Microstructural Investigation of Heat-Treated Ultra-High Performance Concrete for Optimum Production
Abstract
:1. Introduction
2. Experimental Method
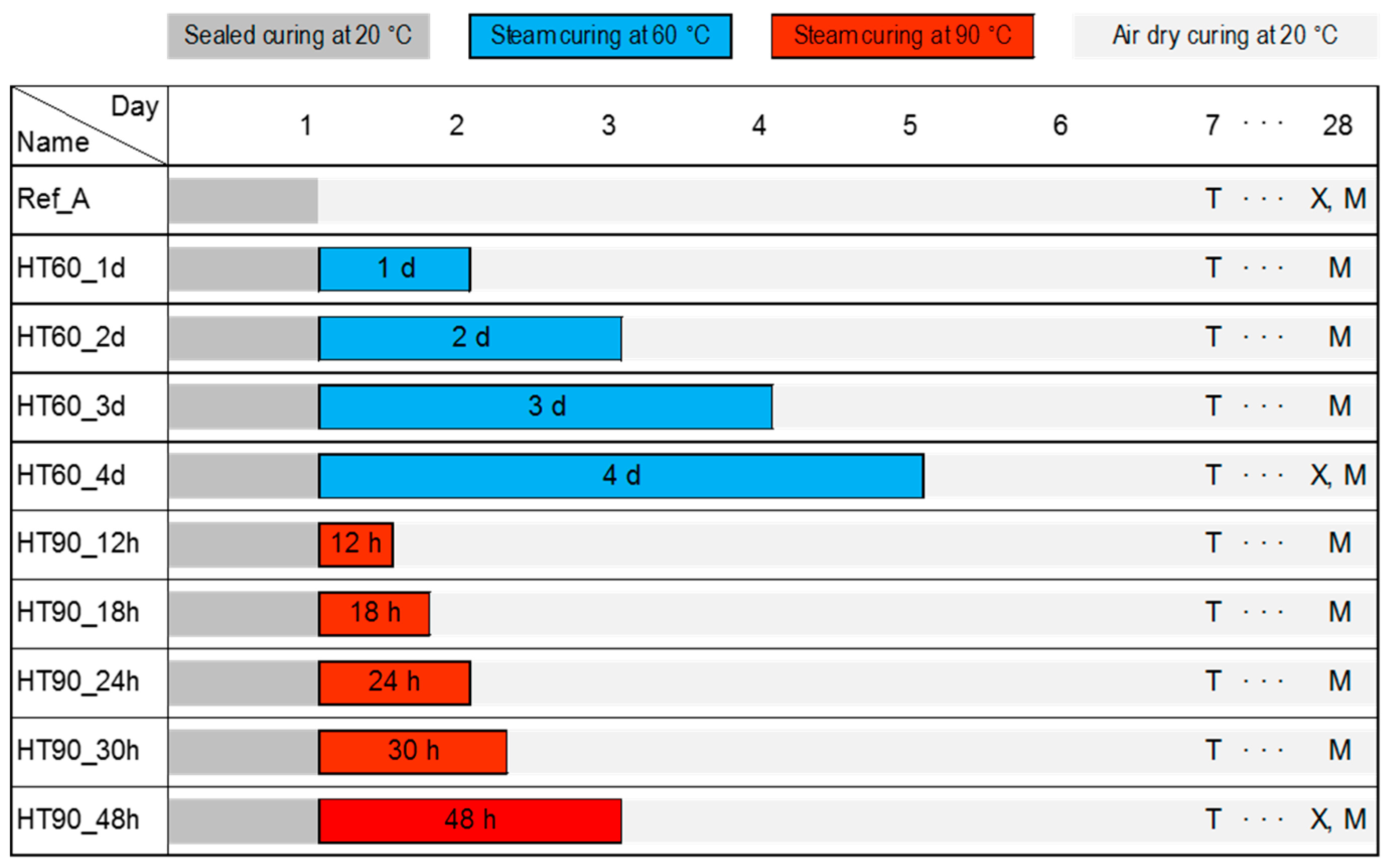
2.1. Preparation of Ultra-High Performance Concrete with Various Heat-Hreatment Conditions
2.2. Experimental Methods
2.2.1. Isothermal Calorimetry
2.2.2. Strength
2.2.3. X-ray Diffraction (XRD) and Thermogravimetric Analysis (TGA)
2.2.4. Pore Size Distribution
3. Results
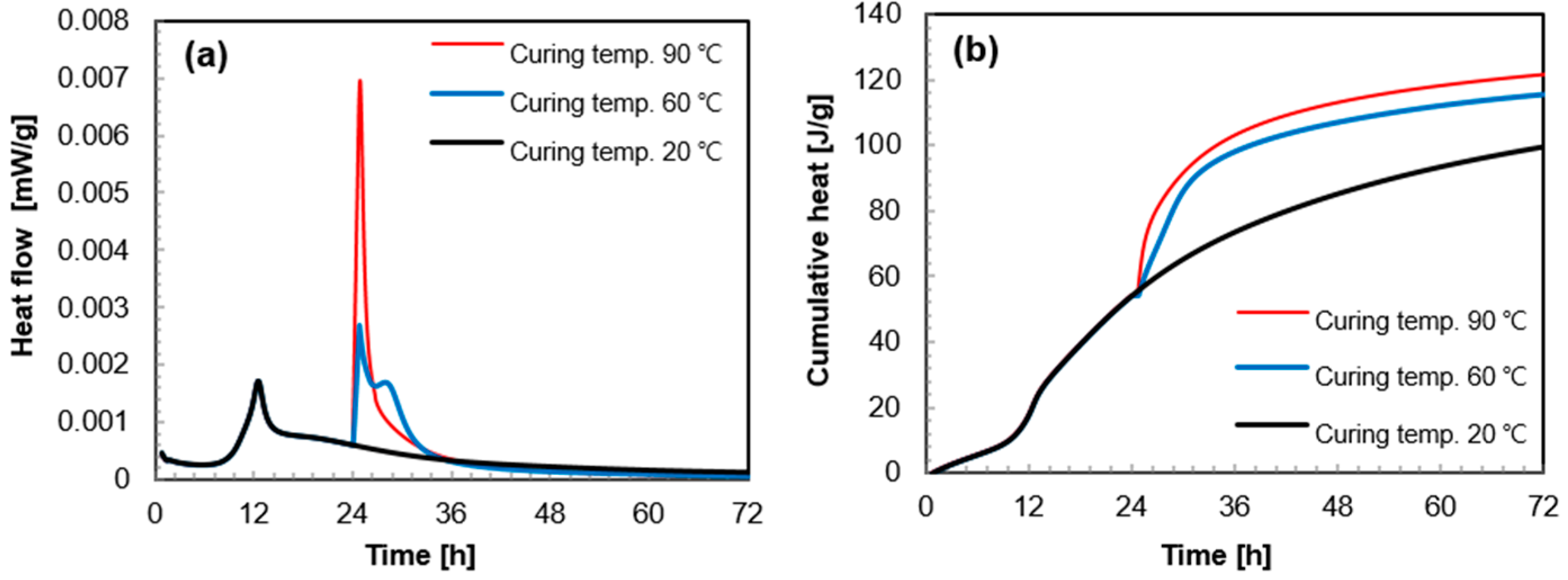
3.1. Heat of Hydration
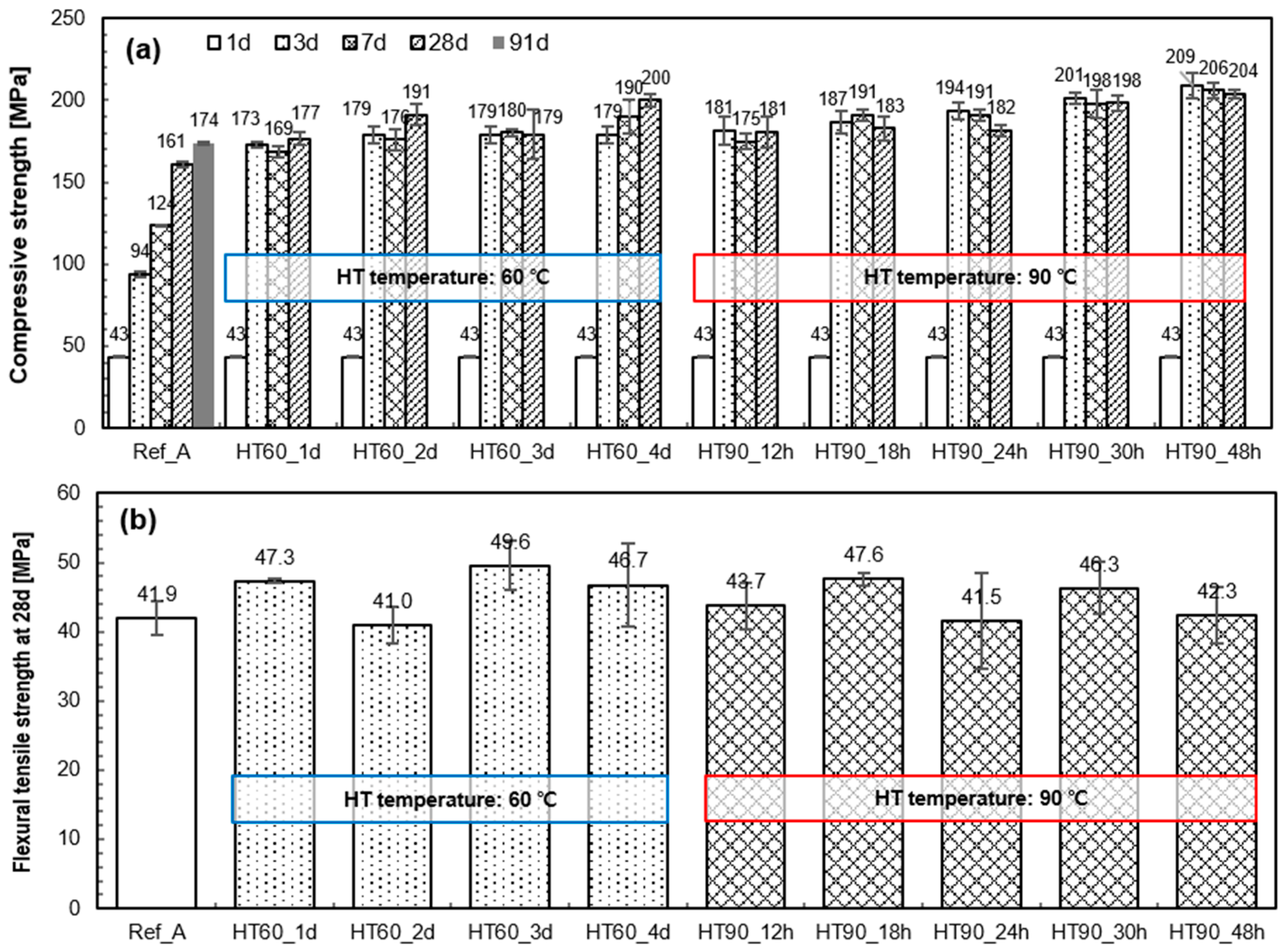
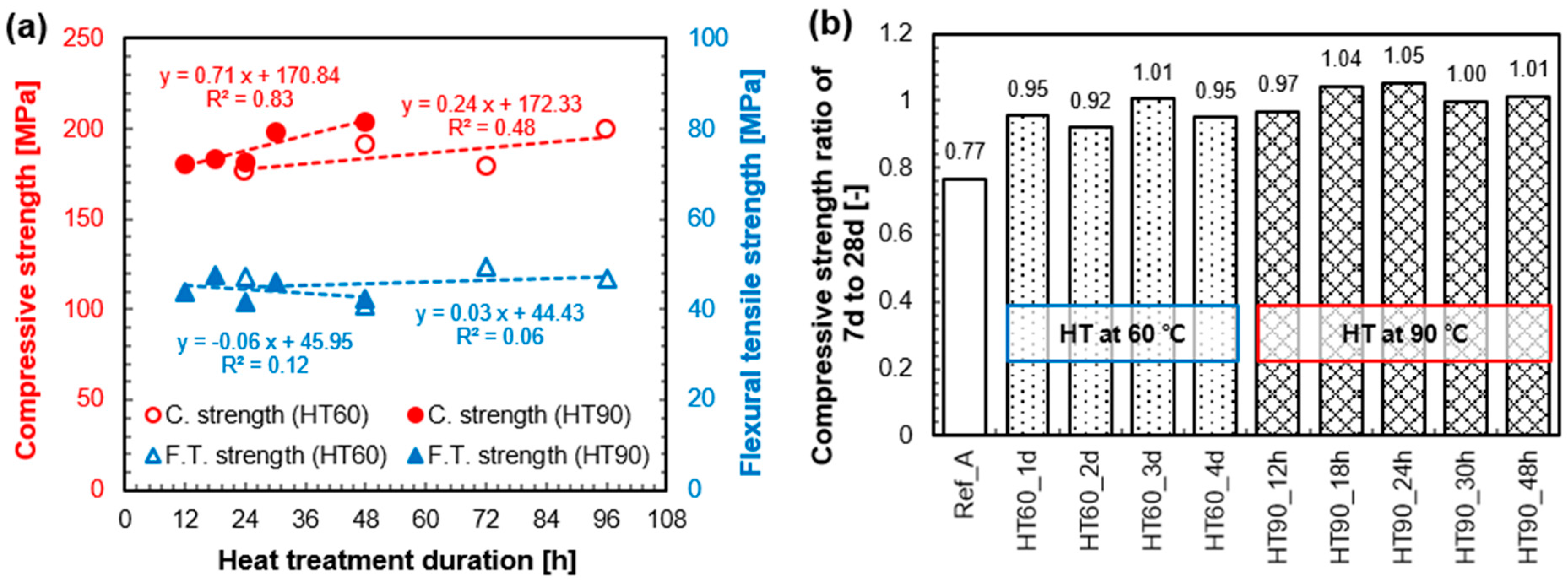
3.2. Mechanical Properties
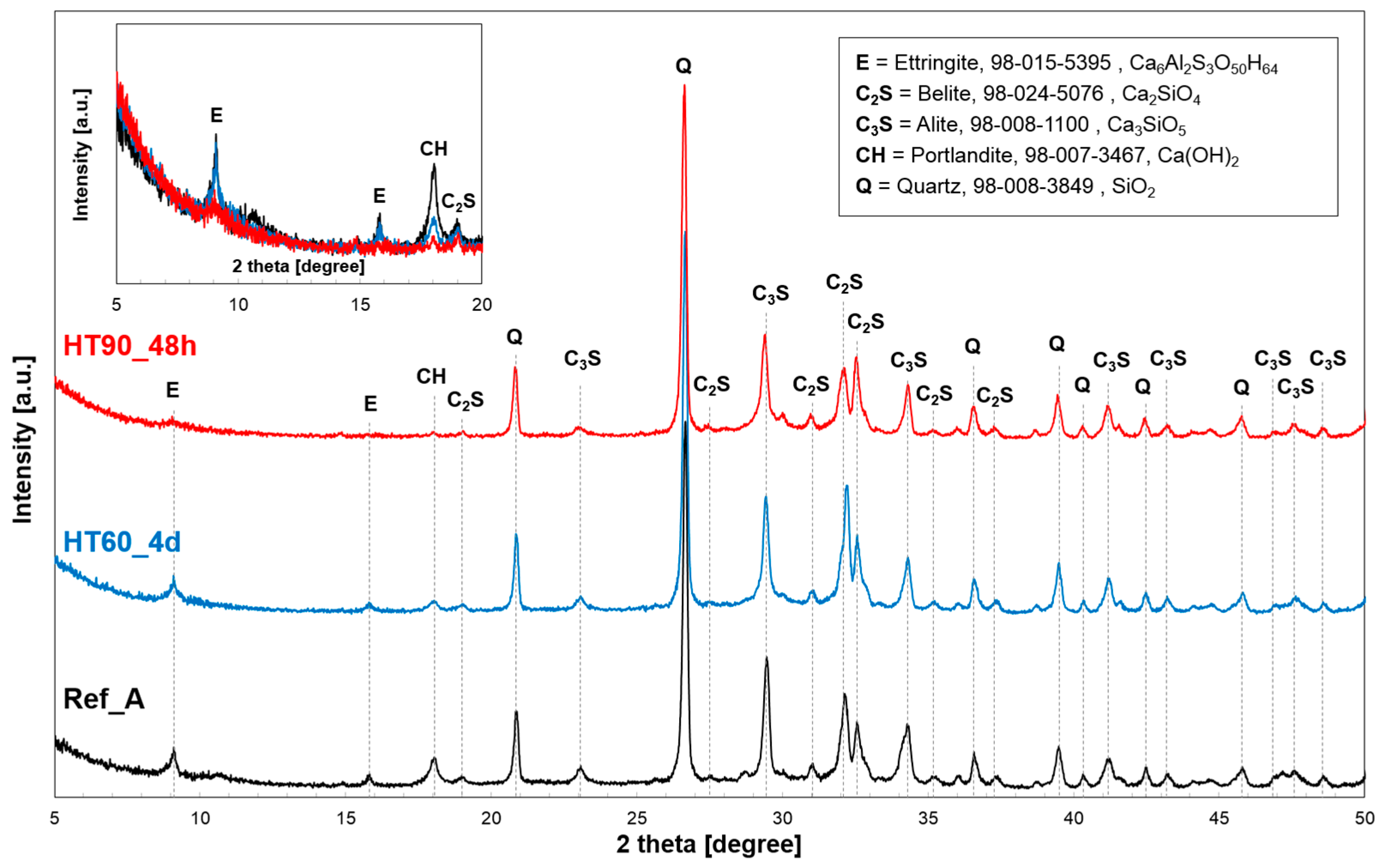
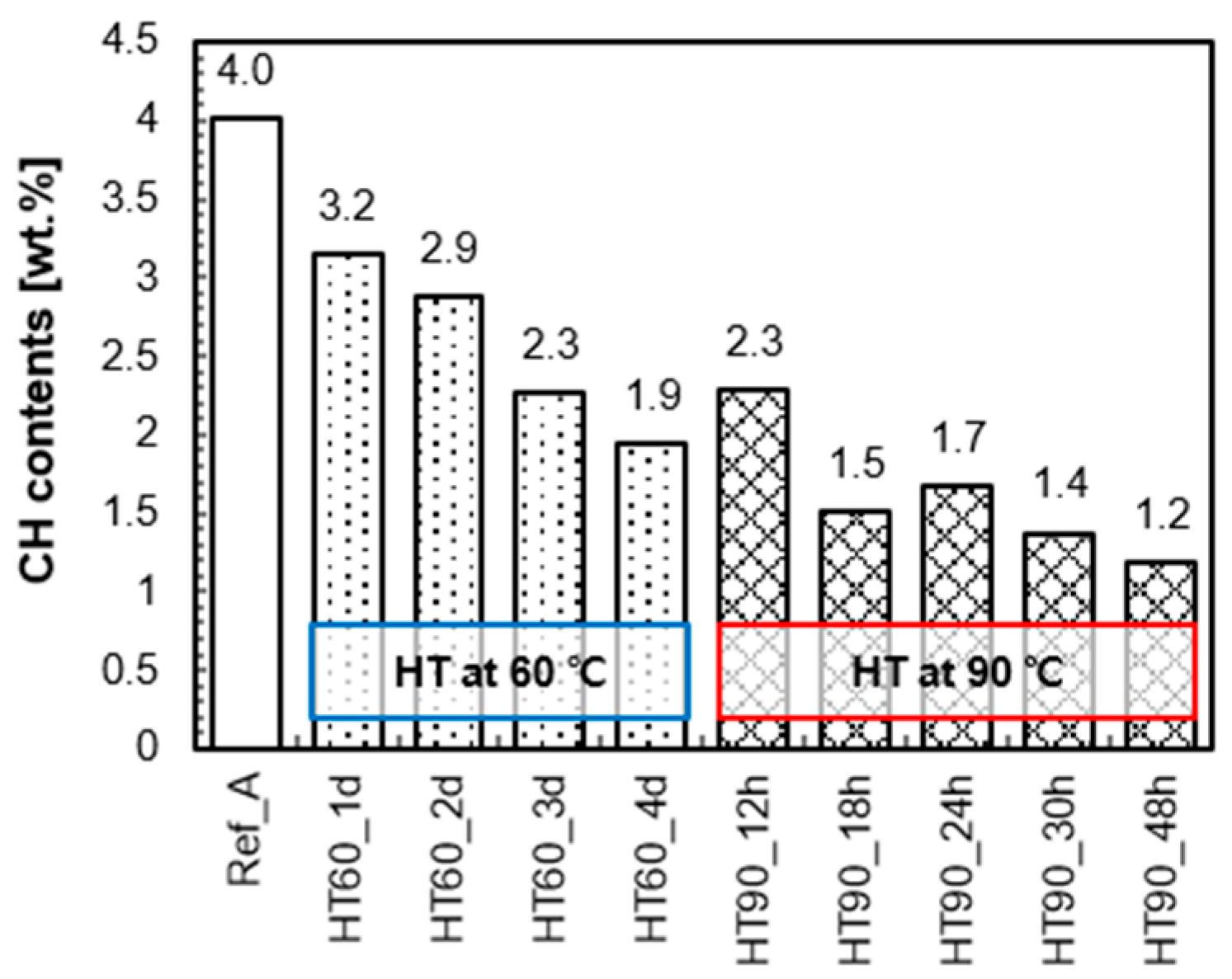
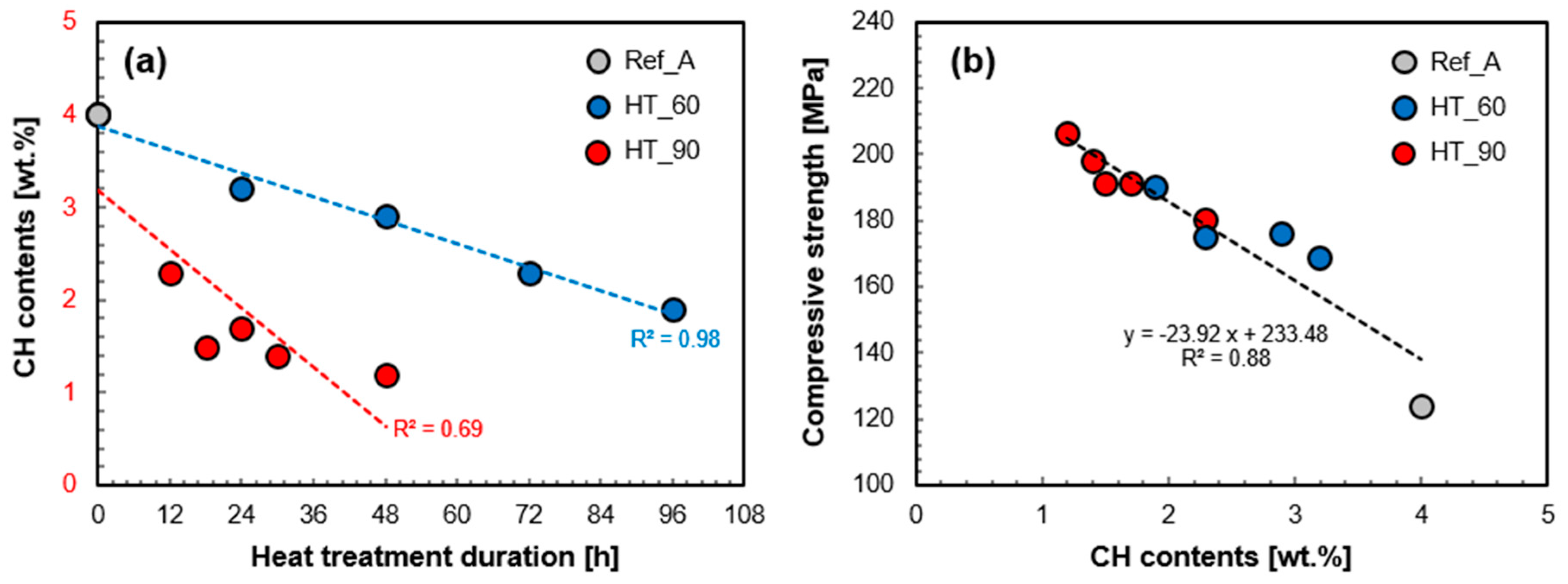
3.3. Mineralogical Analysis
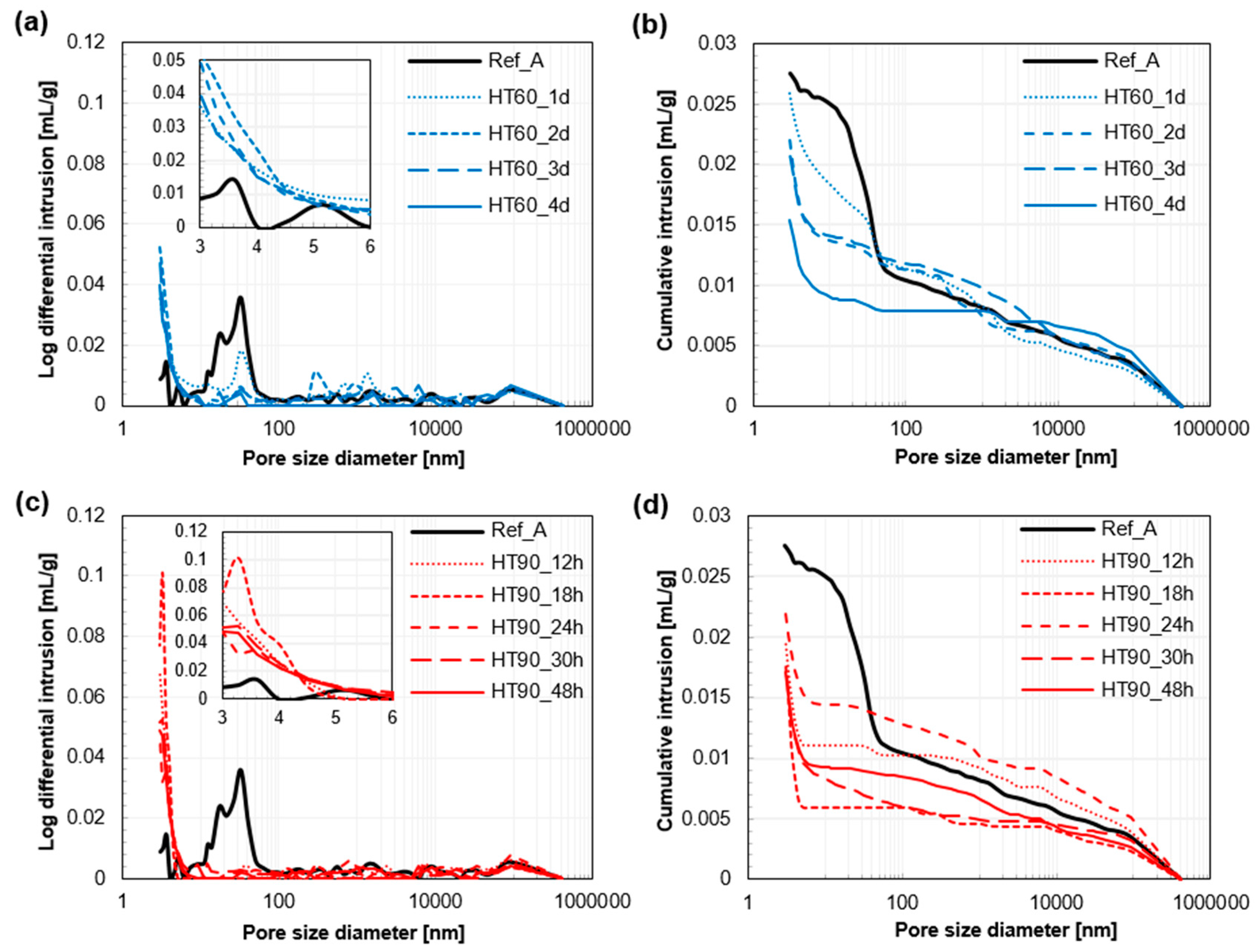
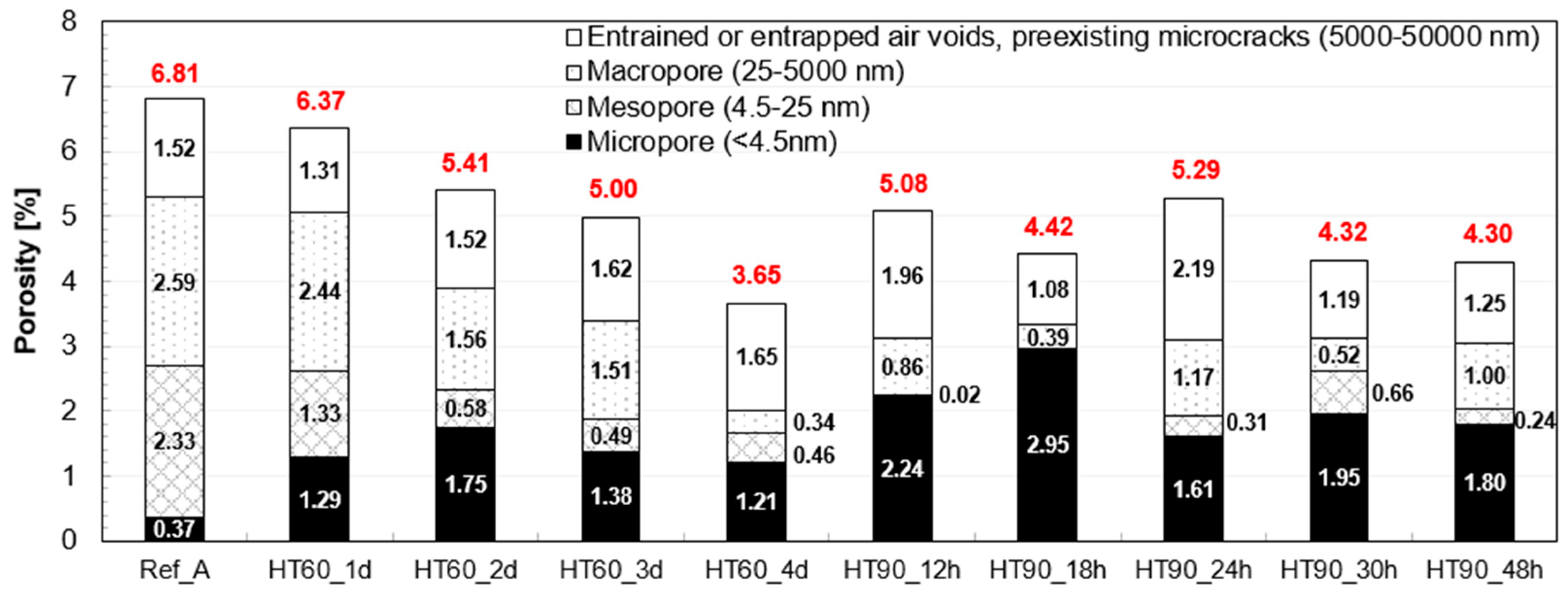
3.4. Pore Structure Analysis
4. Discussion
4.1. Pozzolanic Reaction of UHPC Subject to Heat Treatment
4.2. Benefits of Heat-Treatment on the Mechanical Properties of UHPC
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Association Française de Génie Civil (AFGC). Ultra High Performance Fibre-Reinforced Concrete-Recommendations; Revised edition; AFGC: Paris, France, 2013. [Google Scholar]
- Japan Society of Civil Engineers (JSCE). Recommendations for Design and Construction of Ultra High-Strength Fiber-Reinforced Concrete Structures-Draft; JSCE: Tokyo, Japan, 2004. (In Japanese) [Google Scholar]
