Precipitation Behavior and Quenching Sensitivity of a Spray Deposited Al-Zn-Mg-Cu-Zr Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
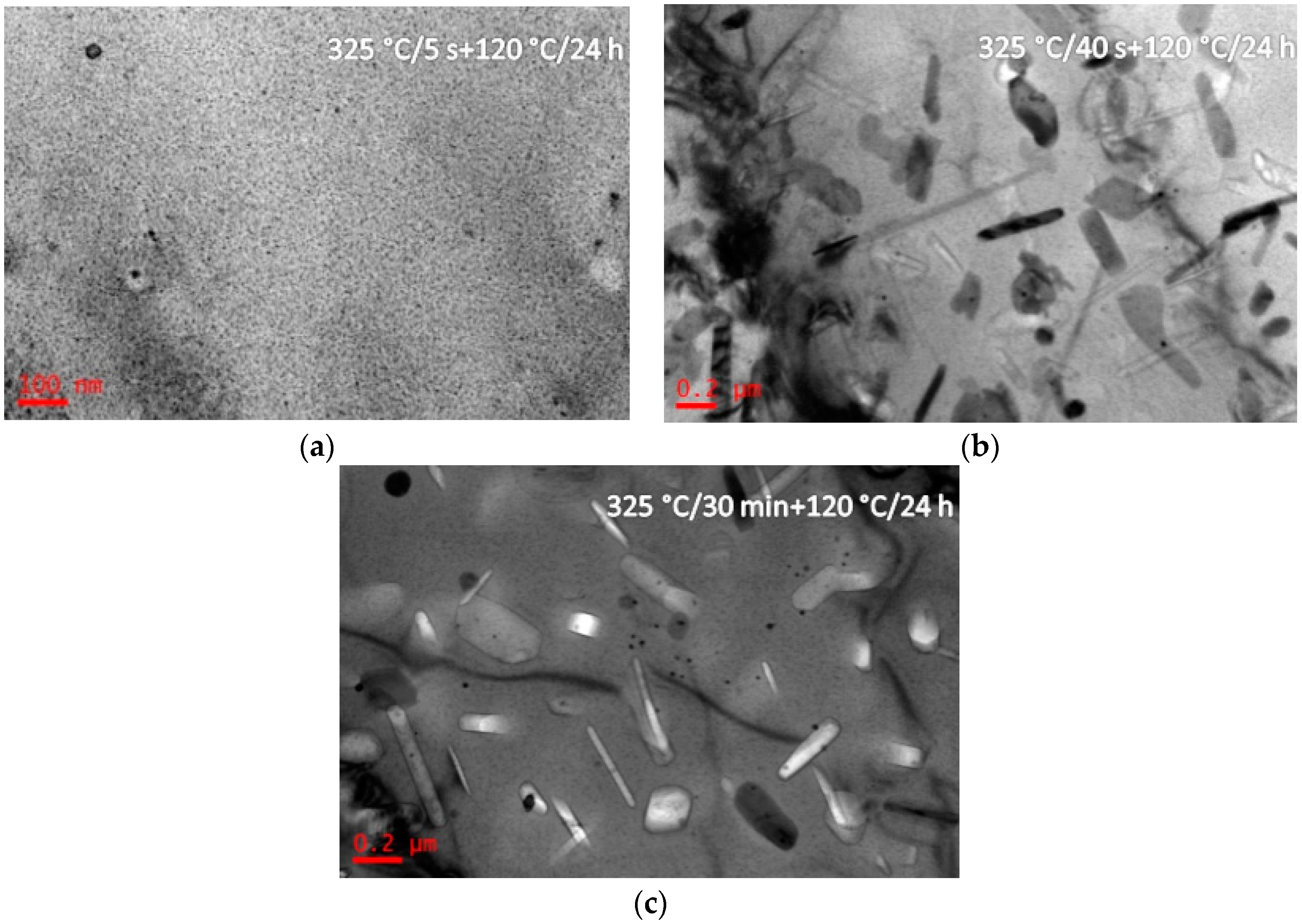
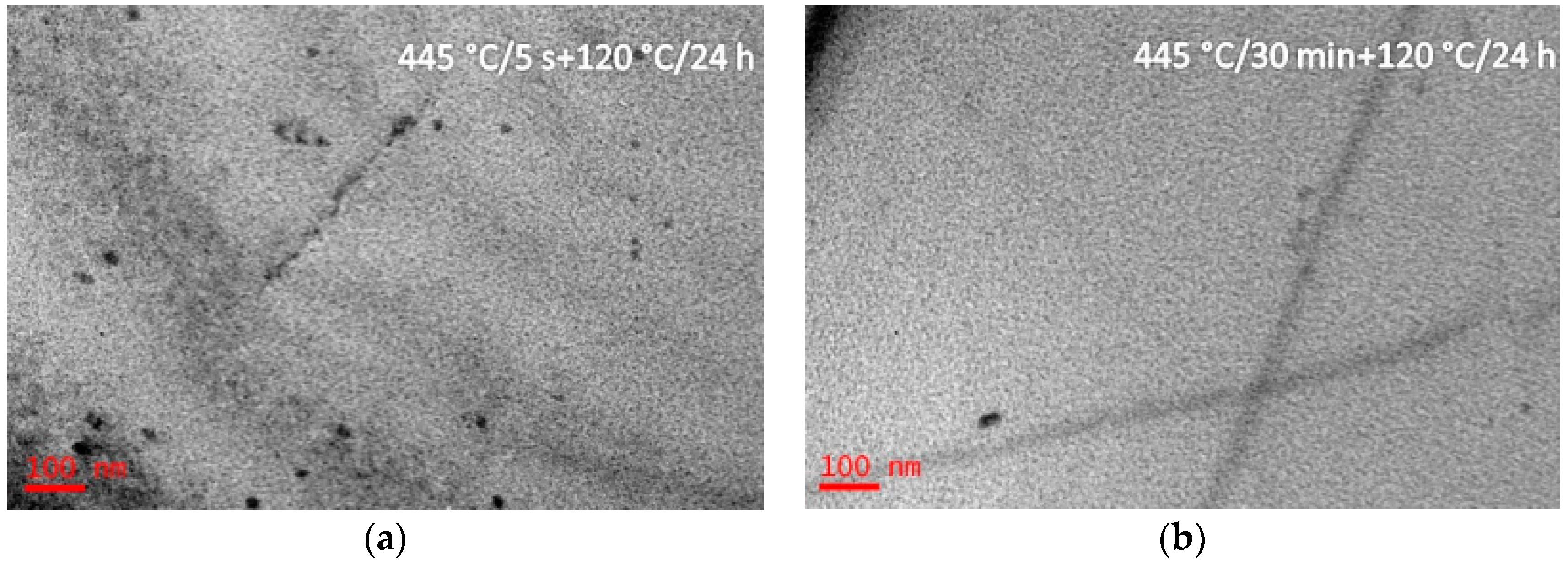
3.1. Microstructure Evolutions during Isothermal Heat Treatment and Aging
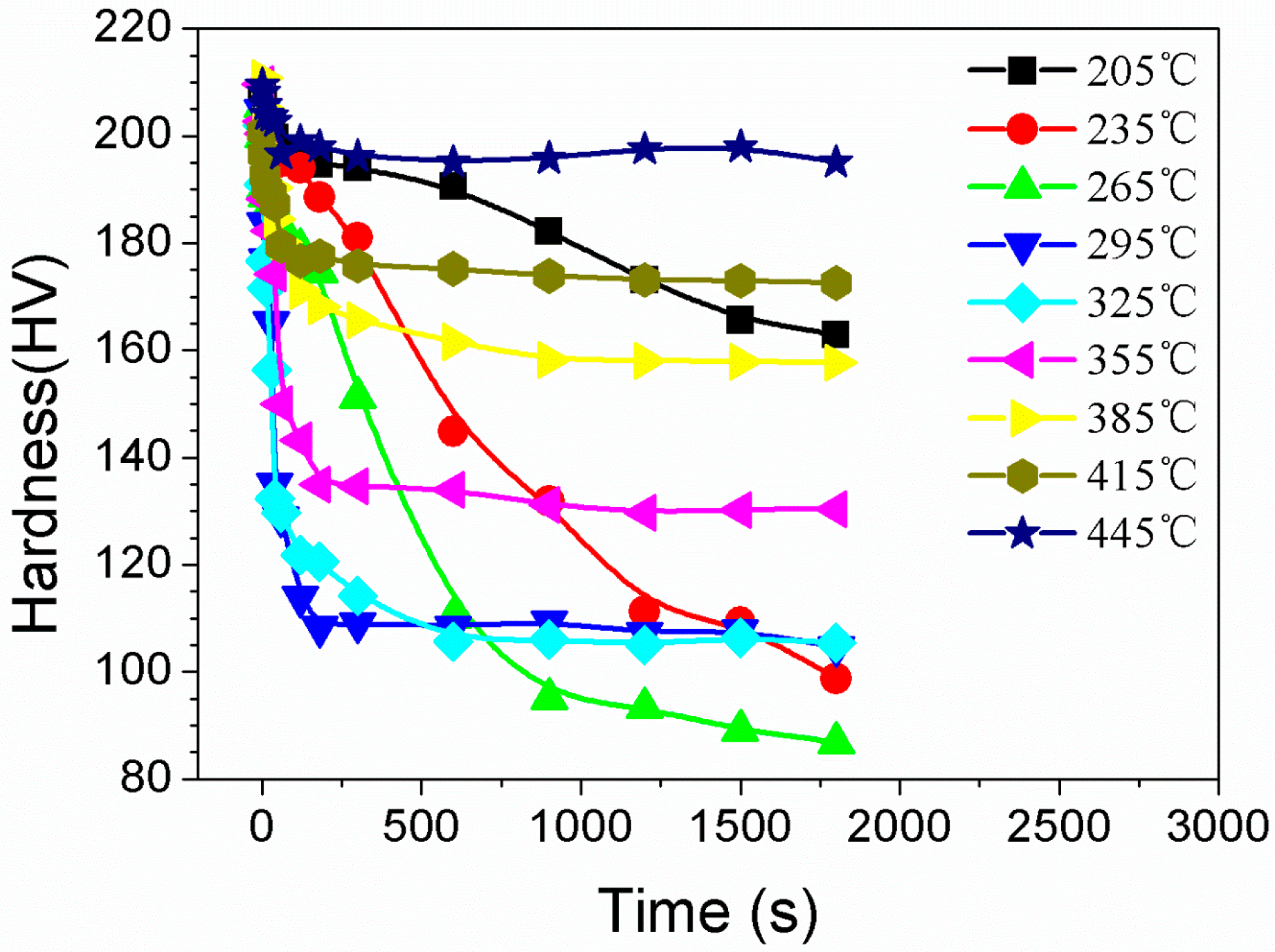
