2.1. N-Doped Carbon Xerogels
Carbon xerogels (CXGs), both with and without melamine, were prepared with two different resorcinol (R) to sodium carbonate (C) ratios (R/C): 130 and 300. Resorcinol to melamine and resorcinol to formaldehyde ratios, along with the amount of solvent were kept constant, as detailed in the experimental section. CXGs are herein named according to the R/C ratio employed, together with an N prefix to indicate N-doped CXGs (i.e., N-CXG-130 stands for N-doped CXG with R/C = 130). Some textural properties of the CXGs and N-CXGs obtained are presented in
Table 1. Bare CXGs (i.e., synthesized without nitrogen precursors) presented high surface areas of 461 and 587 m
2·g
−1, with different pore development as evidenced by N
2 adsorption at partial pressure approaching one. CXG-130 showed a low pore volume of 0.29 cm
3·g
−1, mainly attributed to micropores (80%), whereas CXGs synthesized with higher resorcinol/sodium carbonate ratio, CXG-300, developed a porous structure with a larger pore volume (0.65 cm
3·g
−1) and a predominance of mesopores (85%). The differences in textural development are caused by the growth of resorcinol-formaldehyde monomers during the gelation process, which is known to be favored at low pH, also increasing the density of mesopores in the CXG [
43].
The introduction of melamine in the synthesis of the xerogel has an effect on the textural properties depending on the R/C ratio used. In N-CXG-130, there is an increase of surface area together with a large increase of pore volume when employing melamine. Whereas, a significant decrease of both surface area and pore volume are observed in N-CXG-300. This is due to a higher degree of collapse of the N-doped gel in comparison to the undoped counterpart. Gels containing mixtures of melamine and formaldehyde are more prone to collapse given their higher fragility [
44,
45]. Pérez-Cadenas et al. found that N-doped xerogels present a narrow microporosity [
40]. It appears that a trade-off situation has to be found between the organic gel precursors (resorcinol, melamine) and sodium carbonate for the development of a highly mesoporous structure [
30,
43,
46].
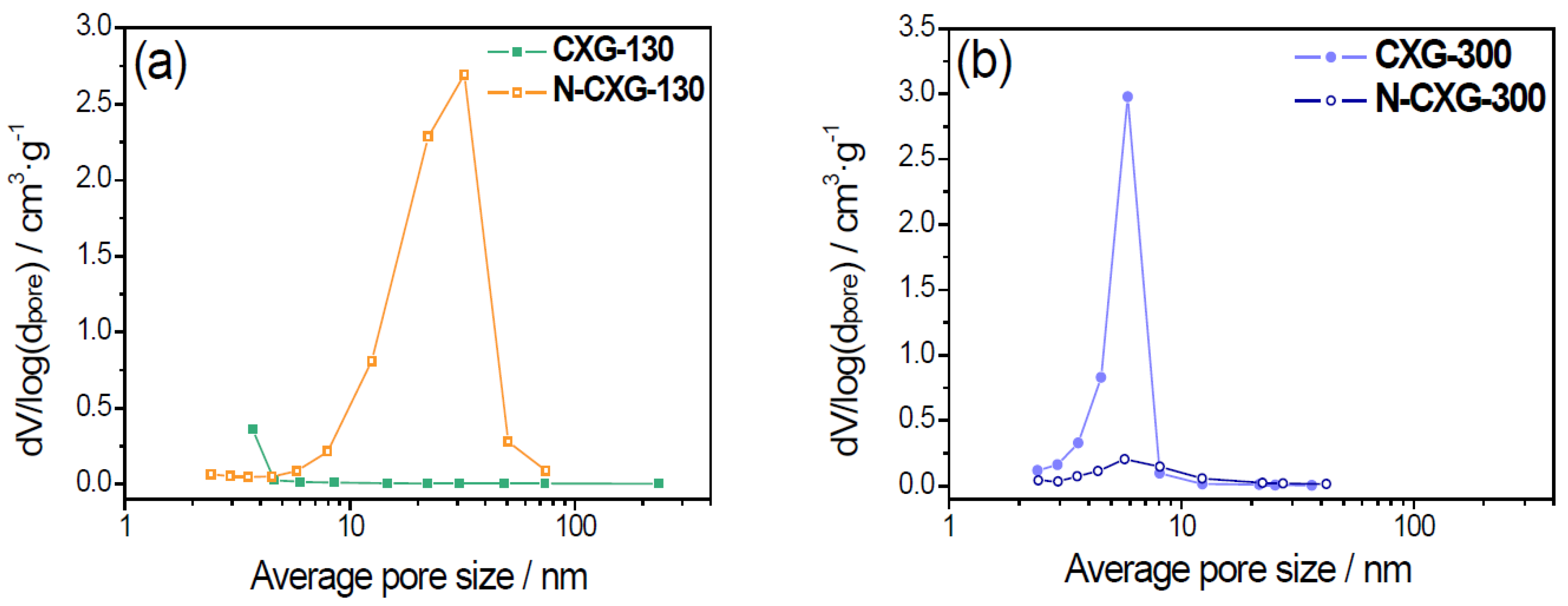
Pore size distribution (PSD), as shown in
Figure 1, corroborates the different effect of introducing melamine depending on the R/C ratio. When R/C is low, N-doping enhances the development of the porous structure. N-CXG-130 has a PSD centered around 30–40 nm. On the other hand, when R/C is high, the introduction of N leads to the collapse of the porous structure and to a lower pore size, being PSD centered around 5–6 nm for N-CXG-300.
The structure of CXGs was investigated with Raman spectroscopy. The main results, including the ratio between the D and G bands, as well as the position of the latter are also summarized in
