Improvement in Wood Bonding Strength of Poly (Vinyl Acetate-Butyl Acrylate) Emulsion by Controlling the Amount of Redox Initiator
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of the Amount of Initiator on the Bonding Strength
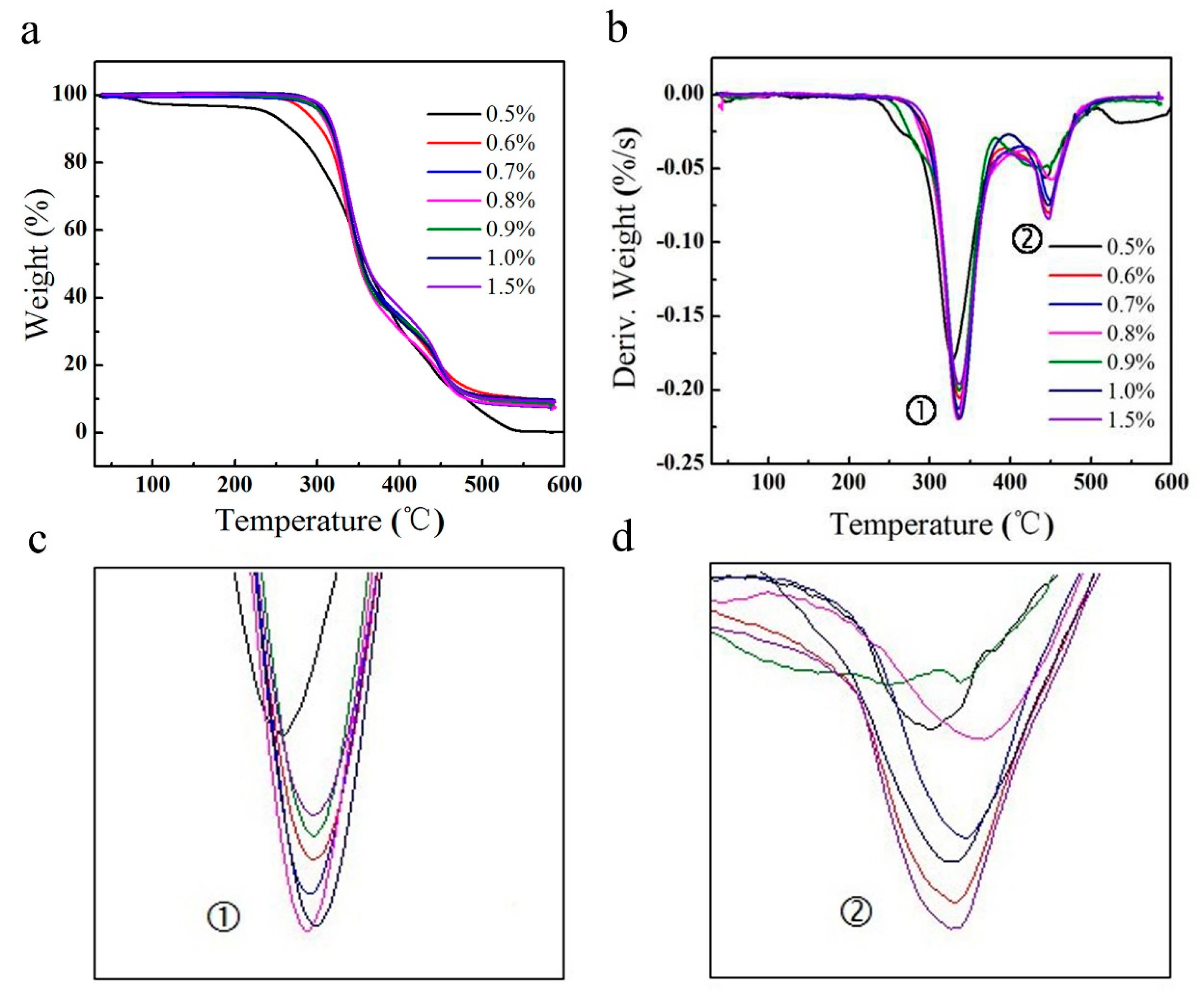
2.2. Thermal Gravimetric Analysis (TGA)
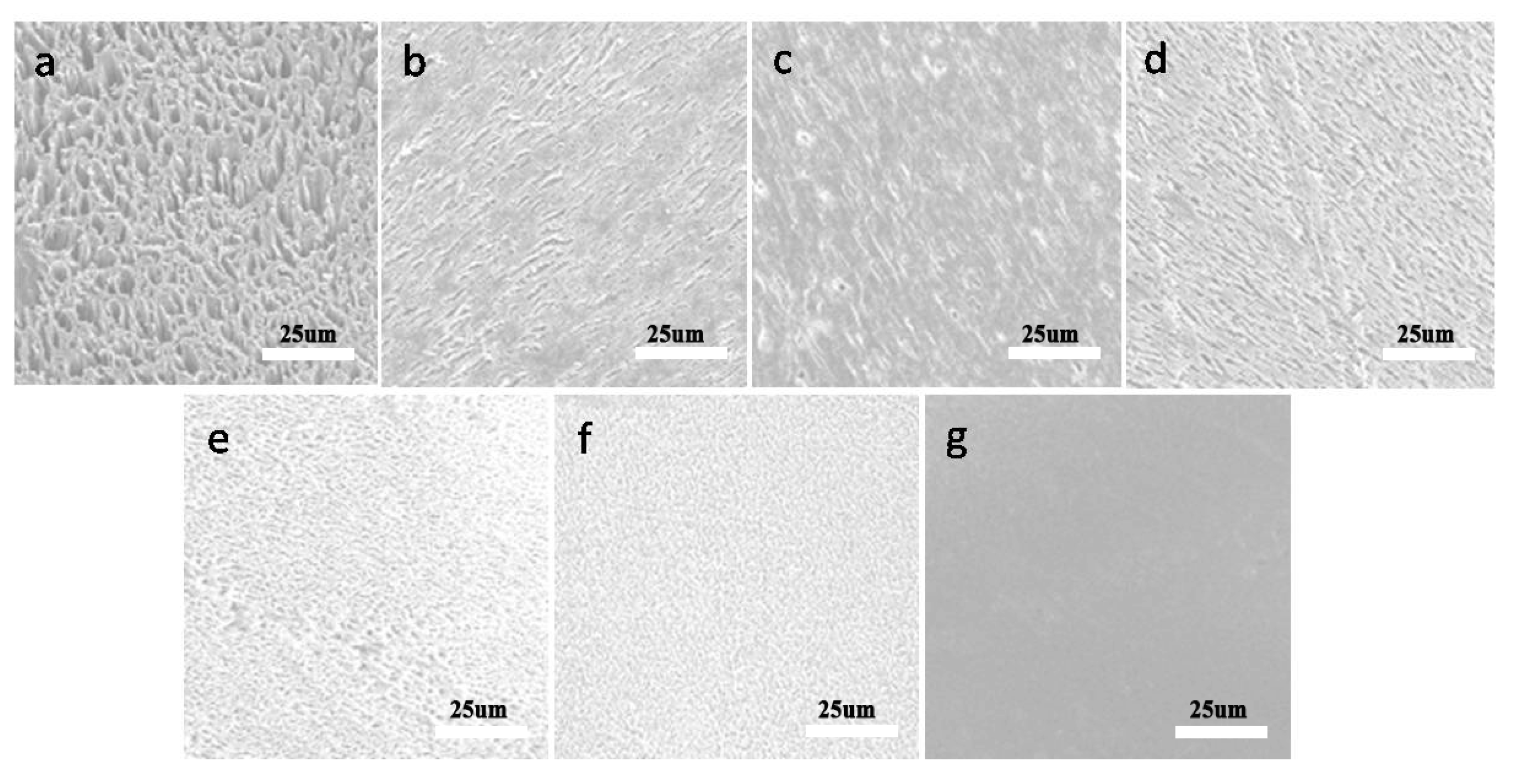
2.3. Scanning Electron Microscopy (SEM) Analysis
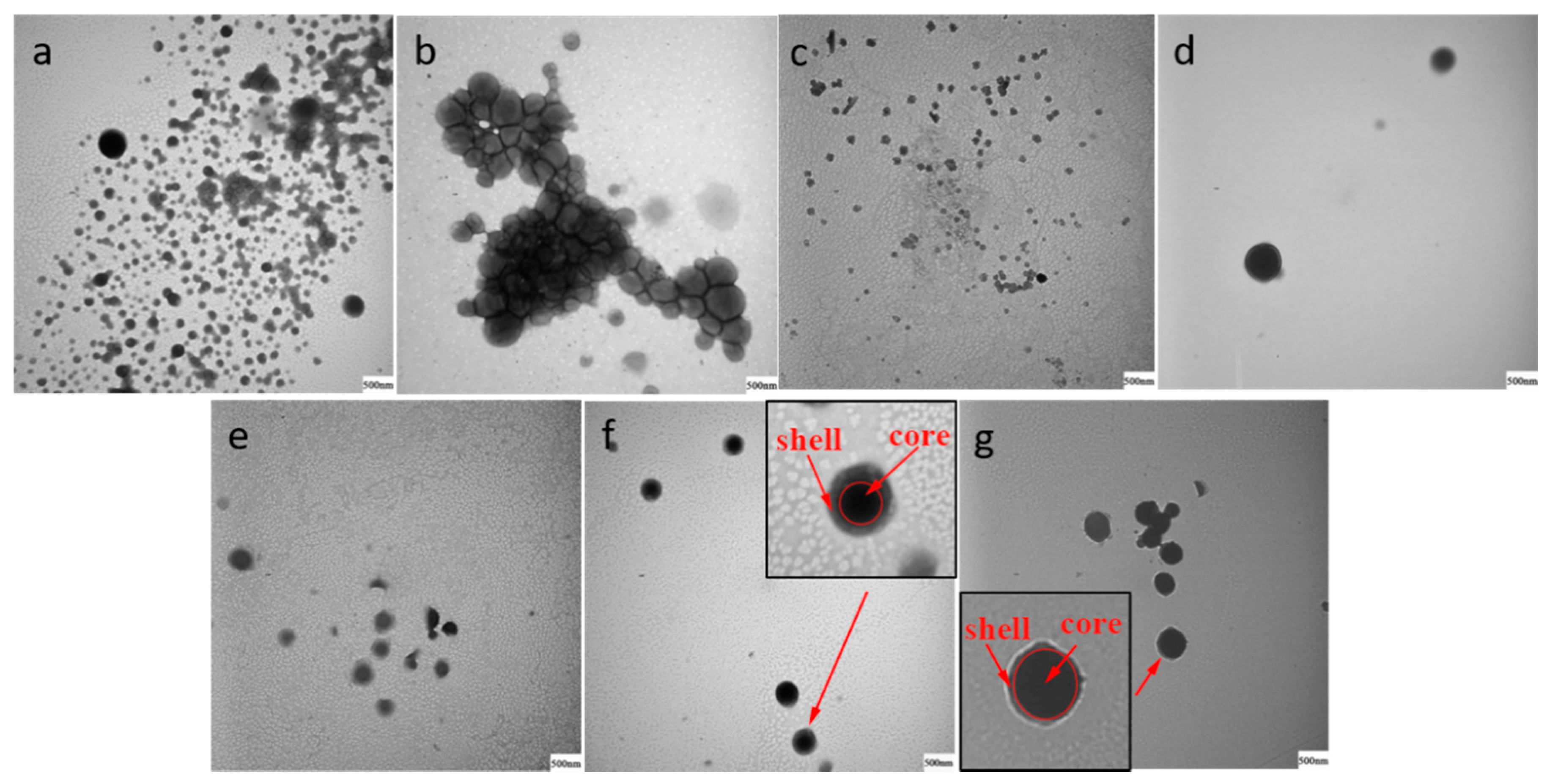
2.4. Transmission Electron Microscopy (TEM) Analysis
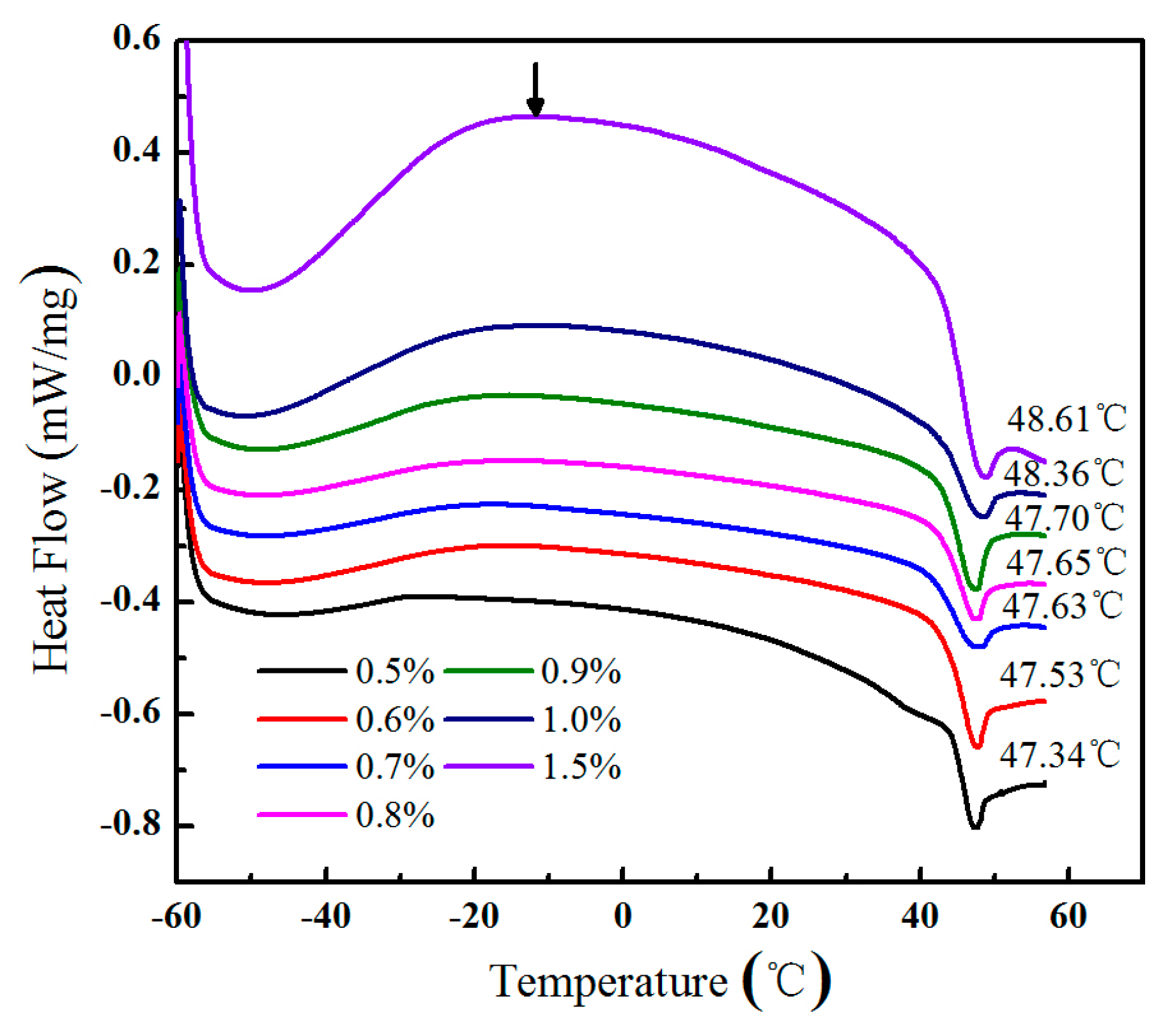
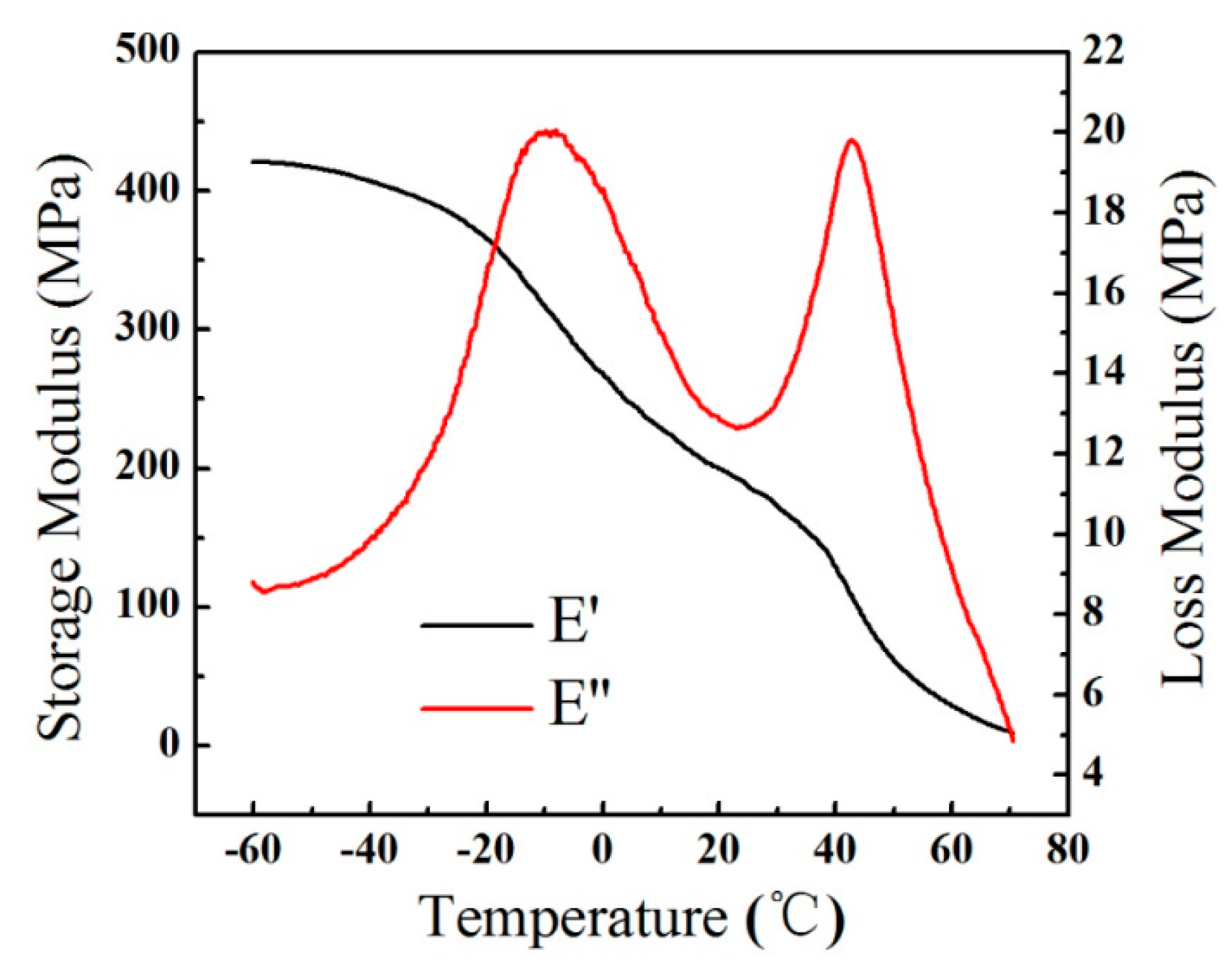
2.5. Differential Scanning Calorimetry (DSC) and Dynamic Mechanical Thermal Analysis (DMA)
3. Experimental
3.1. Materials
3.2. Preparation of Core-Shell Emulsion Adhesives
3.2.1. Preparation of Core-Seed Emulsion
3.2.2. Shell Copolymerization Reaction
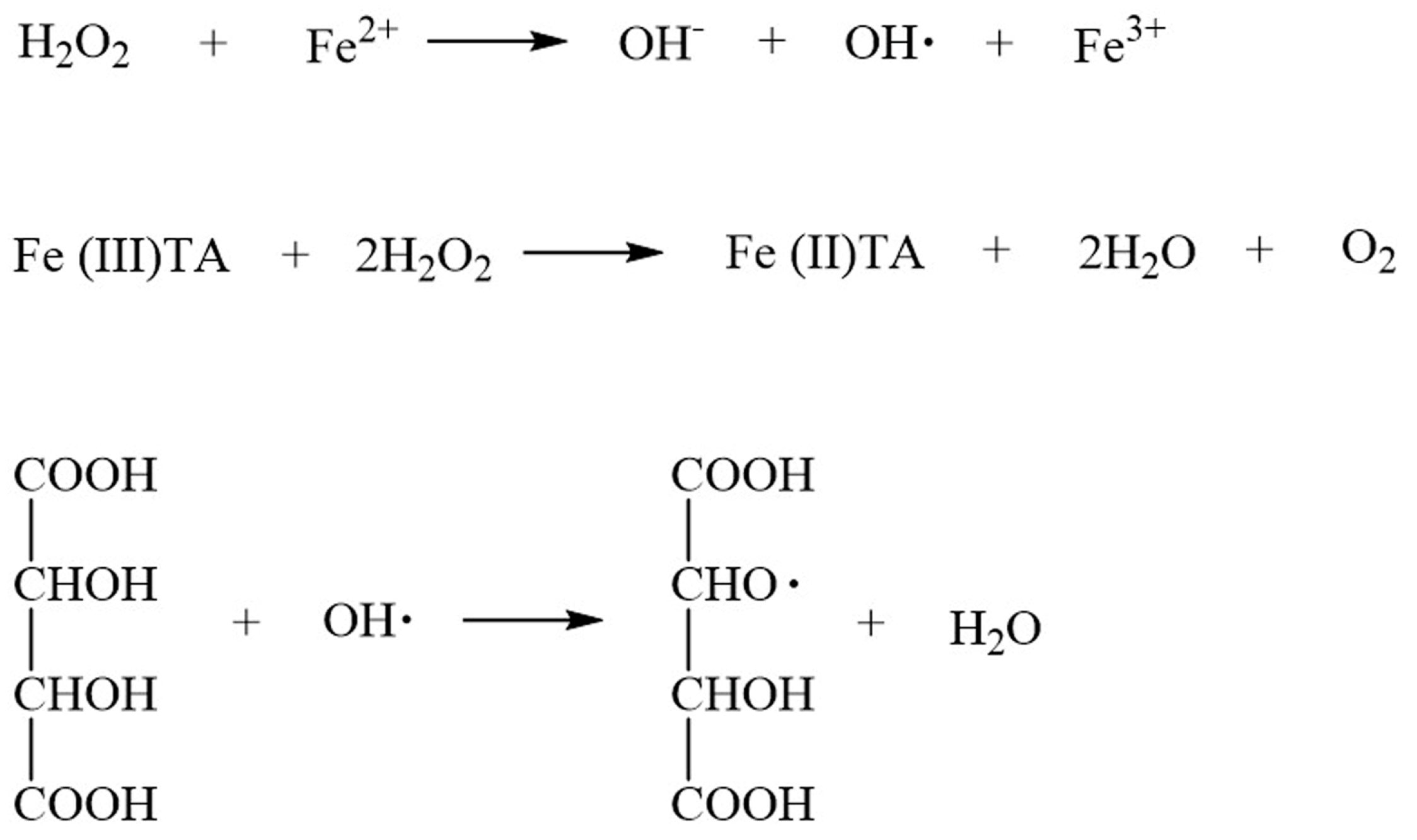
3.3. Mechanism of Initiator
3.4. Preparation and Characterization of Plywood
3.5. Characterization of Emulsion and Particles
3.5.1. Thermal Gravimetric Analysis (TGA)
3.5.2. Scanning Electron Microscopy (SEM)
3.5.3. Transmission Electron Microscopy (TEM)
3.5.4. Differential Scanning Calorimetry (DSC)
3.5.5. Dynamic Mechanical Thermal Analysis (DMA)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pizzi, A. Recent developments in eco-efficient bio-based adhesives for wood bonding: Opportunities and issues. J. Adhes. Sci. Technol. 2006, 20, 829–846. [Google Scholar] [CrossRef]
- Pizzi, A. Bioadhesives for wood and fibres. Rev. Adhes. Adhes. 2013, 1, 88–113. [Google Scholar] [CrossRef]
- Li, X.; Luo, J.; Gao, Q.; Li, J. A sepiolite-based united cross-linked network in a soybean meal-based wood adhesive and its performance. RSC. Adv. 2016, 6, 45158–45165. [Google Scholar] [CrossRef]
