Copper Corrosion and Biocorrosion Events in Premise Plumbing
Abstract
:1. Introduction
2. Copper Issues
3. Copper Processes in Drinking Water Systems
3.1. Electron Transfer Reactions
- (1)
- Simultaneous mechanism2Cu + H2O → Cu2O + 2e− + 2H+Cu → Cu2+ + 2e−
- (2)
- Sequential mechanism2Cu + H2O → Cu2O + 2e− + 2H+Cu2O + 2 H2O → Cu2+ + H2+2e− + 2OH−
- (3)
- Redeposition mechanismCu → Cu2+ + 2e−Cu + Cu2+ + H2O → 2 Cu2O + 2H+
3.2. Copper Speciation Reactions
3.3. Mass Transfer Processes
3.4. Microbial Involvement on Copper Mobility
4. Techniques for Water Chemistry and Surface Characterization
4.1. Techniques and Protocols for Measuring Total and Soluble Copper in Water
4.2. Electrochemical Techniques
4.3. Surface Characterization Techniques
4.4. Techniques for Determination of Microbial Biofilm Populations
5. Hydrodynamic Considerations
5.1. Hydrodynamical Effects
5.2. Flushing Experiments
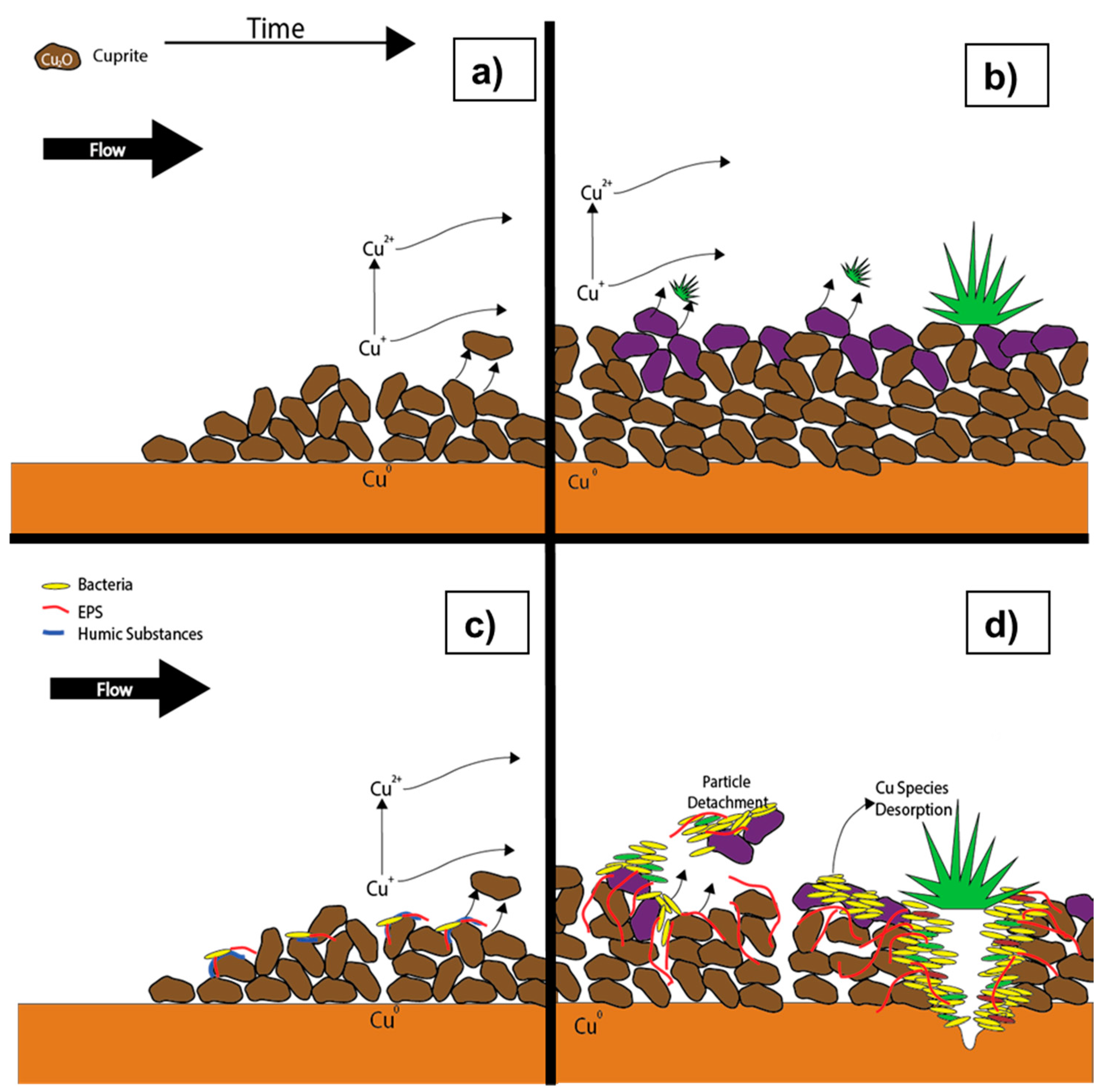
6. Conceptual Corrosion Model
6.1. Conceptual Model During Stagnation
6.2. Conceptual Model During Flow
6.3. Mathematical Modeling
7. Future Challenges
7.1. Early Detection of Corrosion and Biocorrosion
7.2. Quantification of the Problem Extension
7.3. Mathematical Modelling
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Merkel, T.H.; Pehkonen, S.O. General corrosion of copper in domestic drinking water installations: Scientific background and mechanistic understanding. Corros. Eng. Sci. Technol. 2006, 41, 21–37. [Google Scholar] [CrossRef]
- Beech, W.B.; Sunner, J. Biocorrosion: Towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol. 2004, 15, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Keevil, C.W. The physico-chemistry of biofilm-mediated pitting corrosion of copper pipe supplying potable water. Water Sci. Technol. 2004, 49, 91–98. [Google Scholar] [PubMed]
- Walker, J.T.; Dowsett, A.B.; Dennis, P.J.L.; Keevil, C.W. Continuous culture studies of biofilm associated with copper corrosion. Int. Biodeterior. 1991, 27, 121–134. [Google Scholar] [CrossRef]
- Webster, B.J.; Werner, S.E.; Wells, D.B.; Bremer, P.J. Microbiologically influenced corrosion of copper in potable water systems—pH effects. Corrosion 2000, 56, 942–950. [Google Scholar] [CrossRef]
- Oliphant, R.J. Causes of Copper Corrosion in Plumbing Systems; Foundation for Water Research: Buckinghamshire, UK, 2003. [Google Scholar]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Zietz, B.P.; Dieter, H.H.; Lakomek, M.; Schneider, H.; Kessler-Gaedtke, B.; Dunkelberg, H. Epidemiological investigation on chronic copper toxicity to children exposed via the public drinking water supply. Sci. Total Environ. 2003, 302, 127–144. [Google Scholar] [CrossRef]
- Dietrich, A.M.; Glindemann, D.; Pizarro, F.; Gidi, V.; Olivares, M.; Araya, M.; Camper, A.; Duncan, S.; Dwyer, S.; Whelton, A.J.; et al. Health and aesthetic impacts of copper corrosion on drinking water. Water Sci. Technol. 2004, 49, 55–62. [Google Scholar] [PubMed]
- Xu, P.; Huang, S.; Wang, Z.; Lagos, G. Daily intakes of copper, zinc and arsenic in drinking water by population of Shanghai, China. Sci. Total Environ. 2006, 362, 50. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.; Jacobs, S.; Taylor, R.J. The blue water phenomenon. J. Am. Water Works Assoc. 2000, 92, 72–82. [Google Scholar]
- Edwards, M.; Schock, M.R.; Meyer, T.E. Alkalinity, pH, and copper corrosion by-product release. J. Am. Water Works Assoc. 1996, 88, 81–94. [Google Scholar]
- Schock, M.R.; Lytle, D.A.; Clement, J.A. Effect of pH, DIC, Ortophosphaste and Sulphate on Cuprosolvency; NACE International: Houston, TX, USA, 1995. [Google Scholar]
- Calle, G.R.; Vargas, I.T.; Alsina, M.A.; Pasten, P.A.; Pizarro, G.E. Enhanced copper release from pipes by alternating stagnation and flow events. Environ. Sci. Technol. 2007, 41, 7430–7436. [Google Scholar] [CrossRef] [PubMed]
- Olivares, T.E.; Cienfuegos, R.; Vargas, I.T.; Pizarro, G.E. Experimental evidence for enhanced copper release from domestic copper plumbing under hydrodynamic control. Corros. Sci. 2014, 80, 473–481. [Google Scholar] [CrossRef]
- Pizarro, G.E.; Vargas, I.T.; Pasten, P.A.; Calle, G.R. Modeling mic copper release from drinking water pipes. Bioelectrochemistry 2014, 97, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, O.E. An Assessment and Modeling of Copper Plumbing Pipe Failures Due to Pinhole Leaks. Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 19 May 2006. [Google Scholar]
- Pinhole Leaks & Corrosion Control. Available online: https://www.wsscwater.com/water-quality--stewardship/research/pinhole-leaks--corrosion-control.html (accessed on 5 May 2017).
- Lytle, D.A.; Liggett, J. Impact of water quality on chlorine demand of corroding copper. Water Res. 2016, 92, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Araya, M.; Pena, C.; Pizarro, F.; Olivares, M. Gastric response to acute copper exposure. Sci. Total Environ. 2003, 303, 253–257. [Google Scholar] [CrossRef]
