen, X.G. Superhydrophobic aluminum alloy surfaces prepared by chemical etching process and their corrosion resistance properties. Appl. Surf. Sci. 2015, 356, 1012–1024. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Li, J.; Zhou, P.; Zhu, L.; Tang, H. Superhydrophobic copper coating: Switchable wettability, on-demand oil-water separation, and antifouling. Chem. Eng. J. 2017, 327, 849–854. [Google Scholar] [CrossRef]
- Xiang, M.; Jiang, M.; Zhang, Y.; Liu, Y.; Shen, F.; Yang, G.; He, Y.; Wang, L.; Zhang, X.; Deng, S. Fabrication of a novel superhydrophobic and superoleophilic surface by one-step electrodeposition method for continuous oil/water separation. Appl. Surf. Sci. 2018, 434, 1015–1020. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Wu, Y.; Sun, W.; Wang, J.; Sun, H. One-step method for fabrication of superhydrophobic and superoleophilic surface for water-oil separation. Colloids Surf. A 2018, 552, 32–38. [Google Scholar] [CrossRef]
- Zhou, C.; Cheng, J.; Hou, K.; Zhu, Z.; Zheng, Y. Preparation of CuWO4 @Cu2O film on copper mesh by anodization for oil/water separation and aqueous pollutant degradation. Chem. Eng. J. 2017, 307, 803–811. [Google Scholar] [CrossRef]
- Pi, P.; Hou, K.; Zhou, C.; Li, G.; Wen, X.; Xu, S.; Cheng, J.; Wang, S. Superhydrophobic Cu2S@Cu2O film on copper surface fabricated by a facile chemical bath deposition method and its application in oil-water separation. Appl. Surf. Sci. 2016, 396, 566–573. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, Y.; Feng, X.; Wang, Y.; Yue, G.; Jin, S. Fabrication of superhydrophobic-superoleophilic copper mesh via thermal oxidation and its application in oil–water separation. Appl. Surf. Sci. 2016, 367, 493–499. [Google Scholar]
- Song, Y.; Liu, Y.; Zhan, B.; Kaya, C.; Stegmaier, T.; Han, Z.; Ren, L. Fabrication of Bioinspired Structured Superhydrophobic and Superoleophilic Copper Mesh for Efficient Oil-water Separation. J. Bionic Eng. 2017, 14, 497–505. [Google Scholar] [CrossRef]
- Zhang, R. Study on Drag Reduction Mechanism of Nano-Particle Adsorption Microchannel and LBM Simulation; Shanghai University: Shanghai, China, 2012. (In Chinese) [Google Scholar]
- Huang, X. Preparation of Superhydrophobic/Super-Lipophilic Waterborne Epoxy Resin Emulsion Coating and Its Application in Oil-Water Separation Filter Paper; South China University of Technology: Shanghai, China, 2012. (In Chinese) [Google Scholar]



| Serial Number | a | h | k | R2 |
|---|---|---|---|---|
| 1 | −0.44935 | 4.4073 | 8.9446 | 0.89199 |
| 2 | −0.78860 | 8.1127 | 9.5793 | 0.89229 |
| 3 | −0.96367 | 2.8509 | 11.9148 | 0.91121 |
| 4 | −0.66353 | 6.0041 | 11.0044 | 0.80343 |
| 5 | −1.32367 | 8.2791 | 11.0860 | 0.95173 |
| Year | Matrix | Modified Materials | Method | Separation Efficiency (%) | Oil-Water Mixtures | Ref. |
|---|---|---|---|---|---|---|
| 2018 | Cu mesh | Stearic acid (SA) | etching | >97 | benzene, carbon tetrachloride | This work |
| 2018 | Cu mesh | dodecanethiol (DDT) | etching | >98 | cyclohexane, n-hexane | [32] |
| 2017 | Cu mesh | Candle soot | Deposition | 95 | – | [35] |
| 2017 | Cu mesh | CuWO4@Cu2O | Electrochemical anodization | 95 | cyclohexane, chloroform | [43] |
| 2017 | Cu mesh | Cu2S@Cu2O | Chemical bath deposition | >94 | isooctane, chloroform | [44] |
| 2017 | Cu mesh | Dopamine; 1-dodecanethiol | Dip-coating | 90 | cyclohexane, n-hexane | [18] |
| 2017 | Cu mesh | 1-dodecanethiol (HS(CH2)11CH3) | etching | >92 | gasoline, diesel | [45] |
| 2016 | Cu mesh | dodecanthiol (DDT) | thermal oxidation | 95 | dodecane, hexadecane | [46] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, X.; Yang, C.; Zhang, Q.; Yang, S.; Chen, Y.; Zhang, D.; Yuan, X.; Wang, W.; Zhao, Y. Preparation of Parabolic Superhydrophobic Material for Oil-Water Separation. Materials 2018, 11, 1914. https://doi.org/10.3390/ma11101914
Qiao X, Yang C, Zhang Q, Yang S, Chen Y, Zhang D, Yuan X, Wang W, Zhao Y. Preparation of Parabolic Superhydrophobic Material for Oil-Water Separation. Materials. 2018; 11(10):1914. https://doi.org/10.3390/ma11101914
Chicago/Turabian StyleQiao, Xiaoying, Chunyan Yang, Qian Zhang, Shengke Yang, Yangyang Chen, Dan Zhang, Xiaoyu Yuan, Wenke Wang, and Yaqian Zhao. 2018. "Preparation of Parabolic Superhydrophobic Material for Oil-Water Separation" Materials 11, no. 10: 1914. https://doi.org/10.3390/ma11101914