3.2. Hardness Variation
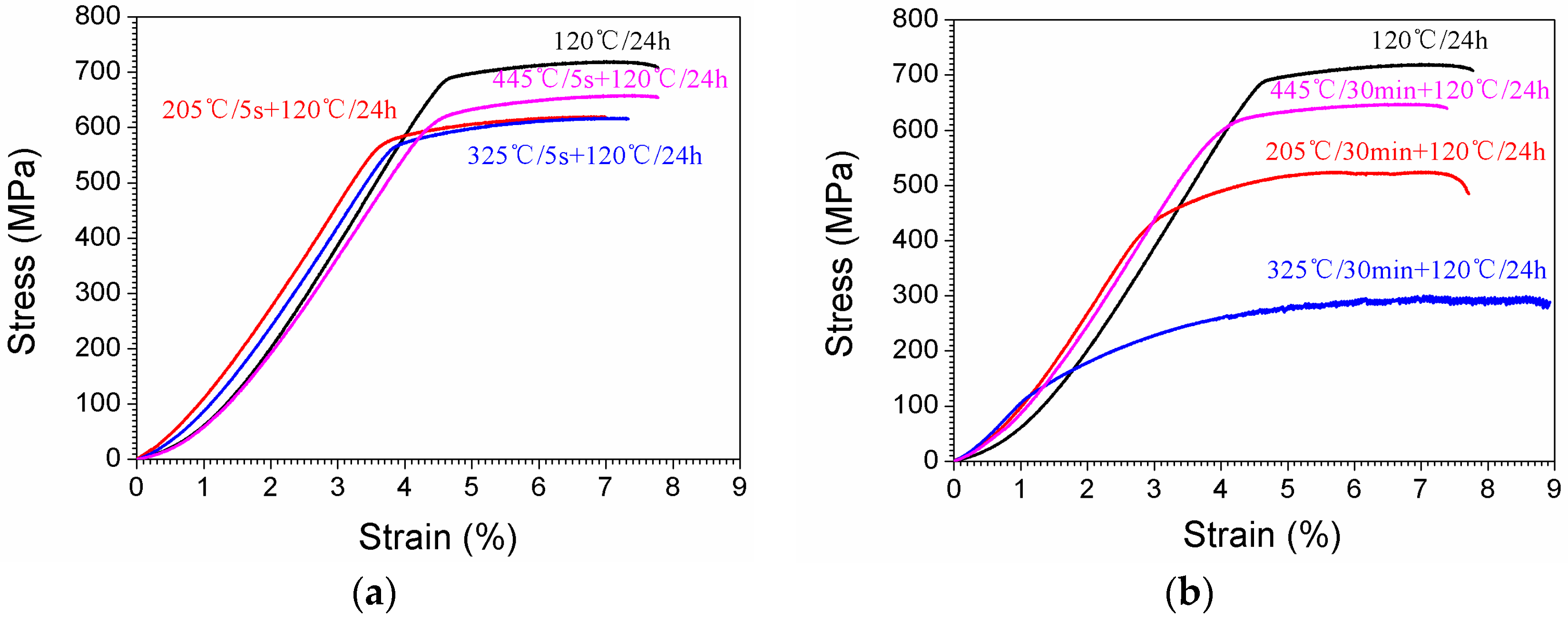
3.3. Stress-Strain Curves
4. Discussion
4.1. The Quenching Sensitivity
4.2. Precipitation Behavior during Isothermal Heat Treatment
4.3. Influence of Precipitates Size on the Strengthening Response
5. Conclusions
- (1)
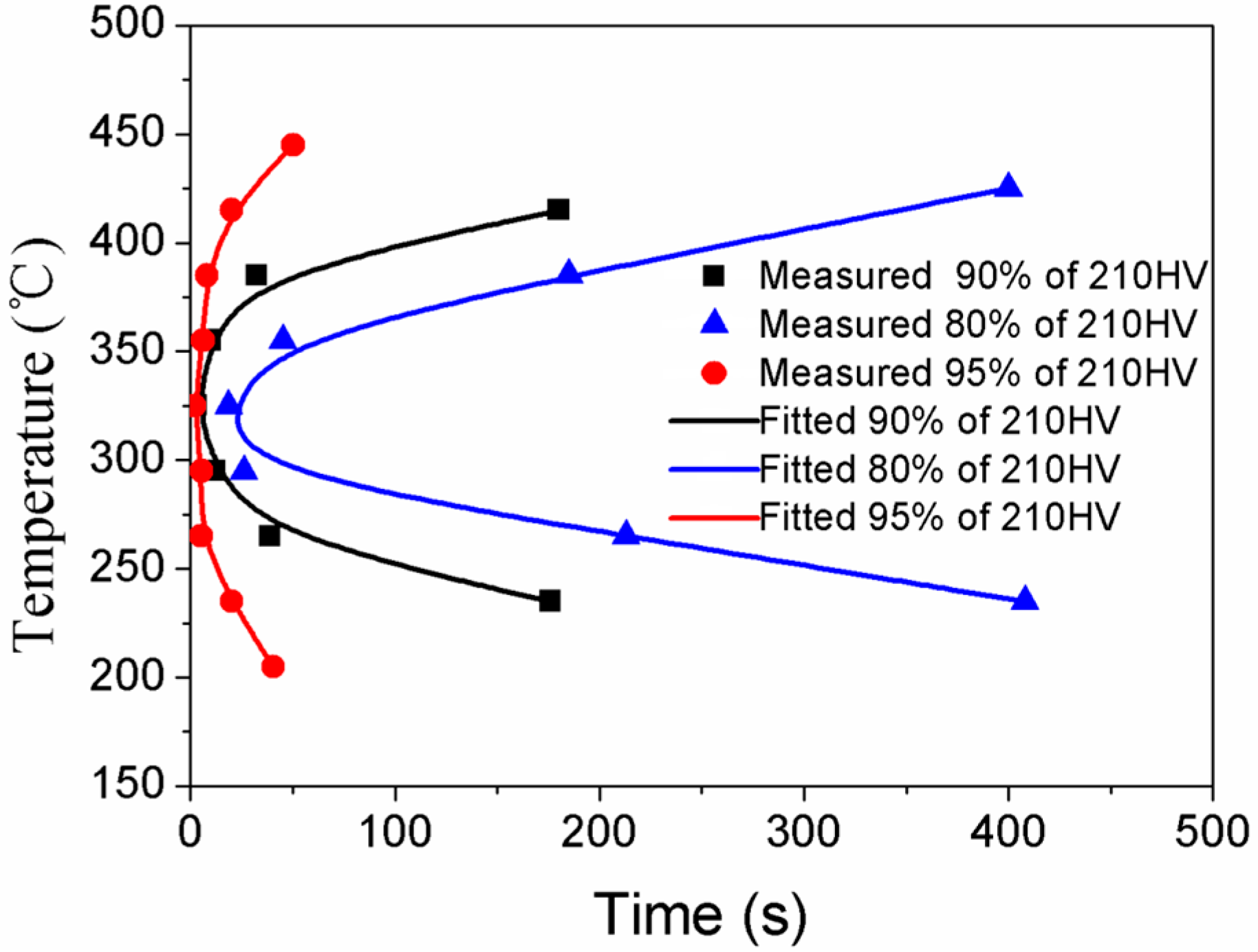
- The hardness and the ultimate tensile strength decreased with the isothermal heat treatment time. TTP curves of the studied alloy have been established, and the mechanism for high quenching sensitivity at the nose temperature zone has been discussed. TTP curves of the studied alloy are “C”-like ones, and the nose-tip temperatures of these curves are around 325 °C.