- Araya, M.; Olivares, M.; Pizarro, F.; Llanos, A.; Figueroa, G.; Uauy, R. Community-based randomized double-blind study of gastrointestinal effects and copper exposure in drinking water. Environ. Health Perspect. 2004, 112, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, N.; Hatano, M. An acute experiment on retrograde intestinal peristalsis with emesis using decerebrated dogs. J. Auton. Nerv. Syst. 1998, 70, 56–65. [Google Scholar] [CrossRef]
- Reisman, D.; Peirano, J.; Lewis, D.; Basu, D.; Hohrseiter, D. Summary Review of the Health Effects Associated with Copper: Health Issue Assessment; Office of Environmental Criteria and Assessment: Washington, DC, USA, 1987.
- W.H.O. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Pettersson, R.; Rasmussen, F. Daily intake of copper from drinking water among young children in Sweden. Environ. Health Perspect. 1999, 107, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Bhave, S. Present interpretation of the role of copper in Indian childhood cirrhosis. Am. J. Clin. Nutr. 1996, 63, S830–S835. [Google Scholar]
- Araya, M.; McGoldrick, M.C.; Klevay, L.M.; Strain, J.J.; Robson, P.; Nielsen, F.; Olivares, M.; Pizarro, F.; Johnson, L.; Poirier, K.A. Determination of an acute no-observed-adverse-effect level (NOAEL) for copper in water. Regul. Toxicol. Pharmacol. 2001, 34, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Araya, M.; Pizarro, F.; Uauy, R. Nausea threshold in apparently healthy individuals who drink fluids containing graded concentrations of copper. Regul. Toxicol. Pharmacol. 2001, 33, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Gotteland, M.; Araya, M.; Pizarro, F.; Olivares, M. Effect of acute copper exposure on gastrointestinal permeability in healthy volunteers. Dig. Dis. Sci. 2001, 46, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, F.; Olivares, M.; Uauy, R.; Contreras, P.; Rebelo, A.; Gidi, V. Acute gastrointestinal effects of graded levels of copper in drinking water. Environ. Health Perspect. 1999, 107, 117–121. [Google Scholar] [CrossRef] [PubMed]
- National research council. Report on copper in drinking water. J. Environ. Health 2000, 63, 41–42. [Google Scholar] [PubMed]
- Olivares, M.; Uauy, R. Limits of metabolic tolerance to copper and biological basis for present recommendations and regulations. Am. J. Clin. Nutr. 1996, 63, 846S–852S. [Google Scholar] [PubMed]
- Pratt, W.B.; Omdahl, J.L.; Sorenson, J. Lack of effects of copper gluconate supplementation. Am. J. Clin. Nutr. 1985, 42, 681–682. [Google Scholar] [PubMed]
- O’Donohue, J.; Reid, M.; Varghese, A.; Portmann, B.; Williams, R. A case of adult chronic copper self-intoxication resulting in cirrhosis. Eur. J. Med. Res. 1999, 4, 252. [Google Scholar] [PubMed]
- Kitzberger, R.; Madl, C.; Ferenci, P. Wilson disease. Metab. Brain Dis. 2005, 20, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Torsdottir, G.; Kristinsson, J.; Sveinbjornsdottir, S.; Snaedal, J.; Johannesson, T. Copper, ceruloplasmin, superoxide dismutase and iron parameters in parkinson’s disease. Pharmacol. Toxicol. 1999, 85, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.F.B. The molecular basis of copper-transport diseases. Trends Mol. Med. 2001, 7, 64–69. [Google Scholar] [CrossRef]
- Brown, D.R.; Kozlowski, H. Biological inorganic and bioinorganic chemistry of neurodegeneration based on prion and alzheimer diseases. Dalton Trans. 2004, 13, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Khiari, D.; Bruchet, A.; Gittelman, T.; Matia, L.; Barrett, S.; Suffett, I.H.; Hund, R. Distribution-generated taste-and-odor phenomena. Water Sci. Technol. 1999, 40, 129–133. [Google Scholar]
- Rigal, S.; Danjou, J. Tastes and odors in drinking water distribution systems related to the use of synthetic materials. Water Sci. Technol. 1999, 40, 203–208. [Google Scholar]
- Dietrich, A.M.; Burlingame, G.A.; Vest, C.; Hopkins, P. Rating method for evaluating distribution-system odors compared with a control. Water Sci. Technol. 2004, 49, 55–60. [Google Scholar] [PubMed]
- Suffet, I.H.M.; Corado, A.; Chou, D.; McGuire, M.J.; Butterworth, S. Awwa taste and odor survey. J. Am. Water Work Assoc. 1996, 88, 168–180. [Google Scholar]
- Secondary Drinking Water Standards: Guidance for Nuisance Chemicals. Available online: https://www.epa.gov/dwstandardsregulations/secondary-drinking-water-standards-guidance-nuisance-chemicals (accessed on 19 August 2016).
- Cohen, J.M.; Kamphake, L.J.; Harris, E.K.; Woodward, R.L. Taste threshold concentrations of metals in drinking water. J. Am. Water Work Assoc. 1960, 52, 660–670. [Google Scholar]