- Korea Concrete Institute (KCI). Design Recommendations for K-UHPC; KCI: Seoul, Korea, 2012. (In Korean) [Google Scholar]
- Toutlemonde, F.; Delort, M. The newly enforced french standard for uhpfrc specification, performance, production, and conformity. In Ultra-High Performance Concrete and High Performance Construction Materials, Proceedings of the 4th International Symposium on Ultra-High Performance Concrete and High Performance Materials, Kassel, Germany, 9–11 March 2016; Kassel University: Kassel, Germany, 2016; pp. 55–56. [Google Scholar]
- National Precast Concrete Association (NPCA). Ultra High Performance Concrete (UHPC): Guide to Manufacturing Architectural Precast UHPC Elements; NPCA: Carmel, IN, USA, 2013; p. 19. [Google Scholar]
- Schachinger, I.; Hilbig, H.; Stengel, T. Effect of curing temperature at an early age on the long-term strength development of UHPC. In Ultra High Performance Concrete (UHPC), Proceedings of the 2nd International Symposium on Ultra High Performance Concrete, Kassel, Germany, 5–7 March 2008; Kassel University: Kassel, Germany, 2008; pp. 205–212. [Google Scholar]
- Selleng, C.; Meng, B.; Fontana, P. Phase composition and strength of thermally treated UHPC. In Ultra-High Performance Concrete and High Performance Construction Materials, Proceedings of the 4th International Symposium on Ultra-High Performance Concrete and High Performance Materials, Kassel, Germany, 9–11 March 2016; Kassel University: Kassel, Germany, 2016; pp. 7–8. [Google Scholar]