Table 1. Doping with N decreases the structural order of the synthesized CXGs, as indicated by the shift of G band to higher frequencies and the increase of the I
D/I
G ratio. Resorcinol and melamine compete in the formaldehyde addition reaction during the gelation process
, i.e., there is a certain amount of un-reacted monomers, leading to an incomplete gelation, and, as a consequence, to a material with a lower structural order. Other authors have also observed this phenomenon [
47,
48,
49]. Podyacheva et al. [
50] published an excellent review reporting different studies concerned with the creation of a higher number of defects on N-doped carbon materials, being these proportional to the doping extent.
The amount of nitrogen in CXGs was determined by elemental analysis.
Table 2 shows the weight percentage of C, N, and H (sulphur content was below the detection limit in all cases). Pristine materials present a small amount of N, below 0.35 wt %, most probably coming from impurities in the carbon precursors. With the use of melamine, weight percentages of nitrogen around 3 wt % are introduced in the carbon xerogel. A slightly higher N content is observed for the N-CXG-300.
The nature of the nitrogen functionalities for N-CXGs was investigated by X-ray photoelectron spectroscopy (XPS). N1s spectra were deconvoluted, as described in [
30] into four components, as shown in
Figure 2, considering the binding energy values detailed in
Table 3.
Table 3 shows the N content, as well as the chemical speciation obtained by XPS. N-CXG-300 possesses a larger amount of N on the surface, in line with elemental analysis. It is believed that N atoms are distributed outside of the primary particles constituting the organic gel, as determined by Pérez-Cadenas et al. [
40]. As gelation occurs in an aqueous media, the hydrophilic amine groups derived from the polymerization, become oriented to the external part of the water-solid interface of the primary particles. This appears to occur in a larger extent for higher R/C ratio, this is, lower than the amount of sodium carbonate (lower pH).
Doped carbon xerogels present comparable contents of pyridinic N, around 30 at %, being slightly more abundant for the N-CXG-300 taking into account the total amount of N (4.5 at %). Larger differences were found in the contribution of pyrrole, graphitic and oxidized N. While graphitic N content is substantially larger in N-CXG-130 (21.3% vs. 7.9%), N-CXG-300 has a greater concentration of both pyrrole and oxidized N. In fact, XPS spectra of
Figure 2, show that the shape of the N1s peak is very similar for both materials, exhibiting two main peaks associated to pyridinic and pyrrolic nitrogen mostly, and differing in the signal at high binding energy (>401 eV), due to the variation in the presence of graphitic and oxidized N.