- Zacarías, I.; Yáñez, C.G.; Araya, M.; Oraka, C.; Olivares, M.; Uauy, R. Determination of the taste threshold of copper in water. Chem. Senses 2001, 26, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Palit, A.; Pehkonen, S.O. Copper corrosion in distribution systems: Evaluation of a homogeneous Cu2O film and a natural corrosion scale as corrosion inhibitors. Corros. Sci. 2000, 42, 1801. [Google Scholar] [CrossRef]
- Boulay, N.; Edwards, M. Role of temperature, chlorine, and organic matter in copper corrosion by-product release in soft water. Water Res. 2001, 35, 683–690. [Google Scholar] [CrossRef]
- Zhou, P.; Hutchison, M.; Scully, J.; Ogle, K. The anodic dissolution of copper alloys: Pure copper in synthetic tap water. Electrochim. Acta 2016, 191, 548–557. [Google Scholar] [CrossRef]
- Ives, D.J.; Rawson, A.E. Copper corrosion III. Electrochemical theory of general corrosion. J. Electrochem. Soc. 1962, 109, 458–462. [Google Scholar] [CrossRef]
- Ives, D.J.; Rawson, A.E. Copper corrosion I. Thermodynamic aspects. J. Electrochem. Soc. 1962, 109, 447–451. [Google Scholar] [CrossRef]
- Ives, D.J.; Rawson, A.E. Copper corrosion II. Kinetic studies. J. Electrochem. Soc. 1962, 109, 452–457. [Google Scholar] [CrossRef]
- Ives, D.J.G.; Rawson, A.E. Copper corrosion IV. The effects of saline additions. J. Electrochem. Soc. 1962, 109, 462–466. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; NACE International: Houston, TX, USA, 1974. [Google Scholar]
- Cong, H.; Scully, J.R. Effect of chlorine concentration on natural pitting of copper as a function of water chemistry. J. Electrochem. Soc. 2010, 157, C200–C211. [Google Scholar] [CrossRef]
- Atlas, D.; Coombs, J.; Zajicek, O. The corrosion of copper by chlorinated drinking waters. Water Res. 1982, 16, 693–698. [Google Scholar] [CrossRef]
- Edwards, M.; Powers, K.; Hidmi, L.; Schock, M.R. The role of pipe ageing in copper corrosion by-product release. Water Sci. Techonl. 2001, 1, 25–32. [Google Scholar]
- Pehkonen, S.O.; Palit, A.; Zhang, X. Effect of specific water quality parameters on copper corrosion. Corrosion 2002, 58, 156–165. [Google Scholar] [CrossRef]
- Hidmi, L.; Edwards, M. Role of temperature and pH in Cu(OH)(2) solubility. Environ. Sci. Technol. 1999, 33, 2607–2610. [Google Scholar] [CrossRef]
- Rushing, J.C.; Edwards, M. The role of temperature gradients in residential copper pipe corrosion. Corros. Sci. 2004, 46, 1883–1894. [Google Scholar] [CrossRef]
- Lagos, G. Corrosion of Copper Plumbing Tubes and the Release of Copper by-Products to Drinking Water; International Copper Association, Ltd.: New York, NY, USA, 2001. [Google Scholar]
- Zhang, X.; Pehkonen, S.O.; Kocherginsky, N.; Andrew Ellis, G. Copper corrosion in mildly alkaline water with the disinfectant monochloramine. Corros. Sci. 2002, 44, 2507–2528. [Google Scholar] [CrossRef]
- Merkel, T.H.; Groß, H.-J.; Wernera, W.; Dahlkeb, T.; Reichertera, S.; Beuchleb, G.; Eberle, S.H. Copper corrosion by-product release in long-term stagnation experiments. Water Res. 2002, 36, 1547–1555. [Google Scholar] [CrossRef]
- Alex, T.; Johannsen, K. Copper in drinking water supplies. The development of a kinetic model to describe the copper by-product release in corrosion tests using din 50931-1. VOM WASSER 2000, 95, 25–36. [Google Scholar]
- Feng, Y.; Teo, W.K.; Siow, K.S.; Tan, K.L.; Hsieh, A.K. The corrosion behaviour of copper in neutral tap water. Part I: Corrosion mechanisms. Corros. Sci. 1996, 38, 369–385. [Google Scholar] [CrossRef]
- Metikos-Hukovic, M.; Babic, R.; Marinovic, A. Spectrochemical characterization of benzotriazole on copper. J. Electrochem. Soc. 1998, 145, 4045–4051. [Google Scholar] [CrossRef]
- Folquer, M.E.; Ribotta, S.B.; Real, S.G.; Gassa, L.M. Study of copper dissolution and passivation processes by electrochemical impedance spectroscopy. Corrosion 2002, 58, 240–247. [Google Scholar] [CrossRef]
- Edwards, M.; Sprague, N. Organic matter and copper corrosion by-product release: A mechanistic study. Corros. Sci. 2001, 43, 1–18. [Google Scholar] [CrossRef]
- Geesey, G.G.; Bremer, P.J. Interactions of exopolymers of corrosive biofilm microorganisms with copper ions. In Proceedings of the NSF-CONICET Workshop on Biofouling and Biocorrosion: Metal/Microbe Interactions, Mar del Plata, Argentina, November 1992; Videla, H.A., Lewandowski, Z., Lutey, R.W., Eds.; Buckman Laboratories International Inc.: Memphis, TN, USA, 1993; pp. 36–41. [Google Scholar]
- Vargas, I.T.; Pavissich, J.P.; Olivares, T.E.; Jeria, G.A.; Cienfuegos, R.A.; Pasten, P.A.; Pizarro, G.E. Increase of the concentration of dissolved copper in drinking water systems due to flow-induced nanoparticle release from surface corrosion by-products. Corros. Sci. 2010, 52, 3492–3503. [Google Scholar] [CrossRef]
- Gagnon, G.A.; Rand, J.L.; O’Leary, K.C.; Rygel, A.C.; Chauret, C.; Andrews, R.C. Disinfectant efficacy of chlorite and chlorine dioxide in drinking water biofilms. Water Res. 2005, 39, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Lehtola, M.J.; Miettinen, K.T.; Keinanen, M.M.; Kekki, T.K.; Laine, O.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. Microbiology, chemistry and biofilm development in a pilot drinking water distribution system with copper and plastic pipes. Water Res. 2004, 38, 3769–3779. [Google Scholar] [CrossRef] [PubMed]