- Heinz, D.; Ludwig, H.-M. Heat treatment and the risk of DEF delayed ettringite formation in UHPC. In Ultra High Performance Concrete (UHPC), Proceedings of the 1st International Symposium on Ultra-High Performance Concrete Kassel, Germany, 13–15 September 2004; Kassel University: Kassel, Germany, 2004; pp. 717–730. [Google Scholar]
- Richard, P.; Cheyrezy, M. Composition of reactive powder concretes. Cem. Concr. Res. 1995, 25, 1501–1511. [Google Scholar] [CrossRef]
- Yazıcı, H.; Deniz, E.; Baradan, B. The effect of autoclave pressure, temperature and duration time on mechanical properties of reactive powder concrete. Constr. Build. Mater. 2013, 42, 53–63. [Google Scholar] [CrossRef]
- Yu, R.; Spiesz, P.; Brouwers, H. Development of an eco-friendly ultra-high performance concrete (UHPC) with efficient cement and mineral admixtures uses. Cem. Concr. Compos. 2015, 55, 383–394. [Google Scholar] [CrossRef]
- Huang, W.; Kazemi-Kamyab, H.; Sun, W.; Scrivener, K. Effect of cement substitution by limestone on the hydration and microstructural development of ultra-high performance concrete (UHPC). Cem. Concr. Compos. 2017, 77, 86–101. [Google Scholar] [CrossRef]