2.2. Pt Catalysts Supported on N-Doped Carbon Xerogels
Pt catalysts supported on CXGs (20 wt % Pt) were synthesized using formic acid as a reducing agent, as detailed in the experimental section. The X-ray diffraction (XRD) patterns (
Figure 3) exhibited the typical face-centered cubic structure of Pt. Catalysts were compared to an internal benchmark, Pt supported on carbon black Vulcan (obtained by the same method). Pt crystal sizes, determined from XRD patterns shown in
Figure 3, range between 3.4 nm and 5.5 nm. Pt content, shown in
Table 4 along with Pt crystal sizes, approaches the nominal 20 wt %. This value is slightly lower for the catalysts supported on CXGs obtained with a R/C = 300.
Upon N-doping of the CXG support, Pt crystal size decreases for CXG-130 sample. It is well known that the variation of carbon support features, apart from nitrogen content, may also influence Pt particle size and distribution, such as the pore size and the surface area [
51,
52,
53]. Nonetheless, the decrease of metal crystal size with N-doping of the support has been also described in the literature [
54,
55]. Some authors claim that nitrogen functionalities provide lone pairs of electrons in a sp
2 orbital in the plane of the carbon ring. Being nitrogen sites less electronegative than oxygen-containing sites (predominating on carbon materials), Pt particles are more strongly anchored to pyridinic N sites, preventing their agglomeration [
55]. Other authors determined that Pt atoms are confined in those sites where N replaces C, which is where Pt nanoparticle nucleation takes place, leading to a smaller crystal size [
56]. This effect of N on crystallite size was not observed for the support characterized by higher R/C ratio, in which there is no significant variation.
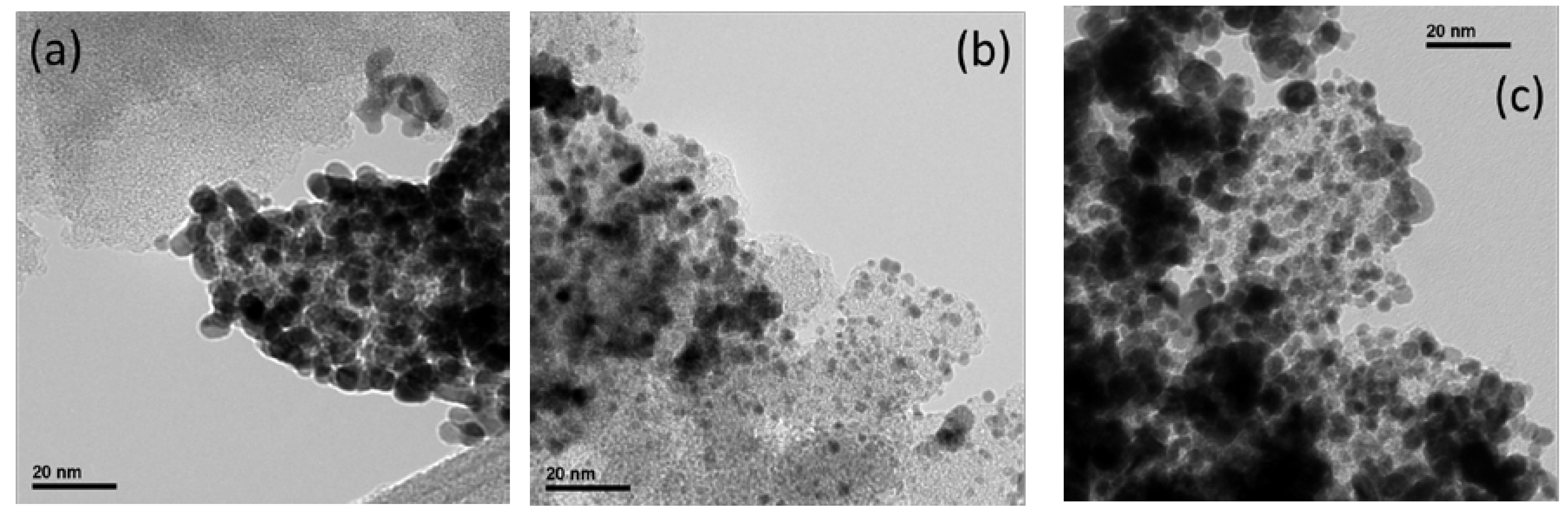
Figure 4 shows representative TEM micrographs for Pt/CXG catalysts. Pt/CXG-130,
Figure 4a, presents some inhomogeneities in Pt particles distribution on the CXG-130 support, including Pt particle agglomerates and small uncatalyzed xerogel regions, due to the preferential growth of metal particles on support defects. The morphology of the CXG-130 xerogel is indeed very compact and dense, consistent with its microporous structure, as previously discussed regarding nitrogen physisorption measurements. Large microporosity, and thus low mesopores availability for Pt deposition, is most probably responsible for the observed poor metal dispersion. The catalyst Pt/N-CXG-130, shown in
Figure 4b, presents a better Pt nanoparticle distribution on the xerogel surface, as a consequence of the support’s larger surface area and pores, with Pt particles of uniform size. Finally, the Pt/N-CXG-300 catalyst, as shown in
Figure 4c, also showed well-distributed areas together with the presence of some agglomerations and clusters of particles.