- Trevors, J.T.; Cotter, C.M. Copper toxicity and uptake in microorganisms. J. Ind. Microbiol. 1990, 6, 77–84. [Google Scholar] [CrossRef]
- Critchley, M.M.; Cromar, N.J.; McClure, N.; Fallowfield, H.J. Biofilms and microbially influenced cuprosolvency in domestic copper plumbing systems. J Appl. Microbiol. 2001, 91, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.; Xi, C.W.; Raskin, L. Microbial ecology of drinking water distribution systems. Curr. Opin. Biotechnol. 2006, 17, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Lehtola, M.J.; Miettinen, I.T.; Lampola, T.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. Pipeline materials modify the effectiveness of disinfectants in drinking water distribution systems. Water Res. 2005, 39, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- LeChevallier, M.W.; Babcock, T.M.; Lee, R.G. Examination and characterization of distribution system biofilms. Appl. Environ. Microbiol. 1987, 53, 2714–2724. [Google Scholar] [PubMed]
- Flemming, H.C. Biofouling in water systems—Cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.J.; Ceri, H.; Turner, R.J. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol. 2007, 5, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The eps matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef] [PubMed]
- Critchley, M.M.; Pasetto, R.; O’Halloran, R.J. Microbiological influences in ‘blue water’ copper corrosion. J. Appl. Microbiol. 2004, 97, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Critchley, M.M.; Cromar, N.J.; McClure, N.C.; Fallowfield, H.J. The influence of the chemical composition of drinking water on cuprosolvency by biofilm bacteria. J. Appl. Microbiol. 2003, 94, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, C.; Fallowfield, H.J. Assessment of microbial involvement in the elevation of copper levels in drinking water. J. Appl. Microbiol. 1998, 85, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Pavissich, J.P.; Vargas, I.T.; Gonzalez, B.; Pasten, P.A.; Pizarro, G.E. Culture dependent and independent analyses of bacterial communities involved in copper plumbing corrosion. J. Appl. Microbiol. 2010, 109, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Burleigh, T.D.; Gierke, C.G.; Fredj, N.; Boston, P.J. Copper tube pitting in santa fe municipal water caused by microbial induced corrosion. Materials 2014, 7, 4321–4334. [Google Scholar] [CrossRef] [PubMed]
- Siedlarek, H.; Wagner, D.; Fischer, W.; Paradies, H. Microbiologically influenced corrosion of copper: The ionic transport properties of biopolymers. Corros. Sci. 1994, 36, 1751–1763. [Google Scholar] [CrossRef]
- Letelier, M.V.; Lagos, G.E.; Reyes, A. Chemical characterization of blue stains in domestic fixtures in contact with drinking water. Environ. Monit. Assess. 2008, 139, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Vargas, I.T.; Alsina, M.A.; Pavissich, J.P.; Jeria, G.A.; Pasten, P.A.; Walczak, M.; Pizarro, G.E. Multi-technique approach to assess the effects of microbial biofilms involved in copper plumbing corrosion. Bioelectrochemistry 2014, 97, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Beech, I.B.; Sunner, J.A.; Hiraoka, K. Microbe-surface interactions in biofouling and biocorrosion processes. Int. Microbiol. 2005, 8, 157–168. [Google Scholar] [PubMed]
- Videla, H.A.; Herrera, L.K. Microbiologically influenced corrosion: Looking to the future. Int. Microbiol. 2005, 8, 169–180. [Google Scholar] [PubMed]
- Little, B.J.; Wagner, P.A.; Lewandowski, Z. Spatial Relationships between Bacteria and Mineral Surfaces. In Geomicrobiology: Interactions between Microbes and Minerals; Ribbe, P.H., Ed.; Mineralogical Society of America: Washington, DC, USA, 1997; Volume 35, pp. 123–159. [Google Scholar]
- Garcia, F.; Lopez, A.L.R.; Guillen, J.C.; Sandoval, L.H.; Gonzalez, C.R.; Castano, V. Corrosion inhibition in copper by isolated bacteria. Anti-Corros. Methods Mater. 2012, 59, 10–17. [Google Scholar] [CrossRef]
- Bremer, P.J.; Geesey, G.G. Laboratory-based model of microbiologically induced corrosion of copper. Appl. Environ. Microbiol. 1991, 57, 1956–1962. [Google Scholar] [PubMed]
- Kip, N.; van Veen, J.A. The dual role of microbes in corrosion. ISME J. 2014, in press. [Google Scholar] [CrossRef] [PubMed]
- Adeloju, S.B.; Hughes, H.C. The corrosion of copper pipes in high chloride-low carbonate mains water. Corros. Sci. 1986, 26, 851. [Google Scholar] [CrossRef]
- Mankowski, G.; Duthil, J.P.; Giusti, A. The pit morphology on copper in chloride- and sulphate-containing solutions. Corros. Sci. 1997, 39, 27–42. [Google Scholar] [CrossRef]