- Park, J.-J.; Yoo, D.-Y.; Park, G.-J.; Kim, S.-W. Feasibility of Reducing the Fiber Content in Ultra-High-Performance Fiber-Reinforced Concrete under Flexure. Materials 2017, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Kazemi-Kamyab, H.; Sun, W.; Scrivener, K. Effect of replacement of silica fume with calcined clay on the hydration and microstructural development of eco-UHPFRC. Mater. Des. 2017, 121, 36–46. [Google Scholar] [CrossRef]
- Yazıcı, H. The effect of curing conditions on compressive strength of ultra high strength concrete with high volume mineral admixtures. Build. Environ. 2007, 42, 2083–2089. [Google Scholar] [CrossRef]
- Afroughsabet, V.; Biolzi, L.; Ozbakkaloglu, T. High-performance fiber-reinforced concrete: A review. J. Mater. Sci. 2016, 51, 6517–6551. [Google Scholar] [CrossRef]
- Heinz, D.; Urbonas, L.; Gerlicher, T. Effect of heat treatment method on the properties of UHPC. In Ultra-High Performance Concrete and Nanotechnology in Construction, Proceedings of the 3rd International Symposium on Ultra High Performance Concrete and Nanotechnology for High Performance Construction Materials, Kassel, Germany, 7–9 March 2012; Kassel University: Kassel, Germany, 2012; pp. 283–290. [Google Scholar]
- Park, J.-S.; Kim, Y.J.; Cho, J.-R.; Jeon, S.-J. Early-age strength of ultra-high performance concrete in various curing conditions. Materials 2015, 8, 5537–5553. [Google Scholar] [CrossRef] [PubMed]
- Zanni, H.; Cheyrezy, M.; Maret, V.; Philippot, S.; Nieto, P. Investigation of hydration and pozzolanic reaction in reactive powder concrete (RPC) using 29 Si NMR. Cem. Concr. Res. 1996, 26, 93–100. [Google Scholar] [CrossRef]