2.3. Activity for the Oxygen Reduction Reaction
The electrocatalytic activity of CXG-supported Pt catalysts was evaluated in a half-cell fitted with a rotating disk electrode (RDE). Catalysts were deposited as a thin film on the surface of the glassy carbon maintaining the Pt loading in all experiments (50 μg·cm
−2), see details in the experimental section.
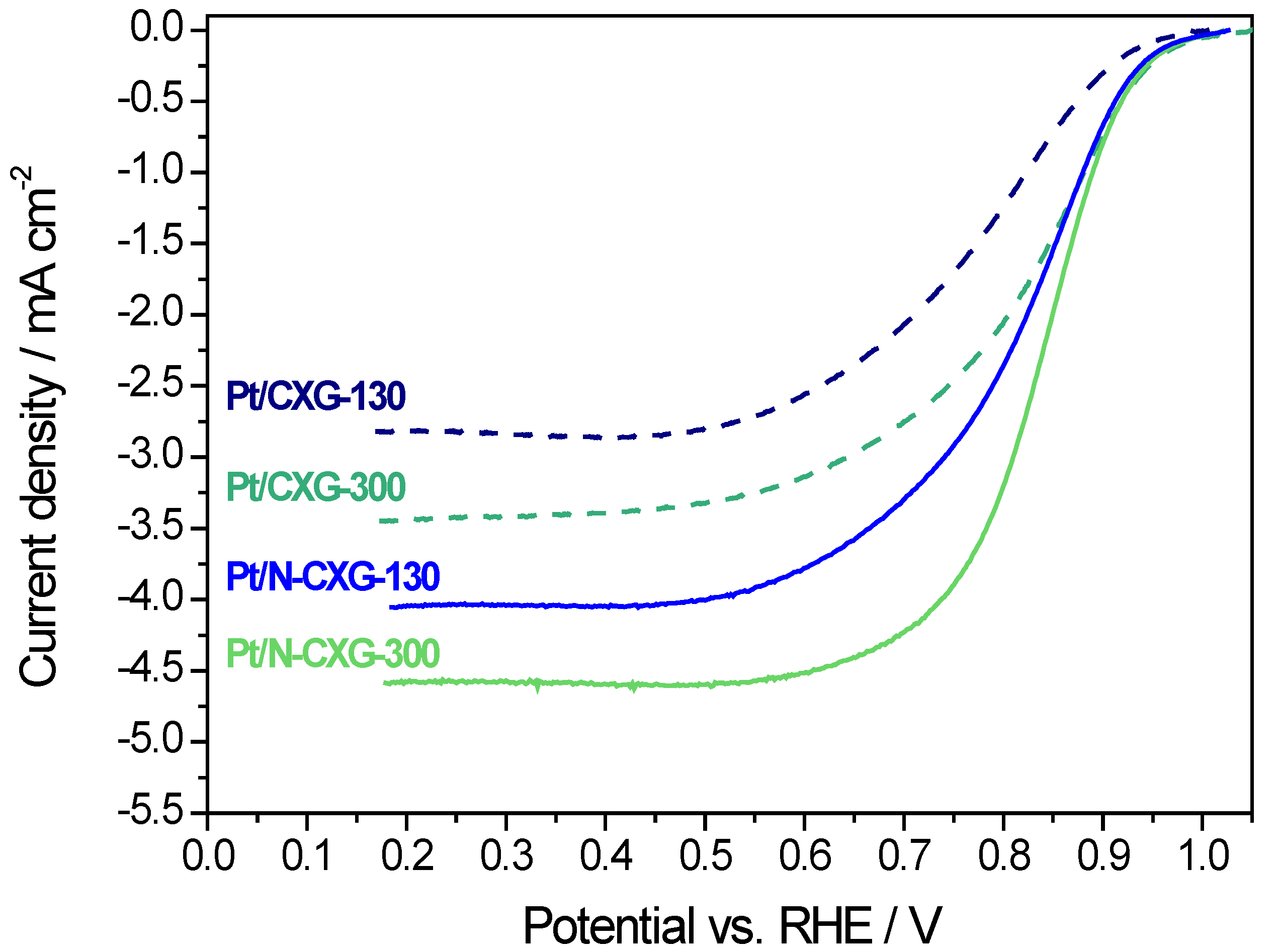
Figure 5 shows the linear sweep voltammetries (LSV) obtained at 1600 rpm in an O
2-saturated 0.5 M H
2SO
4 solution. The positive effect of N-doping is clearly evidenced in the figure. Both Pt/N-CXGs catalysts present better onset and half-wave potentials than their respective undoped counterparts. Moreover, better catalytic behavior is also accompanied by a higher limiting current density, indicating a reduction process approaching 4e- pathway. Among Pt/N-CXGs catalysts, the one supported on N-CXG-300 is more active for the oxygen reduction reaction (ORR) in terms of both limiting current density and onset potential.
Table 5 summarizes several parameters obtained from the RDE measurements along with the number of electrons exchanged (applying the Koutecky-Levich method) in the oxygen reduction reaction, as well as the electrochemical surface area (ECSA) calculated from the adsorption of hydrogen as determined by cyclic voltammetry in the deaerated base electrolyte. There is not a direct correlation between ECSA and electrocatalytic activity, suggesting that the variation in ORR activity is related to the intrinsic activity rather than to the dispersion of Pt. In this context, half-wave potential is a good indicator of the intrinsic activity in the mixed controlled zone (similar contributions from kinetics and diffusion phenomena), and this is not related to the available active sites (ECSA), but to the presence of nitrogen in the carbon