- Chmielova, M.; Seidlerova, J.; Weiss, Z. X-ray diffraction phase analysis of crystalline copper corrosion products after treatment in different chloride solutions. Corros. Sci. 2003, 45, 883–889. [Google Scholar] [CrossRef]
- Callot, P.; Jaegle, A.; Kalt, A.; Nanse, G. Pitting corrosion of copper tubes and carbon deposits: Escs studies. Mater. Corros. 1978, 29, 519–522. [Google Scholar] [CrossRef]
- Lagos, G.E.; Cuadrado, C.A.; Letelier, M.V. Aging of copper pipes by drinking water. J. Am. Water Work Assoc. 2001, 93, 94–103. [Google Scholar]
- Le Gal La Salle, A.; Jardy, A.; Rosset, R.; Keddam, M.; Caramel, A.; Noel, D. Copper corrosion, passivation and protection in aqueous solutions. I. Cyclic mechanism of the corrosion. Mem. Etud. Sci. Rev. Met. 1992, 89, 171–182. [Google Scholar]
- Vargas, I.T.; Alsina, M.A.; Pasten, P.A.; Pizarro, G.E. Influence of solid corrosion by-products on the consumption of dissolved oxygen in copper pipes. Corrosion science. Corros. Sci. 2009, 51, 1030–1037. [Google Scholar] [CrossRef]
- Eriksen, R.S.; Mackey, D.J.; van Dam, R.; Nowak, B. Copper speciation and toxicity in macquarie harbour, tasmania: An investigation using a copper ion selective electrode. Mar. Chem. 2001, 74, 99–113. [Google Scholar] [CrossRef]
- Carvallo, M.J. Evaluación de la capacidad de Sorción de Cobre por Biomasa Usando Electrodo Ise: Implicaciones Para un Modelo de Biocorrosión de Cañerías de Cobre; Pontificia Universidad Católica de Chile: Santiago, Chile, 2005. [Google Scholar]
- Xia, X.; Xie, C.; Cai, S.; Yang, Z.; Yang, X. Corrosion characteristics of copper microparticles and copper nanoparticles in distilled water. Corros. Sci. 2006, 48, 3924–3932. [Google Scholar] [CrossRef]
- Pizarro, F.; Olivares, M.; Araya, M.; Gidi, V.; Uauy, R. Gastrointestinal effects associated with soluble and insoluble copper in drinking water. Environ. Health Perspect. 2001, 109, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.; Edwards, M. Sulfide scale catalysis of copper corrosion. Water Res. 2000, 34, 2798–2808. [Google Scholar] [CrossRef]
- Zhe, Y.; Pehkonen, S.O. Copper corrosion kinetics and mechanisms in the presence of chlorine and orthophosphate. Water Sci. Technol. 2004, 49, 73–81. [Google Scholar] [PubMed]
- Feng, Y.; Teo, W.K.; Siow, K.S.; Hsieh, A.K. The corrosion behaviour of copper in neutral tap water. Part II. Determination of corrosion rates. Corros. Sci. 1996, 38, 387–395. [Google Scholar] [CrossRef]
- Valcarce, M.; De Sanchez, S.; Vazquez, M. A comparative analysis of copper and brass surface films in contact with tap water. J. Mater. Sci. 2006, 41, 1999–2007. [Google Scholar] [CrossRef]
- Arjmand, F.; Adriaens, A. Influence of pH and chloride concentration on the corrosion behavior of unalloyed copper in NaCl solution: A comparative study between the micro and macro scales. Materials 2012, 5, 2439–2464. [Google Scholar] [CrossRef] [Green Version]
- Cong, H.B.; Michels, H.T.; Scully, J.R. Passivity and pit stability behavior of copper as a function of selected water chemistry variables. J. Electrochem. Soc. 2009, 156, C16–C27. [Google Scholar] [CrossRef]
- Burstein, G.; Bi, H.; Kawaley, G. The persistence of inhibition of copper corrosion in tap water. Electrochim. Acta 2016, 191, 247–255. [Google Scholar] [CrossRef]
- Merkel, T.H. Copper corrosion: Understanding and modelling general corrosion. Water Sci. Technol. 2004, 49, 63–71. [Google Scholar] [PubMed]
- Vargas, I.T.; Alsina, M.A.; Pasten, P.A.; Pizarro, G.E. Influence of malachite morphology on copper release in drinking water systems. In Proceedings of the European Corrosion Congress (EUROCORR 2008), Edinburgh, UK, 7–11 September 2008; European Federation of Corrosion: Edinburgh, UK, 2008. [Google Scholar]
- Reyes, A.; Letelier, M.V.; De la Iglesia, R.; Gonzalez, B.; Lagos, G. Biologically induced corrosion of copper pipes in low-pH water. Int. Biodeterior. Biodegrad. 2008, 61, 135–141. [Google Scholar] [CrossRef]
- Adeloju, S.B.; Duan, Y.Y. Influence of bicarbonate ions on stability of copper oxides and copper pitting corrosion. Br. Corros. J. 1994, 29, 315–320. [Google Scholar] [CrossRef]
- Vargas, I.T.; Pasten, P.A.; Pizarro, G.E. Empirical model for dissolved oxygen depletion during corrosion of drinking water copper pipes. Corros. Sci. 2010, 52, 2250–2257. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Sahre, M.; Kautek, W. In-situ grazing incidence X-ray diffractometry observation of pitting corrosion of copper in chloride solutions. Corros. Sci. 1999, 41, 1899–1909. [Google Scholar] [CrossRef]
- Fernandes, P.J.L. Type I pitting of copper tubes from a water distribution system. Eng. Fail Anal. 1998, 5, 35–40. [Google Scholar] [CrossRef]
- Srivastava, A.; Balasubramaniam, R. Microstructural characterization of copper corrosion in aqueous and soil environments. Mater. Charact. 2005, 55, 127–135. [Google Scholar] [CrossRef]