- Reda, M.; Shrive, N.; Gillott, J. Microstructural investigation of innovative UHPC. Cem. Concr. Res. 1999, 29, 323–329. [Google Scholar] [CrossRef]
- Kang, S.-H.; Hong, S.-G.; Moon, J. Absorption kinetics of superabsorbent polymers (SAP) in various cement-based solutions. Cem. Concr. Res. 2017, 97, 73–83. [Google Scholar] [CrossRef]
- Kwon, Y.-H.; Kang, S.-H.; Hong, S.-G.; Moon, J. Acceleration of intended pozzolanic reaction under initial thermal treatment for developing cementless fly ash based mortar. Materials 2017, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-H.; Kang, S.-H.; Hong, S.-G.; Moon, J. Intensified pozzolanic reaction on kaolinite clay-based mortar. Appl. Sci. 2017, 7, 522. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in or [50-mm] Cube Specimens); ASTM C109/C109M; ASTM International: West Conshohocken, PA, USA, 2013; p. 10. [Google Scholar]
- International Organization for Standardization (ISO). Cement—Test Methods—Determination of Strength; ISO 679; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Belsky, A.; Hellenbrandt, M.; Karen, V.L.; Luksch, P. New developments in the Inorganic Crystal Structure Database (ICSD): Accessibility in support of materials research and design. Acta Crystallogr. Sect. B 2002, 58, 364–369. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Zajac, M. Hydration of quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cem. Concr. Compos. 