- (2)
- The studied alloy presented a high quenching sensitivity at the medium-temperature zone (250–400 °C) because in this medium temperature zone the nucleation rate of the precipitates was high and a significant number of precipitates formed. After solid solution treatment, precipitation appeared scarcely as specimens had been isothermal heat treated at the low-temperature zone (<250 °C), and the high-temperature zone (>400 °C), both of above two temperature zones were available for the industry production.
- (3)
- The strengthening phase would precipitate as the sample aged at 120 °C, resulting in high mechanical properties. Precipitates with size of 10 nm would contribute a significant increase in yield strength, while ones larger than 300 nm contribute only little.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huo, W.T.; Shi, J.T.; Hou, L.G.; Zhang, J.S. An improved thermo-mechanical treatment of high-strength Al-Zn-Mg-Cu alloy for effective grain refinement and ductility modification. J. Mater. Process. Technol. 2017, 239, 303–314. [Google Scholar] [CrossRef]
- Subroto, T.; Miroux, A.; Eskin, D.G.; Katgerman, L. Tensile mechanical properties, constitutive parameters and fracture characteristics of an as-cast AA7050 alloy in the near-solidus temperature regime. Mater. Sci. Eng. A 2017, 679, 28–35. [Google Scholar] [CrossRef]
- Shi, C.J.; Lai, J.; Chen, X.G. Microstructural evolution and dynamic softening mechanisms of Al-Zn-Mg-Cu alloy during hot compressive deformation. Materials 2014, 7, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, F.; Zhu, M.L. Study on Mg/Al weld seam based on Zn-Mg-Al ternary alloy. Materials 2014, 7, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.L.; Zurob, H.S.; Purdy, G.R.; Zhang, H. Characterizing precipitate evolution of an Al-Zn-Mg-Cu-based commercial alloy during artificial aging and non-isothermal heat treatments by in situ electrical resistivity monitoring. Mater. Charact. 2016, 117, 47–56. [Google Scholar] [CrossRef]
- Viscusi, A.; Leitão, C.; Rodrigues, D.M.; Scherillo, F.; Squillace, A.; Carrino, L. Laser beam welded joints of dissimilar heat treatable aluminum alloys. J. Mater. Process. Technol. 2016, 236, 48–55. [Google Scholar] [CrossRef]
- Xia, S.H.; Vychigzhanina, L.V.; Wang, J.T.; Alexandrov, I.V.; Sharafutdinov, A.V. Controllable bimodal structures in hypo-eutectoid Cu-Al alloy for both high strength and tensile ductility. Mater. Sci. Eng. A 2008, 490, 471–476. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.Y.; Nie, Z.; Huang, H.; Sun, J.T. Microstructure and mechanical properties of a new Al-Zn-Mg-Cu alloy joints welded by laser beam. Mater. Des. 2015, 83, 451–458. [Google Scholar] [CrossRef]