- Jang, Y.; Huang, J.; Li, K. A new formaldehyde-free wood adhesive from renewable materials. Int. J. Adhes. Adhes. 2011, 31, 754–759. [Google Scholar] [CrossRef]
- Okaya, T.; Suzuki, A.; Kikuchi, K. Importance of grafting in the emulsion polymerization of MMA using PVA as a protective colloid. Effect of initiators. Colloids Surf. A 1999, 153, 123–125. [Google Scholar] [CrossRef]
- Abdollahi, M.; Massoumi, B.; Yousefi, M.R.; Ziaee, F. Free-radical homo-and copolymerization of vinyl acetate and n-butyl acrylate: Kinetic studies by online 1H NMR kinetic experiments. J. Appl. Polym. Sci. 2012, 123, 543–553. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, Z.; Liu, B.; Peng, G.; Niu, J. Synergistic effect between organically modified montmorillonite and ammonium polyphosphate on thermal and flame-retardant properties of poly (butyl acrylate/vinyl acetate) copolymer late. J. Macromol. Sci. Part B 2012, 51, 1089–1099. [Google Scholar] [CrossRef]
- Suma, K.K.; Jacob, S.; Joseph, R. Studies on the effect of nano-TiO2 on vinyl acetate–butyl acrylate latex-based surface coating. Mater. Sci. Eng. B 2010, 16, 254–258. [Google Scholar] [CrossRef]
- Dossi, M.; Liang, K.; Hutchinson, R.A.; Moscatelli, D. Investigation of free-radical copolymerization propagation kinetics of vinyl acetate and methyl methacrylate. J. Phys. Chem. B 2010, 114, 4213–4222. [Google Scholar] [CrossRef] [PubMed]
- Sarac, A.; Yildirim, H. Semi-continuous emulsion copolymerization of vinyl acetate and butyl acrylate using a new protective colloid. Part 1. Effect of different emulsifiers. Polym. Adv. Technol. 2006, 17, 855–859. [Google Scholar] [CrossRef]
