Fabricating a Novel Intragranular Microstructure for Al2O3/GdAlO3 Ceramic Composites
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
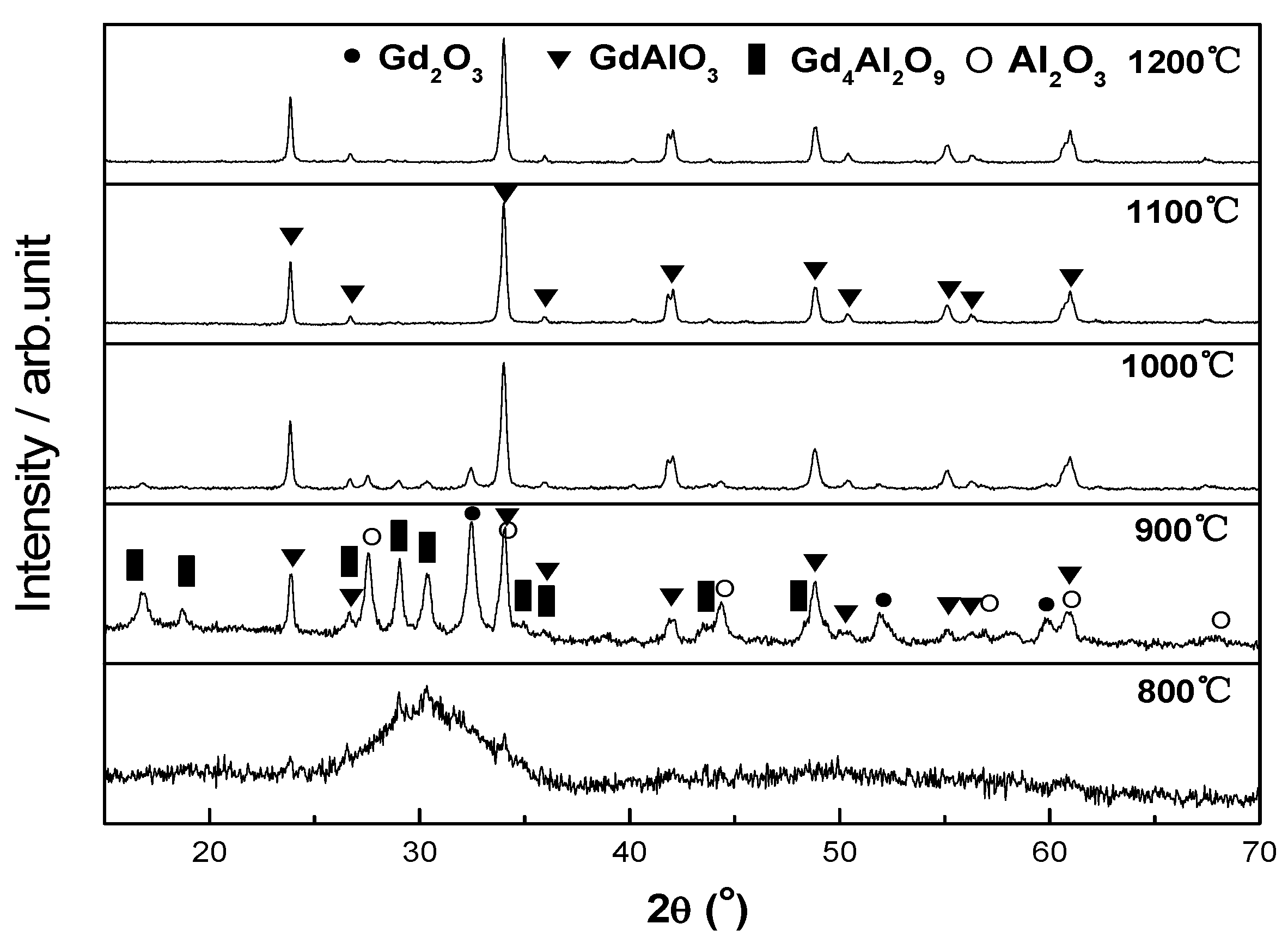
3.1. XRD Patterns of the Powder and Sintered Sample
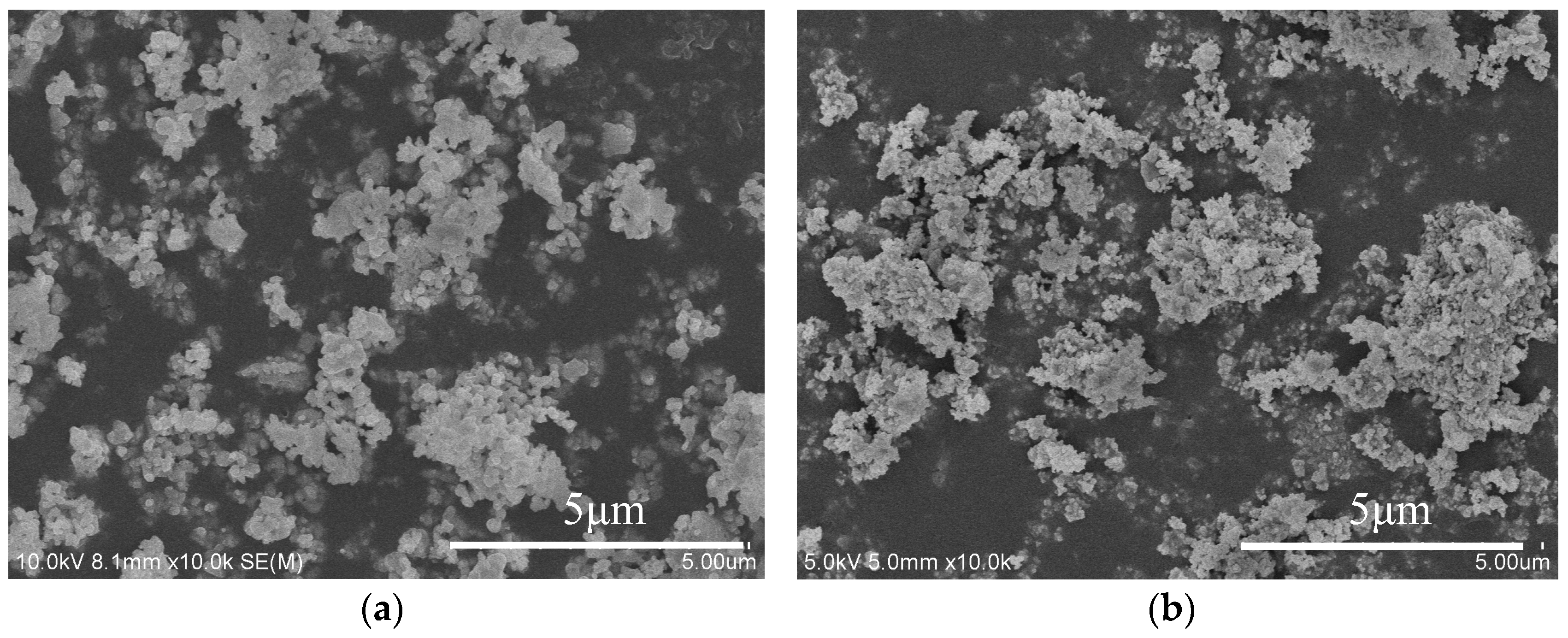
3.2. SEM Micrographs of the Powders
3.3. Surface of Sintered Samples
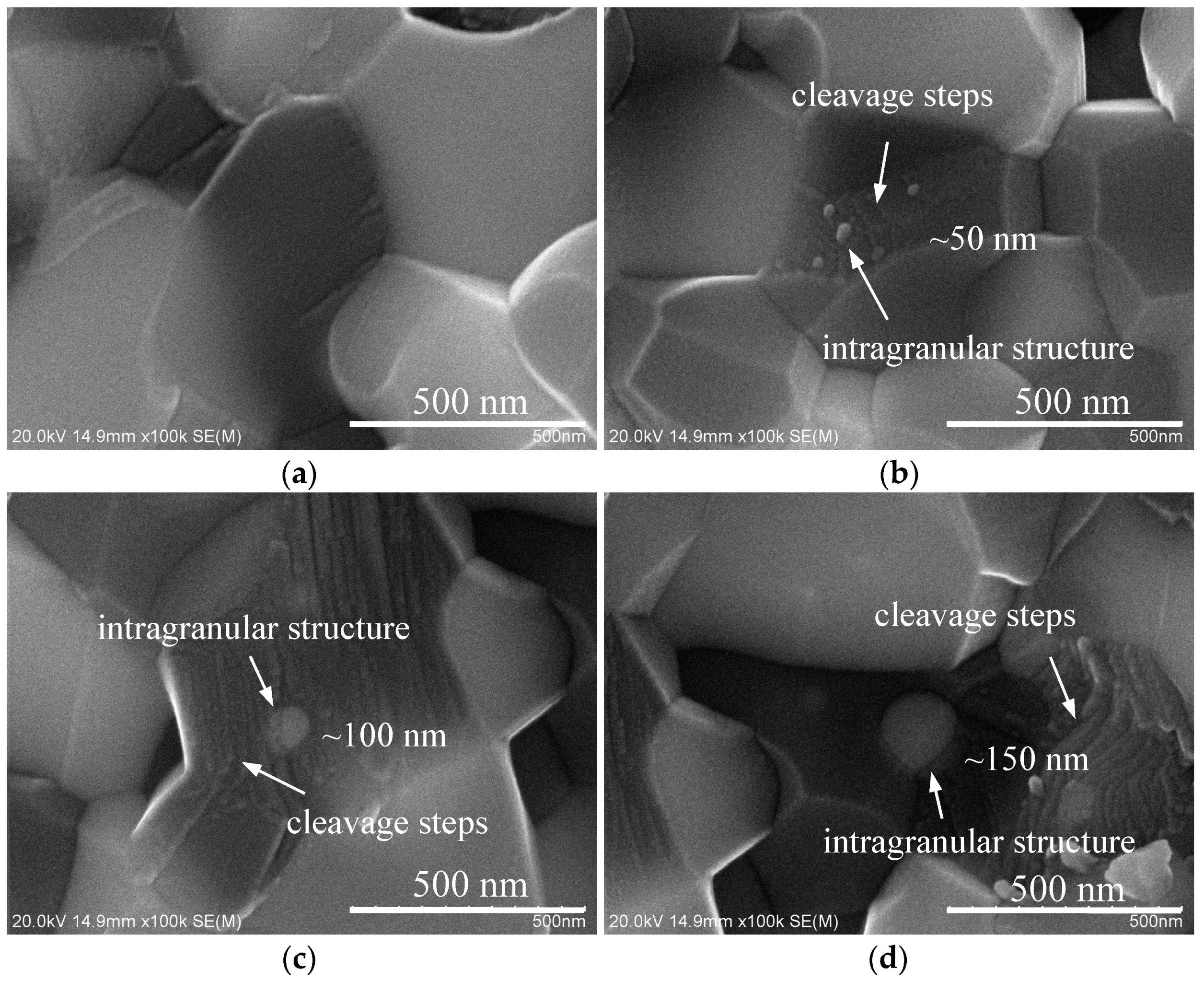
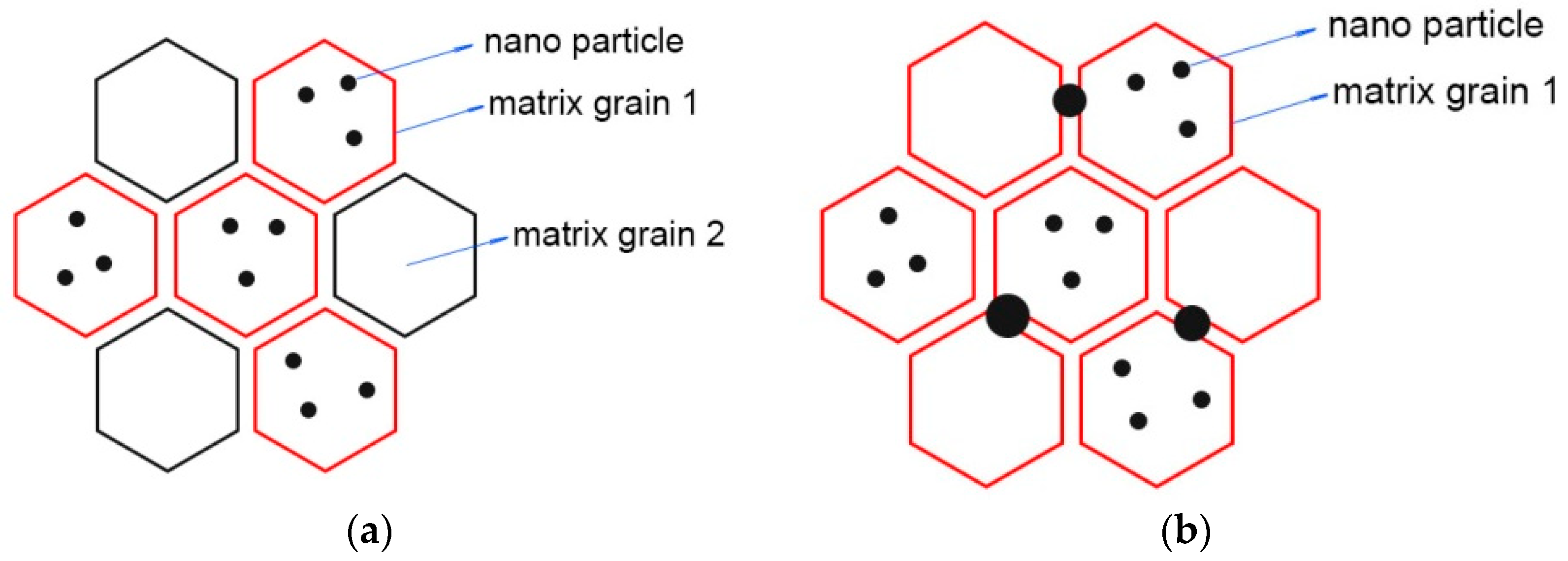
3.4. Fracture Surface of Sintered Samples
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Oh, S.T.; Sando, M.; Niihara, K. Mechanical and magnetic properties of Ni-Co dispersed Al2O3 nanocomposites. J. Mater. Sci. 2001, 36, 1817–1821. [Google Scholar] [CrossRef]
- Niihara, K. New design concept of structural ceramics—Ceramic nanocomposites. J. Ceram. Soc. Jpn. 1991, 99, 974–982. [Google Scholar] [CrossRef]
- Sun, X.D.; Li, J.G. Intragranular particle residual stress strengthening of Al2O3–SiC nanocomposites. J. Am. Ceram. Soc. 2005, 88, 1536–1543. [Google Scholar] [CrossRef]
- Rouxel, T.; Wakai, F.; Brito, M.E.; Iwamoto, A.; Izaki, K. Intragranular crack deflection and crystallographic slip in Si3N4/SiC nanocomposites. J. Eur. Ceram. Soc. 1993, 11, 431–438. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Z.; Zhang, W.; Zhong, Y.; Xian, Q.; Zhang, J.; Wang, J. Mechanical properties of directionally solidified Al2O3/Y3Al5O12 eutectic ceramic prepared by optical floating zone technique. J. Eur. Ceram. Soc. 2018, 38, 3610–3617. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Zhang, H.; Tian, Z.; Du, K.; Wang, J.; Lou, L.; Zhang, J. Mechanism of eutectic growth in directional solidification of an Al2O3/Y3Al5O12 crystal. Scr. Mater. 2016, 116, 44–48. [Google Scholar] [CrossRef]
- Zhang, J.X.; Gao, L.Q. Study on chemical processing for Al2O3/SiCp nano-composites. J. Chin. Ceram. Soc. 2011, 29, 550–553. [Google Scholar]
- Waku, Y.; Nakagawa, N.; Wakamoto, T.; Ohtsubo, H.; Shimizu, K.; Kohtoku, Y. A ductile ceramic eutectic composite with high strength at 1873 K. Nature 1997, 389, 49–52. [Google Scholar] [CrossRef]
- Ohashi, Y.; Yasui, N.; Suzuki, T.; Watanabe, M.; Den, T.; Kamada, K.; Yokota, Y.; Yoshikawa, A. Orientation relationships of unidirectionally aligned GdAlO3/Al2O3 eutectic fibers. J. Eur. Ceram. Soc. 2014, 34, 3849–3857. [Google Scholar] [CrossRef]