2015, 55, 374–382. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, C.; Khayat, K.H. Influence of silica fume content on microstructure development and bond to steel fiber in ultra-high strength cement-based materials (UHSC). Cem. Concr. Compos. 2016, 71, 97–109. [Google Scholar] [CrossRef]
- Taylor, H.; Famy, C.; Scrivener, K. Delayed ettringite formation. Cem. Concr. Res. 2001, 31, 683–693. [Google Scholar] [CrossRef]
- Korpa, A.; Kowald, T.; Trettin, R. Phase development in normal and ultra high performance cementitious systems by quantitative X-ray analysis and thermoanalytical methods. Cem. Concr. Res. 2009, 39, 69–76. [Google Scholar] [CrossRef]
- Lee, H.-S.; Jang, H.-O.; Cho, K.-H. Evaluation of Bonding Shear Performance of Ultra-High-Performance Concrete with Increase in Delay in Formation of Cold Joints. Materials 2016, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials: I. Principles and theoretical background. Cem. Concr. Res. 2001, 31, 647–654. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J. Concrete: Microstructure, Properties and Materials; McGraw-Hill Publishing: London, UK, 2006. [Google Scholar]
- Garas, V.Y.; Kahn, L.F.; Kurtis, K.E. Short-term tensile creep and shrinkage of ultra-high performance concrete. Cem. Concr. Compos. 2009, 31, 147–152. [Google Scholar] [CrossRef]