- Rosdi, M.R.H.; Ariffin, A. Evaluation of flow ability response in EVA emulsion preparation with different vinyl acetate percentage by intrinsic viscosity measurement. Procedia Chem. 2016, 19, 455–461. [Google Scholar] [CrossRef]
- Ahmed, M.; Abd-Elhamid, M.; Sarhan, A.; Hassan, A. Characteristic and thermal stimulated depolarization current of poly (vinyl chloride-co-vinyl acetate-co-2-hydroxy propyl acrylate) Znonanocomposite. Glob. J. Phys. 2017, 5, 585–594. [Google Scholar]
- Zhang, Y.; Pan, S.; Ai, S.; Liu, H.; Wang, H.; He, P. Semi-continuous emulsion copolymerization of vinyl acetate and butyl acrylate in presence of AMPS. Iran. Polym. J. 2014, 23, 103–109. [Google Scholar] [CrossRef]
- Zhao, C.; Peng, G.; Liu, B.; Jiang, Z. Synergistic effect of organically modified layered double hydroxide on thermal and flame-retardant properties of poly (butyl acrylate–vinyl acetate). J. Polym. Res. 2011, 18, 1971–1981. [Google Scholar] [CrossRef]
- Britton, D.J.; Lovell, P.A.; Heatley, F.; Venkatesh, R. Chain transfer to polymer in emulsion copolymerizations. Macromol. Symp. 2001, 175, 95–104. [Google Scholar] [CrossRef]
- Schumacher, H.C.; Alves, M.; Leite, C.A.P.; Santos, J.P.; Neto, É.T.; Murakami, M.M.; Galembeck, F.; do Amaral, M. Cationic latex formation by ionic modification. J. Colloid Interface Sci. 2007, 305, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, W.; Shi, H.; Wu, H.; Xiang, J. Synthesis and characterization of novel poly (AN-BA-DFMA) latex prepared via semi-continuous seeded emulsion polymerization. J. Macromol. Sci. Part A 2012, 49, 744–748. [Google Scholar] [CrossRef]