- Ma, W.; Su, H.; Zhang, J.; Ren, Q.; Liu, H.; Wang, E.; Ren, J.; Lu, Z.; Liu, L.; Fu, H. Effects of composition and solidification rate on growth striations in laser floating zone melted Al2O3/GdAlO3 eutectic ceramics. J. Am. Ceram. Soc. 2018, 101, 3337–3346. [Google Scholar] [CrossRef]
- Xu, C.H.; Sun, D.M. Formation of intragranular nano-structures in micro-sized ceramic composite materials. Mater. Sci. Eng. A 2008, 1, 338–342. [Google Scholar] [CrossRef]
- Wang, X.; Shan, Y.; Gong, H.Y.; Yu, X.G.; Xu, J.; Yin, Y.S. Formation mechanism of intragranular structure in nano-composites. Nonferr. Metall. Soc. 2004, 14, 265–269. [Google Scholar]
- Chaim, R.; Chevallier, G.; Weibel, A.; Estournès, C. Grain growth during spark plasma and flash sintering of ceramic nanoparticles: A review. J. Mater. Sci. 2018, 53, 3087–3105. [Google Scholar] [CrossRef]
- Liu, D.; Gao, Y.; Liu, J.; Liu, F.; Li, K.; Su, H.; Wang, Y.; An, L. Preparation of Al2O3-Y3Al5O12-ZrO2 eutectic ceramic by flash sintering. Scr. Mater. 2016, 114, 108–111. [Google Scholar] [CrossRef]
- Sun, S.; Xu, Q. Effect of calcination temperature on phase transformation and microstructure of Al2O3/GdAlO3 compound powder prepared by Co-precipitation method. Key Eng. Mater. 2011, 512, 535–538. [Google Scholar] [CrossRef]