xerogel. Indeed, the best results in terms of both half-wave potential and number of electrons were obtained for the nitrogen-doped CXG-supported catalysts, similar to our internal reference based on Vulcan carbon black support (Pt/Vulcan). On the other hand, the Pt catalysts supported on the un-doped carbon xerogels resulted in an inefficient reduction mechanism towards 2 e
− pathway, as ascertained from the values summarized in
Table 5 of 2.3 and 2.8 for Pt/CXG-130 and Pt/CXG-300. This could be ascribed to a worse electron transfer from the support to the active phase, or to the higher extent of agglomeration of metal particles as envisaged from TEM images.
In the literature, the effect of different nitrogen moieties on doped carbon-supported electrocatalysts is not yet clear. Some recent studies claim an electronic interaction between nitrogen functionalities and platinum, affecting the surface electronic structure of the latter and modifying the adsorption/desorption of oxygen species [
57,
58]. In our case, the ORR electrochemical activity results evidenced that, the use of N-doped carbon material helps enhancing the activity of the Pt-catalyst, and, this enhancement is more acute when the N-doped CXG possesses a higher amount of N in the form of pyridine and pyrrole. Besides, N-CXG-300 shows a higher ratio of N-pyridine/N-graphitic (3.9 vs. 1.4 for N-CXG-130). Melke et al. observed that platinum-support interactions in N-doped carbon materials might influence several parameters involved in the oxygen reduction, such as Pt particle size, higher electron density of the support with nitrogen, and the prevention of the formation of O-type functional groups [
59]. Therefore, even if the effect of N-doping is still under debate, it appears that a larger amount of pyridinic nitrogen favors the electrocatalytic activity of Pt.