- Pfeifer, C.; Moeser, B.; Weber, C.; Stark, J. Investigations of the pozzolanic reaction of silica fume in Ultra-high performance concrete (UHPC). In Proceedings of the International RILEM Conference on Material Science-MATSCI, Aachen, Germany, 6–8 September 2010; pp. 287–298. [Google Scholar]
- Justs, J.; Wyrzykowski, M.; Bajare, D.; Lura, P. Internal curing by superabsorbent polymers in ultra-high performance concrete. Cem. Concr. Res. 2015, 76, 82–90. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, C.; Khayat, K.H.; Wan, S. Effects of different nanomaterials on hardening and performance of ultra-high strength concrete (UHSC). Cem. Concr. Compos. 2016, 70, 24–34. [Google Scholar] [CrossRef]
- Abbas, S.; Soliman, A.M.; Nehdi, M.L. Exploring mechanical and durability properties of ultra-high performance concrete incorporating various steel fiber lengths and dosages. Constr. Build. Mater. 2015, 75, 429–441. [Google Scholar] [CrossRef]
- Zhou, B.; Uchida, Y. Influence of flowability, casting time and formwork geometry on fiber orientation and mechanical properties of UHPFRC. Cem. Concr. Res. 2017, 95, 164–177. [Google Scholar] [CrossRef]
- Abrishambaf, A.; Pimentel, M.; Nunes, S. Influence of fibre orientation on the tensile behaviour of ultra-high performance fibre reinforced cementitious composites. Cem. Concr. Res. 2017, 97, 28–40. [Google Scholar] [CrossRef]
- Choi, M.S.; Kang, S.-T.; Lee, B.Y.; Koh, K.-T.; Ryu, G.-S. Improvement in predicting the post-cracking tensile behavior of ultra-high performance cementitious composites based on fiber orientation distribution. Materials 2016, 9, 829. [Google Scholar] [CrossRef] [PubMed]