- Kong, X.Z.; Zhu, X.; Jiang, X.; Li, X. Preparation and full characterization of cationic latex of styrene–butyl acrylate. Polymer 2009, 50, 4220–4227. [Google Scholar] [CrossRef]
- Araújo, P.H.H.; Giudici, R.; Sayer, C. Butyl acrylate and vinyl acetate semicontinuous emulsion copolymerizations: Study of stabilization performance. Macromol. Symp. 2004, 206, 179–190. [Google Scholar] [CrossRef]
- Berber, H.; Sarac, A.; Yıldırım, H. Synthesis and characterization of water-based poly (vinyl acetate-co-butyl acrylate) latexes containing oligomeric protective colloid. Polym. Bull. 2011, 66, 881–892. [Google Scholar] [CrossRef]
- Ovando-Medina, V.M.; Díaz-Flores, P.E.; Peralta, R.D.; Mendizábal, E.; Cortez-Mazatan, G.Y. Semicontinuous heterophase copolymerization of vinyl acetate and butyl acrylate. J. Appl. Polym. Sci. 2013, 127, 2458–2464. [Google Scholar] [CrossRef]
- Meng, X.; Liang, L.; Liu, B.; Peng, G.; Wang, B.; Chen, H.; Luo, R. Influence of 2-methylacryloyLxyethyl trimethyl ammonium chloride on the properties of cationic poly (vinyl acetate-butyl acrylate-DMC) copolymer emulsions. J. Macromol. Sci. Part A 2013, 50, 185–192. [Google Scholar] [CrossRef]
- Ovando-Medina, V.M.; Peralta, R.D.; Mendizábal, E.; Martínez-Gutiérrez, H.; Corona-Rivera, M.A. Microemulsion copolymerization of vinyl acetate and butyl acrylate using a mixture of anionic and non-ionic surfactants. Polym. Bull. 2011, 66, 133–146. [Google Scholar] [CrossRef]
- Saraç, A. Semicontinuous emulsion copolymerization of vinyl acetate and butyl acrylate using different initiators and different chain length emulsifiers. Macromol. Symp. 2004, 217, 161–168. [Google Scholar] [CrossRef]