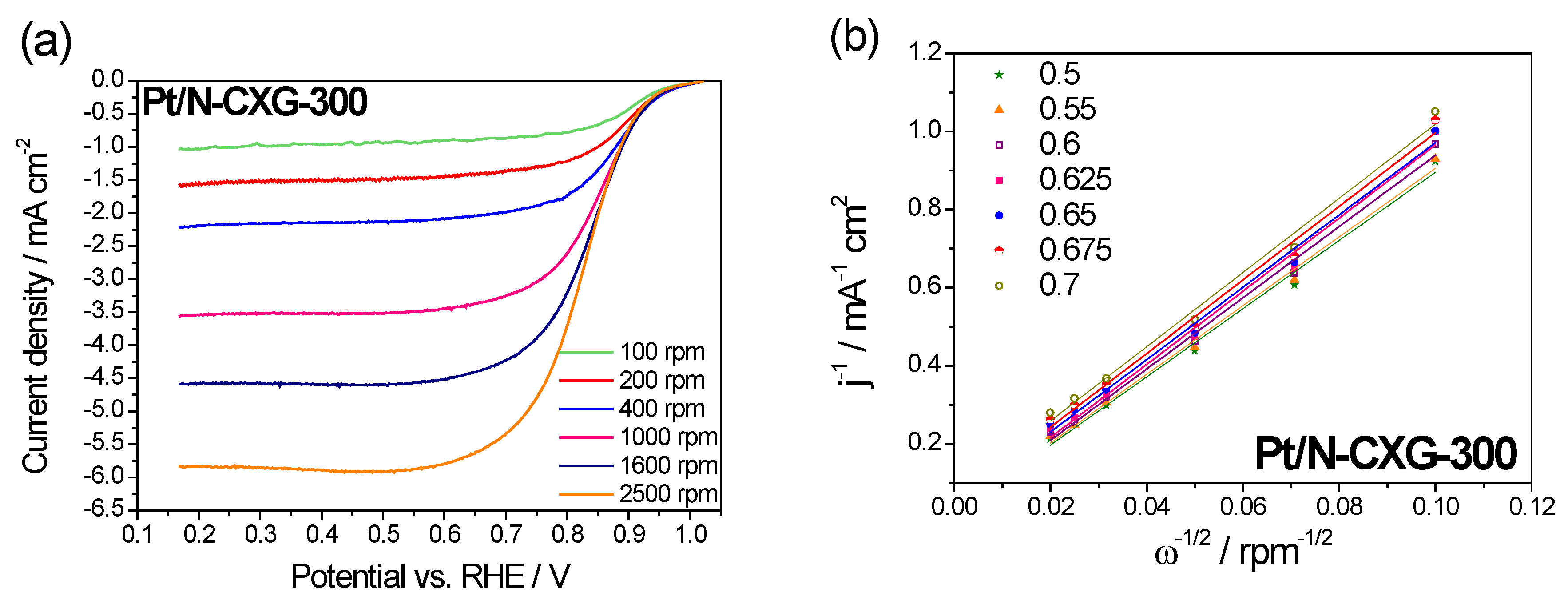
The ORR catalytic pathway for Pt/N-CXGs catalysts was investigated by Koutecky–Levich (K-L) plots obtained from the LSV at various rotating speeds (100–2500 rpm), shown in
Figure 6 and
Figure 7.
Figure 6a and
Figure 7a show how the cathodic current increases when increasing the rotating speed. This is due to an improved oxygen mass transport at the electrode surface.
The Koutecky-Levich equation was employed to calculate the number of electrons transferred per O
2 molecule in the ORR from the slopes of the plots shown in
Figure 6b and
Figure 7b, Pt/N-CXG-130 had a value of n of 3.5 e
− and Pt/N-CXG-300 had a n value of 3.8 e
−. Other studies in the literature have determined that N-doped materials with a higher percentage of graphitic N usually are more active towards a 2 e
− pathway, resulting in a partial reduction of oxygen to hydrogen peroxide, whereas those with higher contents of pyridinic and pyrrolic N lead to direct reduction of O
2 to water via a 4 e
− mechanism, as corroborated by our results. For example, Kurungot et al. stated that pyrrole is essential for oxygen adsorption and subsequent reduction to water via a 4e
− mechanism. Whereas, Ruoff et al. came to the conclusion that pyridinic nitrogen improves offset potential and converts mechanism from 2e
− to 4e
− while activity depends on the amount of graphitic nitrogen [
60].