- Fang, H.C.; Luo, F.H.; Chen, K.H. Effect of intermetallic phases and recrystallization on the corrosion and fracture behavior of an Al-Zn-Mg-Cu-Zr-Yb-Cr alloy. Mater. Sci. Eng. A 2017, 684, 480–490. [Google Scholar] [CrossRef]
- Dong, J.; Cui, J.Z.; Yu, F.X.; Zhao, Z.H.; Zhuo, Y.B. A new way to cast high-alloyed Al-Zn-Mg-Cu-Zr for super-high strength and toughness. J. Mater. Process. Technol. 2006, 171, 399–404. [Google Scholar] [CrossRef]
- Roy, S.; Nataraj, B.R.; Suwas, S.; Kumar, S.; Chattopadhyay, K. Accumulative roll bonding of aluminum alloys 2219/5086 laminates: Microstructural evolution and tensile properties. Mater. Des. 2012, 36, 529–539. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, N.; Sha, G.; Ringer, S.P.; Starink, M.J. Strengthening of an Al-Cu-Mg alloy processed by high-pressure torsion due to clusters, defects and defect-cluster complexes. Mater. Sci. Eng. A 2015, 627, 10–20. [Google Scholar] [CrossRef]
- Shaeri, M.H.; Salehi, M.T.; Seyyedein, S.H.; Abutalebi, M.R.; Park, J.K. Microstructure and mechanical properties of Al-7075 alloy processed by equal channel angular pressing combined with aging treatment. Mater. Des. 2014, 57, 250–257. [Google Scholar] [CrossRef]
- Sun, N.; Apelian, D. Friction stir processing of aluminum cast alloys for high performance applications. J. Miner. Met. Mater. Soc. 2011, 63, 44–50. [Google Scholar] [CrossRef]
- Naeem, H.T.; Mohammed, K.S.; Ahmad, K.R. Effect of friction stir processing on the microstructure and hardness of an aluminum-zinc-magnesium-copper alloy with nickel additives. Phys. Met. Metallogr. 2015, 116, 1035–1046. [Google Scholar] [CrossRef]
- Li, Y.; Koizumi, Y.; Chiba, A. Dynamic recrystallization behavior of biomedical Co-29Cr-6Mo-0.16N alloy with extremely low stacking fault energy. Mater. Sci. Eng. A 2016, 668, 48–54. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Koizumi, Y.; Chiba, A. Dynamic recrystallization behavior of biomedical Co-29Cr-6Mo-0.16N alloy. Mater. Charact. 2016, 118, 50–56. [Google Scholar] [CrossRef]
- Feng, W.; Xiong, B.Q.; Zhang, Y.G.; Liu, H.W.; He, X.Q.; He, H.W.; He, X.Q. Microstructural development of spray-deposited Al-Zn-Mg-Cu alloy during subsequent processing. J. Alloys Compd. 2009, 477, 616–621. [Google Scholar] [CrossRef]
- Jia, Y.D.; Cao, F.Y.; Guo, S.; Ma, P.; Liu, J.S.; Sun, J.F. Hot deformation behavior of spray-deposited Al-Zn-Mg-Cu alloy. Mater. Des. 2014, 53, 79–85. [Google Scholar] [CrossRef]
- Azimi, A.; Shokuhfar, A.; Zolriasatein, A. Nanostructured Al-Zn-Mg-Cu-Zr alloy prepared by mechanical alloying followed by hot pressing. Mater. Sci. Eng. A 2014, 595, 124–130. [Google Scholar] [CrossRef]