- Jaffe, H.L.; Rosenblum, F.M.; Daniels, W. Polyvinyl acetate emulsions for adhesives. In Handbook of Adhesives; Skeist, I., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1989; Volume 3, pp. 381–400. [Google Scholar]
- Grigsby, W.J.; Ferguson, C.J.; Franich, R.A.; Russell, G.T. Evaluation of latex adhesives containing hydrophobic cores and poly (vinyl acetate) shells: Potential to improve poly (vinyl acetate) performance. Int. J. Adhes. Adhes. 2005, 25, 127–137. [Google Scholar] [CrossRef]
- Athawale, V.D.; Kulkarni, M.A. Preparation and properties of urethane/acrylate composite by emulsion polymerization technique. Prog. Org. Coat. 2009, 65, 392–400. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, W.; Meng, H.; Guo, K.; Chen, J.F. Pickering emulsion polymerization: Preparation of polystyrene/nano-SiO2 composite microspheres with core-shell structure. Powder Technol. 2009, 190, 393–400. [Google Scholar] [CrossRef]
- Chern, C.S. Emulsion polymerization mechanisms and kinetics. Prog. Polym. Sci. 2006, 31, 443–486. [Google Scholar] [CrossRef]
- D’hooge, D.R.; Van Steenberge, P.H.M.; Derboven, P.; Reyniers, M.F.; Marin, G.B. Model-based design of the polymer microstructure: Bridging the gap between polymer chemistry and engineering. Polym. Chem. 2005, 6, 7081–7096. [Google Scholar] [CrossRef]
- D’hooge, D.R.; Van Steenberge, P.H.M.; Derboven, P.; Reyniers, M.F.; Marin, G.B. The strength of multi-scale modeling to unveil the complexity of radical polymerization. Prog. Polym. Sci. 2016, 58, 59–89. [Google Scholar] [CrossRef]
- Desmet, G.B.; Marien, Y.W.; Van Steenberge, P.H.M.; D’hooge, D.R.; Reyniers, M.F.; Marin, G.B. Ab initio based kinetic Monte Carlo analysis to unravel the propagation kinetics in vinyl acetate pulsed laser polymerization. Polym. Chem. 2017, 8, 7143–7150. [Google Scholar] [CrossRef]