- Shim, J.J.; Kim, J.G. Copper corrosion in potable water distribution systems: Influence of copper products on the corrosion behavior. Mater. Lett. 2004, 58, 2002–2006. [Google Scholar] [CrossRef]
- Kautek, W.; Geuß, M.; Sahre, M.; Zhao, P.; Mirwald, S. Multi-method analysis of the metal/electrolyte interface: Scanning force microscopy (SFM), quartz microbalance measurements (QMB), fourier transform infrared spectroscopy (FTIR) and grazing incidence X-ray diffractometry (GIXD) at a polycrystalline copper electrode. Surf. Interface Anal. 1997, 25, 548–560. [Google Scholar]
- Christy, A.G.; Lowe, A.; Otieno-Alego, V.; Stoll, M.; Webster, R.D. Voltammetric and raman microspectroscopic studies on artificial copper pits grown in simulated potable water. J. Appl. Electrochem. 2004, 34, 225–233. [Google Scholar] [CrossRef]
- Paradies, H.H.; Hänßel, I.; Fischer, W.; Wagner, D. Microbiologically Induced Corrosion on Copper Pipes; International Copper Research Association: New York, NY, USA, 1990. [Google Scholar]
- Frenkel, A.I.; Korshin, G.V. Exafs studies of the chemical state of lead and copper in corrosion products formed on the brass surface in potable water. J. Synchrotron Radiat. 1999, 6, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Higashi, Y.; Tomita, M.; Ichino, T. Microstructural analysis of artificially formed patinas on copper. Electrochem. Solid State Lett. 2002, 5, B28–B31. [Google Scholar] [CrossRef]
- Fonsati, M.; Zucchi, F.; Trabanelli, G. Study of corrosion inhibition of copper in 0.1 m NaCl using the eqcm technique. Electrochim. Acta 1998, 44, 311–322. [Google Scholar] [CrossRef]
- Brusic, V.; Frisch, M.; Eldridge, B.; Novak, F.; Kaufman, F.; Rush, B.; Frankel, G. Copper corrosion with and without inhibitors. J. Electrochem. Soc. 1991, 138, 2253–2259. [Google Scholar] [CrossRef]
- Lewandowski, B.R.; Lytle, D.A.; Garno, J.C. Nanoscale investigation of the impact of pH and orthophosphate on the corrosion of copper surfaces in water. Langmuir 2010, 26, 14671–14679. [Google Scholar] [CrossRef] [PubMed]
- Abelev, E.; Starosvetsky, D.; Ein-Eli, Y. Potassium sorbate—A new aqueous copper corrosion inhibitor electrochemical and spectroscopic studies. Electrochim. Acta 2007, 52, 1975–1982. [Google Scholar] [CrossRef]
- Frost, R.L. Raman spectroscopy of selected copper minerals of significance in corrosion. Spectrochim. Acta Mol. Biomol. Spectrosc. 2003, 59, 1195. [Google Scholar] [CrossRef]
- Martiny, A.C.; Jorgensen, T.M.; Albrechtsen, H.J.; Arvin, E.; Molin, S. Long-term succession of structure and diversity of a biofilm formed in a model drinking water distribution system. Appl. Environ. Microbiol. 2003, 69, 6899–6907. [Google Scholar] [CrossRef] [PubMed]
- Amann, R.I.; Ludwig, W.; Schleifer, K.H. Phylogenetic identification and in-situ detection of individual microbial-cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [PubMed]
- Bremer, P.J.; Webster, B.J.; Wells, D.B. Biocorrosion of copper in potable water. J. Am. Water Work Assoc. 2001, 93, 82–91. [Google Scholar]
- Bermont-Bouis, D.; Janvier, M.; Grimont, P.A.D.; Dupont, I.; Vallaeys, T. Both sulfate-reducing bacteria and enterobacteriaceae take part in marine biocorrosion of carbon steel. J. Appl. Microbiol. 2007, 102, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Fang, H.H.P.; Ko, B.C.B. Methanogen population in a marine biofilm corrosive to mild steel. Appl. Microbiol. Biotechnol. 2003, 63, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Marchand, E.A.; Roberts, D.; Peccia, J. In situ assessment of active thiobacillus species in corroding concrete sewers using fluorescent rna probes. Int. Biodeterior. Biodegrad. 2002, 49, 271–276. [Google Scholar] [CrossRef]
- Chen, S.; Wang, P.; Zhang, D. Corrosion behavior of copper under biofilm of sulfate-reducing bacteria. Corros. Sci. 2014, 87, 407–415. [Google Scholar] [CrossRef]
- Beale, D.J.; Dunn, M.S.; Marney, D. Application of gc-ms metabolic profiling to ‘blue-green water’ from microbial influenced corrosion in copper pipes. Corros. Sci. 2010, 52, 3140–3145. [Google Scholar] [CrossRef]
- Beale, D.J.; Dunn, M.S.; Morrison, P.D.; Porter, N.A.; Marlow, D.R. Characterisation of bulk water samples from copper pipes undergoing microbially influenced corrosion by diagnostic metabolomic profiling. Corros. Sci. 2012, 55, 272–279. [Google Scholar] [CrossRef]
- Beale, D.J.; Karpe, A.V.; Jadhav, S.; Muster, T.H.; Palombo, E.A. Omics-based approaches and their use in the assessment of microbial-influenced corrosion of metals. Corros. Rev. 2016, 34, 1–15. [Google Scholar] [CrossRef]
- DIN. Corrosion of Metals—Corrosion Testing of Drinking Water Distribution Systems—Part 1: Determining Changes to the Composition of Drinking Water; German National Standard; Deutsches Institut Fur Normung E.V.: Berlin, Germany, 1999. [Google Scholar]