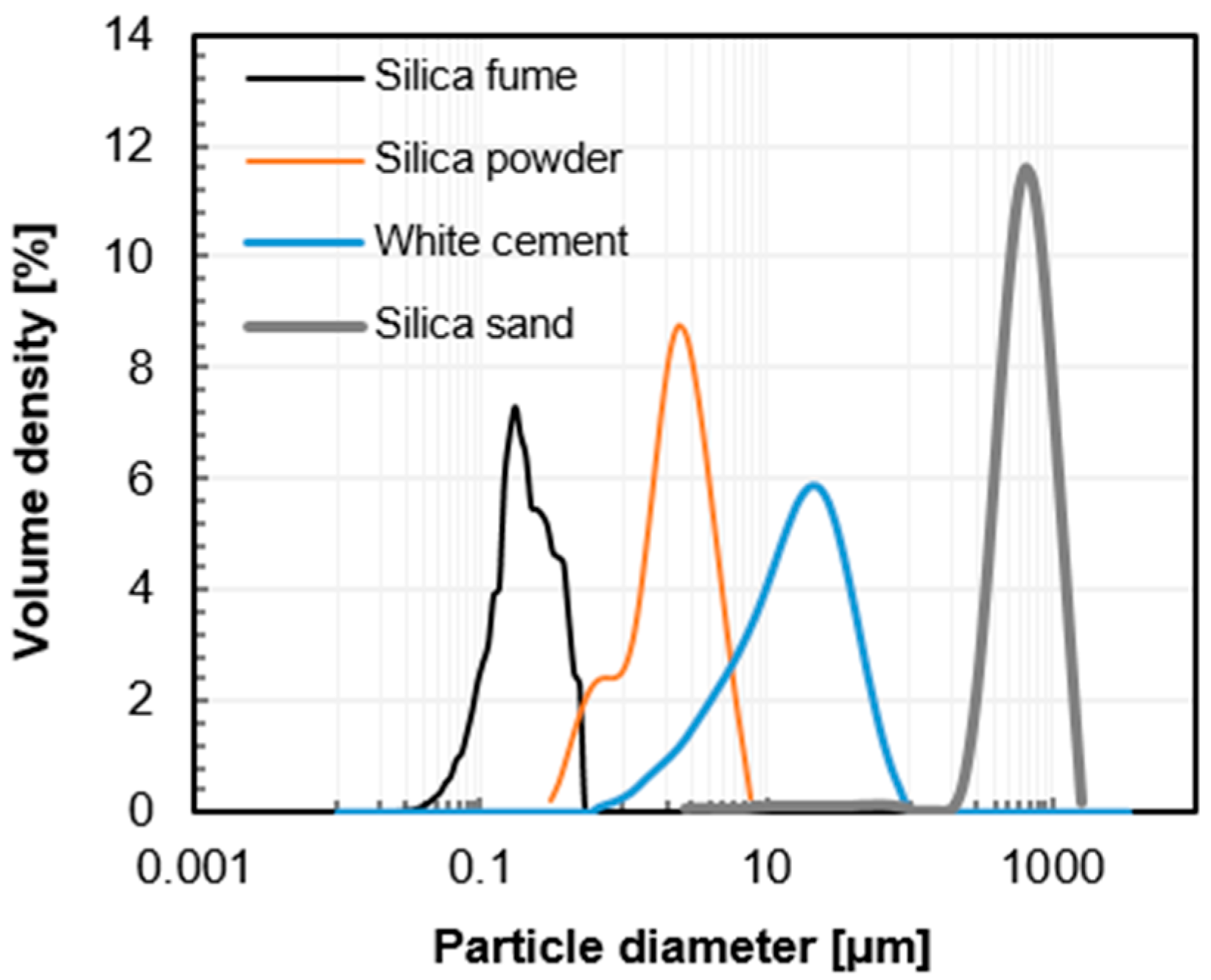
| Cement | Silica Fume | Silica Powder | Silica Sand | Water | Superplasticizer 1 | Steel Fiber [vol %] 2 |
|---|---|---|---|---|---|---|
| 1 | 0.25 | 0.35 | 1.1 | 0.253 | 0.012 | 2 |
| Chemical Composition | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | K2O | SO3 | SrO | LOI 1 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| White cement | 21.4 | 4.63 | 0.358 | 1.27 | 65.8 | 0.13 | 2.89 | 0.076 | 3.4 | 99.95 |
| Silica fume | 96.90 | 0.29 | 0.15 | 0.18 | 1.54 | 0.64 | - | - | 0.02 | 99.72 |
| Silica powder | 97.70 | 0.49 | 0.05 | 0.21 | 1.37 | 0.02 | - | - | 0.02 | 99.86 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-H.; Lee, J.-H.; Hong, S.-G.; Moon, J. Microstructural Investigation of Heat-Treated Ultra-High Performance Concrete for Optimum Production. Materials 2017, 10, 1106. https://doi.org/10.3390/ma10091106
Kang S-H, Lee J-H, Hong S-G, Moon J. Microstructural Investigation of Heat-Treated Ultra-High Performance Concrete for Optimum Production. Materials. 2017; 10(9):1106. https://doi.org/10.3390/ma10091106
Chicago/Turabian StyleKang, Sung-Hoon, Ji-Hyung Lee, Sung-Gul Hong, and Juhyuk Moon. 2017. "Microstructural Investigation of Heat-Treated Ultra-High Performance Concrete for Optimum Production" Materials 10, no. 9: 1106. https://doi.org/10.3390/ma10091106