- Guo, S.; Wan, W.; Chen, C.; Chen, W.H. Thermal decomposition kinetic evaluation and its thermal hazards prediction of AIBN. J. Therm. Anal. Calorim. 2013, 113, 1169–1176. [Google Scholar] [CrossRef]
- Jiang, H.; Zang, N.; Qian, X.; Fu, Z. Thermal stability of potassium supersulphate and sodium supersulphate. J. Chem. Ind. Eng. China 2006, 57, 2798. [Google Scholar]
- Larsen, M.B.; Boydston, A.J. Investigations in fundamental and applied polymer mechanochemistry. Macromol. Chem. Phys. 2016, 217, 354–364. [Google Scholar] [CrossRef]
- Misra, B.N.; Mehta, I.K.; Khetarpal, R.C. Grafting onto cellulose. VIII. Graft copolymerization of poly (ethylacrylate) onto cellulose by use of redox initiators. Comparison of initiator reactivities. J. Polym. Sci. Pol. Chem. 1984, 22, 2767–2775. [Google Scholar] [CrossRef]
- Daniels, E.S.; Dimonie, V.L.; El-Aasser, M.S.; Vanderhoff, J.W. Preparation of ABS (acrylonitrile/butadiene/styrene) latexes using hydroperoxide redox initiators. J. Appl. Polym. Sci. 1990, 41, 2463–2477. [Google Scholar] [CrossRef]
- Hayashi, S. Preparations and Properties of Porous Poly (vinyl alcohol)-Poly (vinyl acetate) Composites. In Handbook of Engineering Polymeric Materials; Cheremisinoff, P., Ed.; Marcel Dekker: New York, NY, USA, 2016; Volume 13, pp. 167–177. ISBN 0-8247-9799-X. [Google Scholar]
- Kong, X.Z.; Pichot, C.; Guillot, J. Characterization of particle surface and morphology in vinyl acetate-butyl acrylate emulsion copolymers—Influence of the copolymerization pathway. Colloid Polym. Sci. 1987, 265, 791–802. [Google Scholar] [CrossRef]