- Lagos, G.E.; Maggi, L.C.; Peters, D.; Reveco, F. Model for estimation of human exposure to copper in drinking water. Sci. Total Environ. 1999, 239, 49–70. [Google Scholar] [CrossRef]
- Poulson, B. Advances in understanding hydrodynamic effects on corrosion. Corros. Sci. 1993, 35, 655–665. [Google Scholar] [CrossRef]
- Fries, J.S. Predicting interfacial diffusion coefficients for fluxes across the sediment-water interface. J. Hydraul. Eng. 2007, 133, 267–272. [Google Scholar] [CrossRef]
- Higashino, M.; Stefan, H.G. Diffusive boundary layer development above a sediment-water interface. Water Environ. Res. 2004, 76, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, N.; Hondzo, M. Diffusional mass transfer at sediment-water interface. J. Environ. Eng. 1999, 125, 192–200. [Google Scholar] [CrossRef]
- Jeria, G.A.; Vargas, I.T.; Walczak, M.M.; Pastén, P.A.; Pizarro, G.E. Effect of hydrodynamic conditions on copper release in drinking water systems. In Proceedings of the European Corrosion Congress (EUROCORR 2010), Moscow, Russia, 13–17 September 2010. [Google Scholar]
- Harrison, D.B.; Nicholas, D.M.; Evans, G.M. Pitting corrosion of copper tubes in soft drinking waters: Corrosion mechanism. J. Am. Water Work Assoc. 2004, 96, 67–76. [Google Scholar]
- Geesey, G.G.; Bremer, P.J.; Fischer, W.; Wagner, D.; Keevil, C.W.; Walker, J.T.; Chamberlain, A.H.L.; Angell, P. Unusual types of pitting corrosion of copper tubes in potable water systems. In Biofouling and Biocorrosion in Industrial Water Systems; Geesey, G.G., Lewandowski, Z., Flemming, H.C., Eds.; Lewis Publishers: New York, NY, USA, 1994; pp. 243–263. [Google Scholar]
- De Chialvo, M.G.; Zerbino, J.; Marchiano, S.; Arvia, A. Correlation of electrochemical and ellipsometric data in relation to the kinetics and mechanism of Cu2O electroformation in alkaline solutions. J. Appl. Electrochem. 1986, 16, 517–526. [Google Scholar] [CrossRef]
- Feng, Y.; Siow, K.S.; Teo, W.K.; Tan, K.L.; Hsieh, A.K. Corrosion mechanisms and products of copper in aqueous solutions at various pH values. Corrosion 1997, 53, 389–398. [Google Scholar] [CrossRef]
- Taxen, C.; Letelier, M.V.; Lagos, G. Model for estimation of copper release to drinking water from copper pipes. Corros. Sci. 2012, 58, 267–277. [Google Scholar] [CrossRef]
- Wagner, D.; Chamberlain, A.H.L. Microbiologically influenced copper corrosion in potable water with emphasis on practical relevance. Biodegradation 1997, 8, 177–187. [Google Scholar]
- Battin, T.J.; Kaplan, L.A.; Newbold, J.D.; Hansen, C.M.E. Contributions of microbial biofilms to ecosystem processes in stream mesocosms. Nature 2003, 426, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Arens, P.; Tuschewitzki, G.J.; Wollmann, H.; Follner, H.; Jacobi, H. Indicators for microbiologically induced corrosion of copper pipes in a cold water plumbing system. Zbl. Hyg. Umweltmed. 1995, 196, 444–454. [Google Scholar]
- Walker, J.T.; Wagner, D.; Fischer, W.; Keevil, C.W. Rapid detection of biofilm on corroded copper pipes. Biofouling 1994, 8, 55–63. [Google Scholar] [CrossRef]
- Waines, P.L.; Moate, R.; Moody, A.J.; Allen, M.; Bradley, G. The effect of material choice on biofilm formation in a model warm water distribution system. Biofouling 2011, 27, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Noguera, D.R.; Pizarro, G.; Regan, J.M. Modeling biofilms. In Microbial Biofilms; Ghannoum, M.A., O’Toole, G.A., Eds.; ASM Press: Washington, DC, USA, 2004; pp. 222–249. [Google Scholar]
- Xavier, J.B.; Picioreanu, C.; van Loosdrecht, M.C.M. A framework for multidimensional modelling of activity and structure of multispecies biofilms. Environ. Microbiol. 2005, 7, 1085–1103. [Google Scholar] [CrossRef] [PubMed]
- Picioreanu, C.; Kreft, J.U.; van Loosdrecht, M.C.M. Particle-based multidimensional multispecies biofilm model. Appl. Environ. Microbiol. 2004, 70, 3024–3040. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.; Gilbert, E.S.; Eliashberg, N.; Keasling, J.D. A three-dimensional, stochastic simulation of biofilm growth and transport-related factors that affect structure. Microbiology 2003, 149, 2859–2871. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, G.; Griffeath, D.; Noguera, D.R. Quantitative cellular automaton model for biofilms. J. Environ. Eng. 2001, 127, 782–789. [Google Scholar] [CrossRef]
- Pizarro, G.E.; Teixeira, J.o.; Sepúlveda, M.; Noguera, D.R. Bitwise implementation of a two-dimensional cellular automata biofilm model. J. Comput. Civ. Eng. 2005, 19, 258–268. [Google Scholar] [CrossRef]
- Guibaud, G.; Tixier, N.; Bouju, A.; Baudu, M. Relation between extracellular polymers’ composition and its ability to complex cd, Cu and Pb. Chemosphere 2003, 52, 1701–1710. [Google Scholar] [CrossRef]
- Jolley, J.G.; Geesey, G.G.; Hankins, M.R.; Wright, R.B.; Wichlacz, P.L. In situ, real-time ft-ir/cir/atr study of the biocorrosion of copper by gum arabic, alginic acid, bacterial culture supernatant and pseudomonas atlantica exopolymer. Appl. Spectrosc. 1989, 43, 1062–1067. [Google Scholar] [CrossRef]