Pt/N-CXGs were also investigated in a gas diffusion electrode (GDE) in a half-cell configuration and a low Pt loading (0.1 mg·cm
−2) in order to assess their stability and performance under conditions more similar to a practical application. The catalysts were subjected to a 1000 cycles between 0.6 and 1.2 V vs. RHE of potential.
Figure 6 shows the polarization curves at the beginning and at the end of the stress test (BoT and EoT, respectively). As evidenced in
Figure 8a,b, the catalyst based on the N-doped CXG (Pt/N-CXG-130) presents a decay of performance of about 45%, which is larger that the decay obtained with its un-doped counterpart, of about 30% (Pt/CXG-130). These catalysts present similar physico-chemical features, i.e., similar support BET surface area, a parameter that greatly influences the resistance towards degradation. On the other hand, Pt/N-CXG-130 presents a lower Pt crystal size (4.3 nm vs. 5.9 nm for the Pt/CXG-130), which may also contribute to its lower stability [
32]. On the other hand, Pt/N-CXG-300 (
Figure 8c) presents almost the same performance at the beginning and at the end of the 1000 cycles. Compared with the Pt/N-CXG-130, presenting a higher decay of performance, this catalyst is supported on N-CXG-130, with a higher specific surface area compared to the N-CXG-300 support (500 m
2·g
−1 vs. 390 m
2·g
−1). The higher the surface area the faster the oxidation of carbon, which may have led to a higher corrosion of the CXG-130, and N-CXG-130 supported catalysts but it does not explain itself the excellent stability for Pt/N-CXG-300.
In the literature, the reason why catalysts supported on N-doped materials present higher stability is still not clear. Some authors refer that it is due to a better chemical binding between the support and the noble metal particles, which results in an enhanced durability [
61,
62]. In the present case, only the catalyst supported on the N-CXG-300 shows a remarkable stability. Pt/Vulcan suffers a significant decay of performance, but less acute than Pt/N-CXG-130. It is well-documented that carbon supports with a higher graphitic content are more corrosion resistant, while excess oxygen groups can lead to an acceleration of the carbon support corrosion rate [
63]. Vulcan is characterized by a low Brunauer-Emmet-Teller (BET) surface area (250 m
2·g
−1) when compared to CXGs (≈380–500 m
2·g
−1). Besides, the lower the crystal size, the higher the corrosion rate. The remarkable durability of Pt/N-CXG-300 might be due to a combination of low surface area and appropriate nitrogen speciation on the surface, presumably within mesopores. As a consequence, the density of pyrrolic and pyridinic nitrogen groups per surface area is much higher, allowing Pt nanoparticles for an improved anchorage, thus hindering the coarsening of the active phase by the dissolution and redeposition of the noble metal particles (Ostwald ripening mechanism). Computational studies that will be the focus of a future work, will help to determine if the nature of N groups has any influence on the durability.
In order to better identify the causes of catalyst degradation, the ECSA was calculated from the hydrogen adsorption in the deaerated electrolyte by means of cyclic voltammetry before and after the degradation tests (
Table 6).
Pt/N-CXG-300 suffers the lowest decay of ECSA, which is comparable to that of Pt/Vulcan. Whereas, Pt/Vulcan showed a decrease of current density that is proportional to the decay of ECSA, the Pt/N-CXG-300 showed a negligible variation of current. This means that the intrinsic activity in terms of current per unit of Pt surface area increased after the degradation test, balancing the loss of ECSA. On the other hand, Pt/N-CXG-130 and its un-doped counterpart, lost almost half of their ECSA, directly impacting the performance. Thus, using a carbon xerogel with well-defined porosity and nitrogen functionalities appears as a good strategy to improve the degradation resistance of Pt catalysts despite the decrease of electrochemical surface area and the loss of Pt active sites availability.