- Sun, P.Q.; Liu, D.Z.; Zhao, K.; Chen, G.T. Development of particle morphology simulating of emulsion copolymerization of vinyl acetate and butyl acrylate. Acta Polym. Sin. 1998, 5, 542–548. [Google Scholar]
- Li, G.; Wang, T.; Fu, X.; Gao, T.M.; Huang, M.F. Study of core-shell structure poly (vinyl acetate-butyl acrylate) emulsion particles modification. Adv. Mater. Res. 2014, 1061–1062, 277–282. [Google Scholar] [CrossRef]
- Kong, X.Z.; Pichot, C.; Guillot, J. Kinetics of emulsion copolymerization of vinyl acetate with butyl acrylate. Eur. Polym. J. 1988, 24, 485–492. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Yuan, T.Q.; Sun, R.C. Lignin–phenol–formaldehyde resin adhesives prepared with biorefinery technical lignins. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Z. Test Methods of Evaluating the Properties of Wood-Based Panels and Surface Decorated Wood-Based Panels; China National Standard, GB/T 17657; Chinese Standards Press: Beijing, China, 2013. [Google Scholar]
| PVA | Bonding Strength (MPa) | Solid Content (%) | pH |
|---|---|---|---|
| 5% | 0.77 | 12.7 | 3.21 |
| 10% | 1.08 | 14.4 | 3.26 |
| 15% | 1.64 | 23.3 | 3.55 |
| 20% | 2.06 | 25.7 | 3.58 |
| 25% | 1.92 | 21.9 | 3.36 |
| VAc/BA | Bonding Strength (MPa) | Solid Content (%) | pH |
|---|---|---|---|
| 1:1 | 1.83 | 17.3 | 3.18 |
| 2:1 | 2.06 | 25.7 | 3.58 |
| 3:1 | 2.18 | 26.6 | 3.23 |
| 4:1 | 2.34 | 28.0 | 3.12 |
| 5:1 | 2.31 | 27.5 | 3.26 |
| Initiator Dosage | Bonding Strength (MPa) | Solid Content (%) | pH | Viscosity (mPa·s) |
|---|---|---|---|---|
| 0.5% | 2.34 | 28.0 | 3.12 | 44,320 ± 1000 |
| 0.6% | 2.41 | 29.9 | 3.30 | 39,430 ± 1000 |