- Harada, Y.; Ayabe, K.; Uekawa, N.; Kojima, T.; Kakegawa, K.; Kim, S.J. Formation of GdAlO3-Al2O3 composite having fine pseudo-eutectic microstructure. J. Eur. Ceram. Soc. 2008, 28, 2941–2946. [Google Scholar] [CrossRef]
- Chaudhury, S.; Parida, S.C.; Pillai, K.T.; Mudher, K.S. High-temperature X-ray diffraction and specific heat studies on GdAlO3, Gd3Al5O12 and Gd4Al2O9. J. Solid State Chem. 2007, 180, 2393–2399. [Google Scholar] [CrossRef]
- Wang, H.Z.; Gao, L.; Li, W.Q.; Kawaoka, H.; Niihara, K. Preparation and Microstructure of Al2O3-YAG Composites. J. Inorg. Mater. 2001, 16, 169–172. [Google Scholar]
- Paneto, F.J.; Pereira, J.L.; Lima, J.O.; Jesus, E.J.; Silva, L.A.; Lima, E.S.; Cabral, R.F.; Santos, C. Effect of porosity on hardness of Al2O3-Y3Al5O12 ceramic composite. Int. J. Refract. Met. Hard Mater. 2015, 48, 365–368. [Google Scholar] [CrossRef]
- Wang, H.Z.; Gao, L.; Guo, J.K. Effect of nanoscale SiC particles on the microstructure of Al2O3 ceramics. Ceram. Int. 2000, 26, 391–396. [Google Scholar] [CrossRef]