- Yan, L.M.; Shen, J.; Li, Z.B.; Li, J.P.; Yan, X.D. Microstructure evolution of Al-Zn-Mg-Cu-Zr alloy during hot deformation. Rare Met. 2010, 29, 426–432. [Google Scholar] [CrossRef]
- Li, F.X.; Liu, Y.Z.; Yi, J.H. Microstructural evolution of gas atomized Al-Zn-Mg-Cu-Zr powders during semi-solid rolling process. Trans. Nonferrous Met. Soc. China 2014, 24, 2475–2481. [Google Scholar] [CrossRef]
- Mathur, P.; Apelian, D.; Lawley, A. Analysis of the spray deposition process. Acta Metall. 1989, 37, 429–443. [Google Scholar] [CrossRef]
- Li, H.C.; Cao, F.Y.; Guo, S.; Ning, Z.L.; Liu, Z.Y.; Jia, Y.D.; Scudino, S.; Gemming, T.; Sun, J.F. Microstructures and properties evolution of spray-deposited Al-Zn-Mg-Cu-Zr alloys with scandium addition. J. Alloys Compd. 2017, 691, 482–488. [Google Scholar] [CrossRef]
- Liu, B.; Lei, Q.; Xie, L.Q.; Wang, M.P.; Li, Z. Microstructure and mechanical properties of high product of strength and elongation Al-Zn-Mg-Cu-Zr alloys fabricated by spray deposition. Mater. Des. 2016, 96, 217–223. [Google Scholar] [CrossRef]
- Yu, H.C.; Wang, M.P.; Sheng, X.F.; Li, Z.; Chen, L.B.; Lei, Q.; Chen, C.; Jia, Y.L.; Xiao, Z.; Chen, W.; et al. Microstructure and tensile properties of large-size 7055 aluminum billets fabricated by spray forming rapid solidification technology. J. Alloys Compd. 2013, 578, 208–214. [Google Scholar] [CrossRef]
- Dorward, R.C.; Beerntsen, D.J. Grain structure and quench-rate effects on strength and toughness of AA7050 Al-Zn-Mg-Cu-Zr alloy plate. Metall. Mater. Trans. A 1995, 26, 2481–2484. [Google Scholar] [CrossRef]
- Sharma, M.M.; Amateau, M.F.; Eden, T.J. Mesoscopic structure control of spray formed high strength Al-Zn-Mg-Cu alloys. Acta Mater. 2005, 53, 2919–2924. [Google Scholar] [CrossRef]
- Liu, S.D.; Zhang, X.M.; Chen, M.A.; You, J.H.; Zhang, X.Y. Effect of Zr content on quench sensitivity of AIZnMgCu alloys. Trans. Nonferrous Met. Soc. China 2007, 17, 787–792. [Google Scholar] [CrossRef]
- Cai, Y.H.; Liang, R.G.; Su, Z.P.; Zhang, J.S. Microstructure of spray formed Al-Zn-Mg-Cu alloy with Mn addition. Trans. Nonferrous Met. Soc. China 2011, 21, 9–14. [Google Scholar] [CrossRef]
- Shen, L.N.; Li, Z.; Dong, Q.Y.; Xiao, Z.; Li, S.; Lei, Q. Microstructure evolution and quench sensitivity of Cu-10Ni-3Al-0.8 Si alloy during isothermal treatment. J. Mater. Res. 2015, 30, 736–744. [Google Scholar] [CrossRef]
- Sheng, X.F.; Lei, Q.; Xiao, Z.; Wang, M. Hot deformation behavior of a spray deposited Al-8.31Zn-2.07Mg-2.46Cu-0.12Zr alloy. Metals 2017, 7, 299. [Google Scholar] [CrossRef]
- Lia, X.Z.; Hansena, V.; Gjonnesa, J.; Wallenbergb, L.R. HREM study and structure modeling of the η′ phase, the hardening precipitates in commercial Al-Zn-Mg alloys. Acta Mater. 1999, 47, 2651–2659. [Google Scholar] [CrossRef]