| 0.7% | 2.53 | 30.1 | 3.52 | 37,850 ± 1000 |
| 0.8% | 2.55 | 30.4 | 3.57 | 40,050 ± 500 |
| 0.9% | 2.76 | 31.8 | 3.56 | 38,520 ± 500 |
| 1.0% | 2.97 | 33.4 | 3.56 | 36,270 ± 200 |
| 1.5% | 2.78 | 34.9 | 3.53 | 38,530 ± 200 |
| CPVAc 1 | 2.55 | 32.5 | 3.55 | - 2 |
| Initiator Ratio | First Degradation Temperature (°C) | Secondary Degradation Temperature (°C) | ||||
|---|---|---|---|---|---|---|
| T0-1 | Tp-1 | Tf-1 | T0-2 | Tp-2 | Tf-2 | |
| 0.5% | 293.6 | 328.5 | 337.2 | 378.7 | 443.6 | 507.8 |
| 0.6% | 309.8 | 336.5 | 349.3 | 384.5 | 447.5 | 515.1 |
| 0.7% | 311.8 | 335.7 | 347.1 | 381.4 | 449.4 | 505.1 |
| 0.8% | 306.8 | 336.6 | 343.1 | 379.4 | 447.4 | 514.6 |
| 0.9% | 313.1 | 336.7 | 345.6 | 377.5 | 447.6 | 500.6 |
| 1.0% | 308.7 | 338.3 | 344.8 | 377.3 | 451.3 | 505.6 |
| 1.5% | 310.1 | 337.2 | 351.4 | 388.5 | 447.4 | 503.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Pang, B.; Yang, S.; Fang, W.; Yang, S.; Yuan, T.-Q.; Sun, R.-C. Improvement in Wood Bonding Strength of Poly (Vinyl Acetate-Butyl Acrylate) Emulsion by Controlling the Amount of Redox Initiator. Materials 2018, 11, 89. https://doi.org/10.3390/ma11010089
Zhang Y, Pang B, Yang S, Fang W, Yang S, Yuan T-Q, Sun R-C. Improvement in Wood Bonding Strength of Poly (Vinyl Acetate-Butyl Acrylate) Emulsion by Controlling the Amount of Redox Initiator. Materials. 2018; 11(1):89. https://doi.org/10.3390/ma11010089
Chicago/Turabian StyleZhang, Yun, Bo Pang, Sen Yang, Wei Fang, Sheng Yang, Tong-Qi Yuan, and Run-Cang Sun. 2018. "Improvement in Wood Bonding Strength of Poly (Vinyl Acetate-Butyl Acrylate) Emulsion by Controlling the Amount of Redox Initiator" Materials 11, no. 1: 89. https://doi.org/10.3390/ma11010089