- Palmero, P.; Pulci, G.; Marra, F.; Valente, T.; Montanaro, L. Al2O3/ZrO2/Y3Al5O12 Composites: A High-Temperature Mechanical Characterization. Materials 2015, 8, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Zhai, X.J.; Fu, Y.; Lu, Z.J.; Bi, S.W. Lattice thermal expansion coefficients of combustion synthesized alpha- Al2O3 nanoparticles. J. Inorg. Mater. 2005, 20, 755–758. [Google Scholar]
- Matsuo, H.; Mitsuhara, M.; Ikeda, K.; Hata, S.; Nakashima, H. Electron microscopy analysis for crack propagation behavior of alumina. Int. J. Fatigue 2010, 32, 592–598. [Google Scholar] [CrossRef]
- Xu, Y.R.; Zangvil, A.; Kerber, A. SiC nanoparticle-reinforced Al2O3 matrix composites: Role of intra- and intergranular particles. J. Eur. Ceram. Soc. 1997, 17, 921–928. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Xu, Q. Fabricating a Novel Intragranular Microstructure for Al2O3/GdAlO3 Ceramic Composites. Materials 2018, 11, 1879. https://doi.org/10.3390/ma11101879
Sun S, Xu Q. Fabricating a Novel Intragranular Microstructure for Al2O3/GdAlO3 Ceramic Composites. Materials. 2018; 11(10):1879. https://doi.org/10.3390/ma11101879
Chicago/Turabian StyleSun, Shuai, and Qiang Xu. 2018. "Fabricating a Novel Intragranular Microstructure for Al2O3/GdAlO3 Ceramic Composites" Materials 11, no. 10: 1879. https://doi.org/10.3390/ma11101879