- Fischer, W.R.; Wagner, D.; Siedlarek, H. Microbiologically influenced corrosion in potable water installations: An engineering approach to developing countermeasures. Mater. Perform. 1995, 34, 50–54. [Google Scholar]
- Picioreanu, C.; van Loosdrecht, M.C.M. A mathematical model for initiation of microbiologically influenced corrosion by differential aeration. J. Electrochem. Soc. 2002, 149, B211–B223. [Google Scholar] [CrossRef]
- Efird, K.D. Effect of fluid dynamics on the corrosion of copper-base alloys in sea water. Corrosion 1977, 33, 3–8. [Google Scholar] [CrossRef]
- Yabuki, A.; Murakami, M. Breakaway properties of film formed on copper and copper alloys in erosion-corrosion by mass transfer equation. Mater. Corros. 2008, 59, 25. [Google Scholar] [CrossRef]
- Lehtola, M.J.; Laxander, M.; Miettinen, I.T.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. The effects of changing water flow velocity on the formation of biofilms and water quality in pilot distribution system consisting of copper or polyethylene pipes. Water Res. 2006, 40, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Malki, B.; Baroux, B. Computer simulation of the corrosion pit growth. Corros. Sci. 2005, 47, 171. [Google Scholar] [CrossRef]
- Ha, H.; Taxen, C.; Cong, H.B.; Scully, J.R. Effect of applied potential on pit propagation in copper as function of water chemistry. J. Electrochem. Soc. 2011, 159, C59–C73. [Google Scholar] [CrossRef]
- Newman, J.S. Electrochemical Systems; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1973. [Google Scholar]
- Seyeux, A.; Maurice, V.; Marcus, P. Oxide film growth kinetics on metals and alloys I. Physical model. J. Electrochem. Soc. 2013, 160, C189–C196. [Google Scholar] [CrossRef]





| Study | Country | Metallic Taste (mg/L of Cu) | |
|---|---|---|---|
| (In Distilled Water) | (In Tap Water) | ||
| Cohen et al. [45] * | U.S. | 6.6 *** | 13 ** |
| Zacarias et al. [46] * | Chile | 2.5 | 2.6 |
| Dietrich et al. [10] | U.S. | 0.5 | |
| By-Product | Chemical Formula | References |
|---|---|---|
| cuprite | Cu2O | [14,98,99,100,117,119] |
| tenorite | CuO | [14,99,117] |
| malachite | Cu2(OH)2CO3 | [14,99,100,117] |
| cuprous chloride | CuCl | [97,99,119] |
| cupric chloride | CuCl2 | [14,98] |
| atacamite | Cu2Cl(OH)3 | [97,98,99] |
| antlerite | Cu3SO4(OH)4 | [127] |
| brochantite | Cu4SO4(OH)6 | [97] |
| posnjakite | Cu4(OH)6SO4·H2O | [14] |
| langite | Cu4(OH)6 SO4·2H2O | [100] |
| Technique | Information | References |
|---|---|---|
| X-ray diffraction (XRD) | Crystalline identity of the corrosion scale. Requires certain knowledge about the chemical composition of the scale. | (See Table 2 for References) |
| Quartz crystal microbalance (QCM) | Evolution of the corrosion scale mass as a result of by-product deposition. | [123,128] |
| Time-of-Flight Secondary Ion Mass Spectroscopy (TOF-SIMS) | Atomic and molecular structure of organic and inorganic components of the scale. | [129,130] |
| X-ray Spectroscopy | ||
| Energy dispersive X-ray spectroscopy (EDS) | Chemical composition of the corrosion scale. Often coupled with electron microscopy (EM). | [120,121] |
| X-ray photoelectron spectroscopy (XPS) | Chemical composition and speciation in the corrosion scale. | [97,122,131] |
| X-ray absorption spectroscopy (XAS) | Coordination chemistry and short-range ordering of a given element inside the corrosion scale. | [15,102,126] |
| Vibrational Spectroscopy | ||
| Fourier transformed Infrared spectroscopy (FT-IR) | Molecular structure of organic and inorganic components of the scale. | [123,128] |
| Raman spectroscopy | Molecular structure of organic and inorganic components in the scale. | [124,132] |
| Electrochemical techniques | ||
| Cyclic voltammetry | Scale composition based on its electrochemical properties. | [131] |
| Linear sweep voltammetry | Short-term experiment of accelerated pitting corrosion under different solutions. | [124] |
| Electrochemical impedance spectroscopy (EIS) | Electrochemical characterization and stability of formed films. | [47,122] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, I.T.; Fischer, D.A.; Alsina, M.A.; Pavissich, J.P.; Pastén, P.A.; Pizarro, G.E. Copper Corrosion and Biocorrosion Events in Premise Plumbing. Materials 2017, 10, 1036. https://doi.org/10.3390/ma10091036
Vargas IT, Fischer DA, Alsina MA, Pavissich JP, Pastén PA, Pizarro GE. Copper Corrosion and Biocorrosion Events in Premise Plumbing. Materials. 2017; 10(9):1036. https://doi.org/10.3390/ma10091036
Chicago/Turabian StyleVargas, Ignacio T., Diego A. Fischer, Marco A. Alsina, Juan P. Pavissich, Pablo A. Pastén, and Gonzalo E. Pizarro. 2017. "Copper Corrosion and Biocorrosion Events in Premise Plumbing" Materials 10, no. 9: 1036. https://doi.org/10.3390/ma10091036