- Liu, J.Z.; Chen, J.H.; Yang, X.B.; Ren, S.; Wu, C.L.; Xu, H.Y.; Zou, J. Revisiting the precipitation sequence in Al-Zn-Mg-based alloys by high-resolution transmission electron microscopy. Scr. Mater. 2010, 63, 1061–1064. [Google Scholar] [CrossRef]
- Totten, G.E.; MacKenzie, D.S. Handbook of Aluminum; Marcel Dekker Inc.: New York, BY, USA, 2003; Volume 1, pp. 287–288. ISBN 0824704940. [Google Scholar]
- Lityńska-Dobrzyńska, L.; Dutkiewicz, J.; Maziarz, W.; Góral, A. Microstructure of rapidly solidified Al-12Zn-3Mg-1.5Cu alloy with Zr and Sc additions. Mater. Trans. 2011, 52, 309–314. [Google Scholar] [CrossRef]
- Robinson, J.S.; Cudd, R.L.; Tanner, D.A.; Dolan, G.P. Quench sensitivity and tensile property inhomogeneity in 7010 forgings. J. Mater. Process. Technol. 2001, 119, 261–267. [Google Scholar] [CrossRef]
- Evancho, J.W.; Staley, J.T. Kinetics of precipitation in aluminum alloys during continuous cooling. Metall. Trans. 1974, 5, 43–47. [Google Scholar]
- Archambaulta, P.; Godard, D. High temperature precipitation kinetics and ttt curve of a 7xxx alloy by in-situ electrical resistivity measurements and differential calorimetry. Scr. Mater. 2000, 42, 675–680. [Google Scholar] [CrossRef]
- Nie, B.; Liu, P.; Zhou, T.; Xie, Y.J. Evaluation of the quenching sensitivity of Al-Zn-Mg-Cu-Zr aluminum alloys by mole fraction of equilibrium phases. In Proceedings of the 13th International Conference on Aluminum Alloys (ICAA13), Pittsburgh, PA, USA, 3–7 June 2012; Weiland, H., Rollett, A.D., Cassada, W.A., Eds.; Springer: Cham, Switzerland, 2012; pp. 331–336. [Google Scholar] [CrossRef]
- Liu, S.D.; Zhong, Q.M.; Zhang, Y.; Liu, W.J.; Zhang, X.M.; Deng, Y.L. Investigation of quench sensitivity of high strength Al-Zn-Mg-Cu alloys by time-temperature-properties diagrams. Mater. Des. 2010, 31, 3116–3120. [Google Scholar] [CrossRef]
- Deng, Y.L.; Wan, L.; Zhang, Y.Y.; Zhang, X.M. Influence of Mg content on quench sensitivity of Al-Zn-Mg-Cu aluminum alloys. J. Alloys Compd. 2011, 509, 4636–4642. [Google Scholar] [CrossRef]
- Du, Z.W.; Sun, Z.M.; Shao, B.L.; Zhou, T.T.; Chen, C.Q. Quantitative evaluation of precipitates in an Al-Zn-Mg-Cu alloy after isothermal aging. Mater. Charact. 2006, 56, 121–128. [Google Scholar] [CrossRef]
- Yang, X.B.; Chen, J.H.; Liu, J.Z.; Liu, P.; Qin, F.; Cheng, Y.L.; Wu, C.L. Spherical constituent particles formed by a multistage solution treatment in Al-Zn-Mg-Cu alloys. Mater. Charact. 2013, 83, 79–88. [Google Scholar] [CrossRef]
- Yang, W.C.; Ji, S.X.; Zhang, Q.; Wang, M.P. Investigation of mechanical and corrosion properties of an Al-Zn-Mg-Cu alloy under various ageing conditions and interface analysis of η′ precipitate. Mater. Des. 2015, 85, 752–761. [Google Scholar] [CrossRef]
- Yang, W.C.; Ji, S.X.; Wang, M.P.; Li, Z. Precipitation behavior of Al-Zn-Mg-Cu alloy and diffraction analysis from η′ precipitates in four variants. J. Alloys Compd. 2014, 610, 623–629. [Google Scholar] [CrossRef]
- Cao, L.F.; Wang, M.P.; Guo, M.X.; Li, Z. Heat treatment of modified 6005A alloy for vehicles. Trans. Mater. Heat Treat. 2004, 25, 619–622. [Google Scholar]
- Yu, H.C.; Wang, M.P.; Jia, Y.L.; Xiao, Z.; Chen, C.; Lei, Q.; Li, Z.; Chen, W.; Zhang, H.; Wang, Y.G.; et al. High strength and large ductility in spray-deposited Al-Zn-Mg-Cu alloys. J. Alloys Compd. 2014, 601, 120–125. [Google Scholar] [CrossRef]
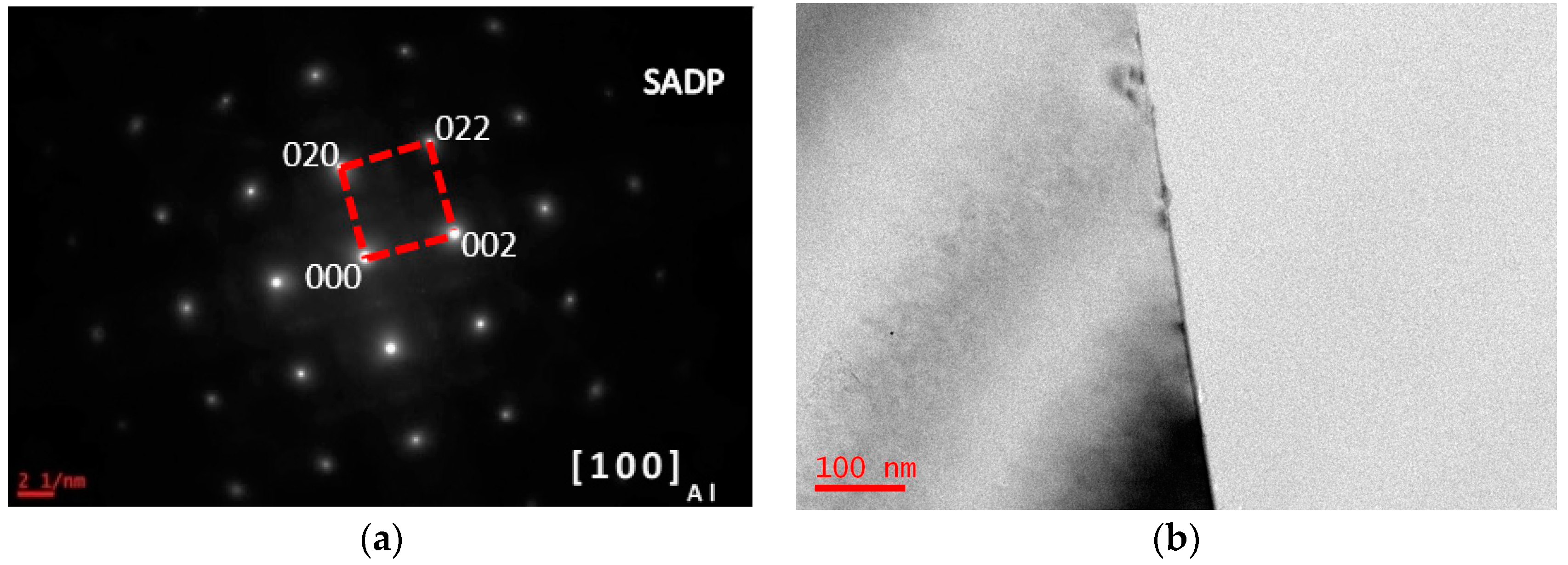
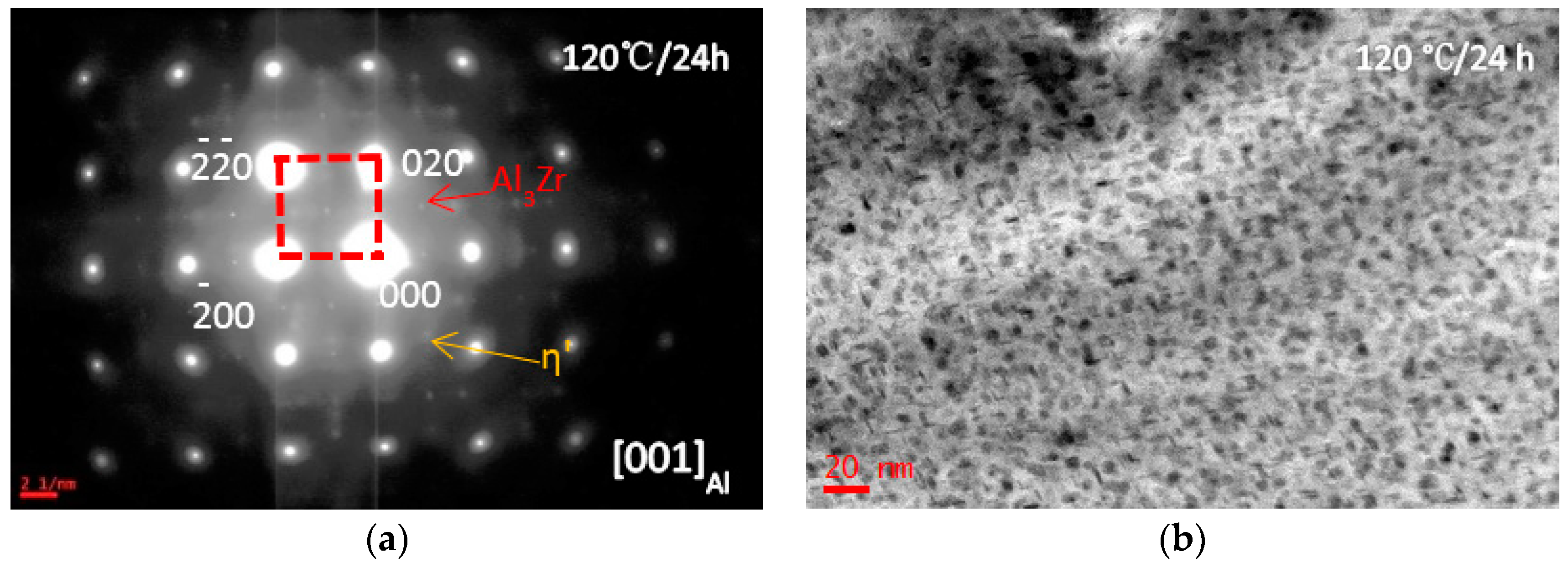
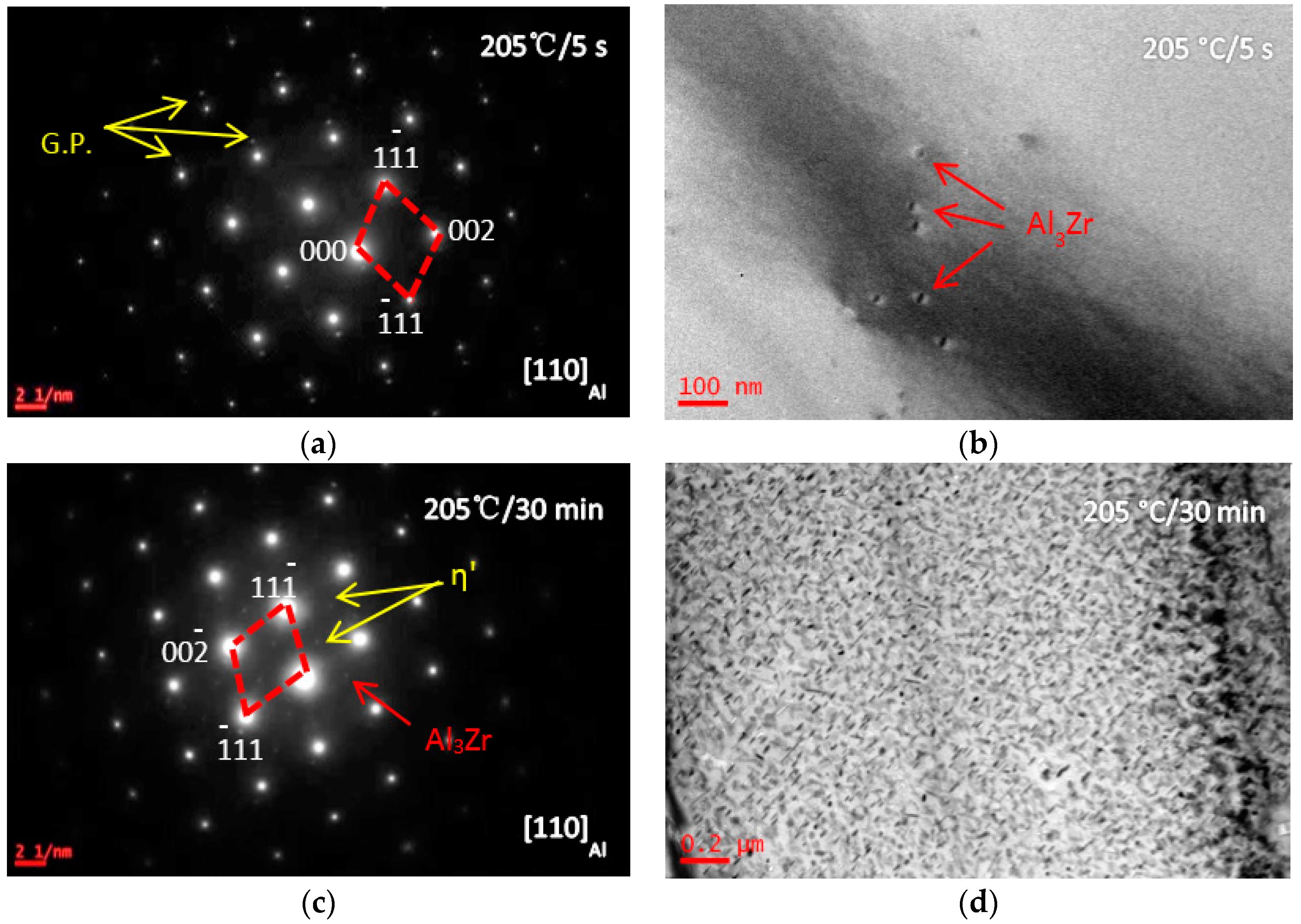


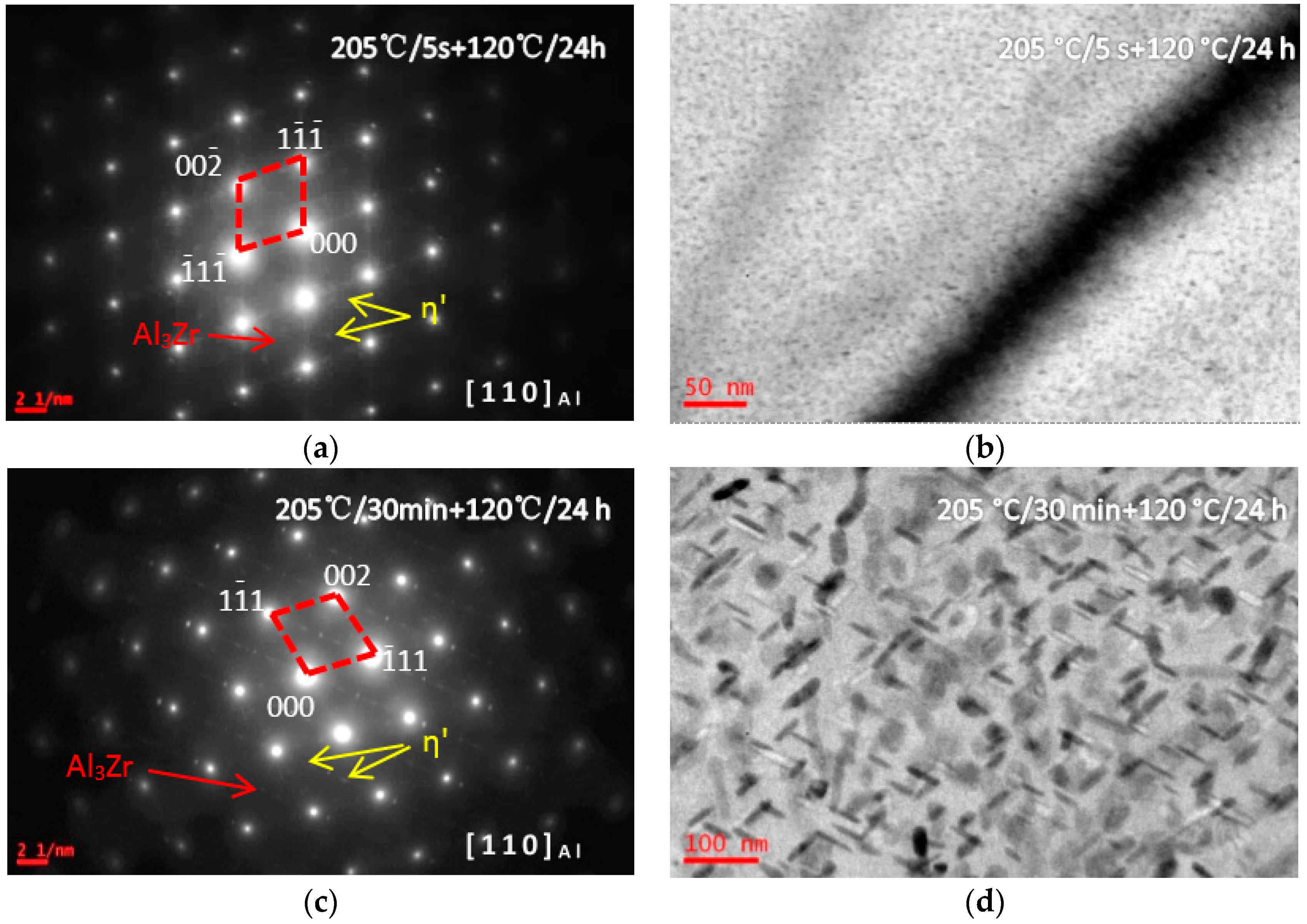
| Composition | Al | Zn | Mg | Cu | Zr | Fe | Si | Ti | Mn | Cr |
|---|---|---|---|---|---|---|---|---|---|---|
| wt % | Bal. | 8.31 | 2.07 | 2.46 | 0.12 | 0.078 | 0.056 | 0.005 | 0.005 | 0.003 |
| at % | Bal. | 3.68 | 2.45 | 1.12 | 0.038 | 0.04 | 0.057 | 0.003 | 0.003 | 0.002 |
| Sample No. | Solution Treatment | Isothermal Heat Treatment | Aging Treatment |
|---|---|---|---|
| A | 470 °C/1 h | / | / |
| B | 470 °C/1 h | / | 120 °C/24 h |
| C | 470 °C/1 h | 205 °C/5 s (30 min) | / |
| D | 470 °C/1 h | 325 °C/5 s (40 s), (30 min) | / |
| E | 470 °C/1 h | 445 °C/5 s, (30 min) | / |
| F | 470 °C/1 h | 205 °C/5 s (30 min) | 120 °C/24 h |
| G | 470 °C/1 h | 325 °C/5 s (40 s), (30 min) | 120 °C/24 h |
| H | 470 °C/1 h | 445 °C/5 s (30 min) | 120 °C/24 h |
| Sample No. | Elongation (%) | Ultimate Tensile Strength (MPa) | Yield Strength (MPa) |
|---|---|---|---|
| B | 7.7 | 719.8 | 699.8 |
| C/5 s | 6.9 | 620.2 | 598.2 |
| D/5 s | 7.3 | 604.4 | 578.3 |
| E/5 s | 7.7 | 647.5 | 621.9 |
| F/30 min | 7.7 | 524.5 | 506.4 |
| G/30 min | 8.9 | 300.1 | 267.6 |
| H/30 min | 7.3 | 647.5 | 621.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, X.; Lei, Q.; Xiao, Z.; Wang, M. Precipitation Behavior and Quenching Sensitivity of a Spray Deposited Al-Zn-Mg-Cu-Zr Alloy. Materials 2017, 10, 1100. https://doi.org/10.3390/ma10091100
Sheng X, Lei Q, Xiao Z, Wang M. Precipitation Behavior and Quenching Sensitivity of a Spray Deposited Al-Zn-Mg-Cu-Zr Alloy. Materials. 2017; 10(9):1100. https://doi.org/10.3390/ma10091100
Chicago/Turabian StyleSheng, Xiaofei, Qian Lei, Zhu Xiao, and Mingpu Wang. 2017. "Precipitation Behavior and Quenching Sensitivity of a Spray Deposited Al-Zn-Mg-Cu-Zr Alloy" Materials 10, no. 9: 1100. https://doi.org/10.3390/ma10091100





