Preparation and Chemical/Microstructural Characterization of Azacrown Ether-Crosslinked Chitosan Films
Abstract
:1. Introduction
2. Results and Discussion
2.1. Basic Characteristics of the Chitosan Starting Material
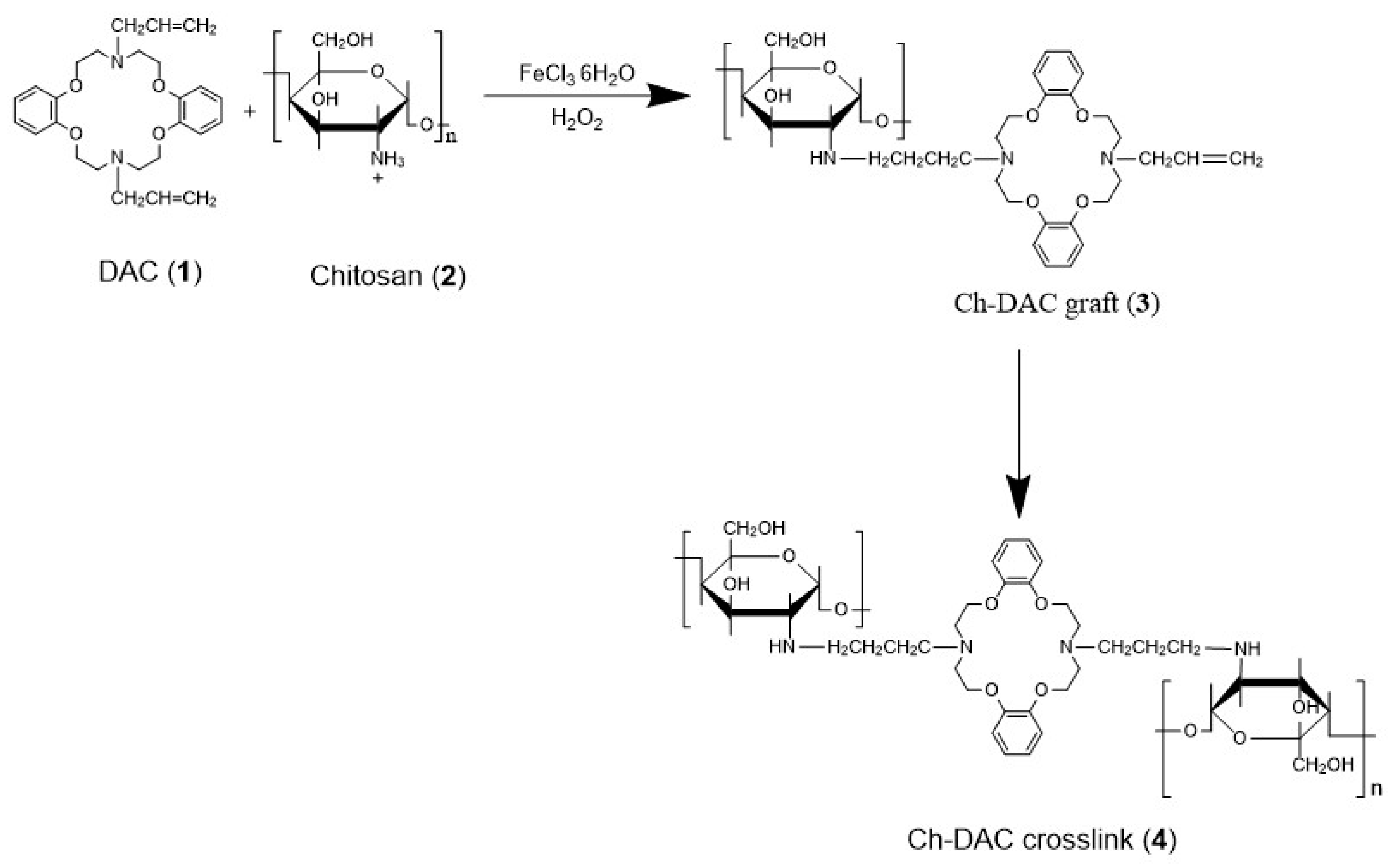
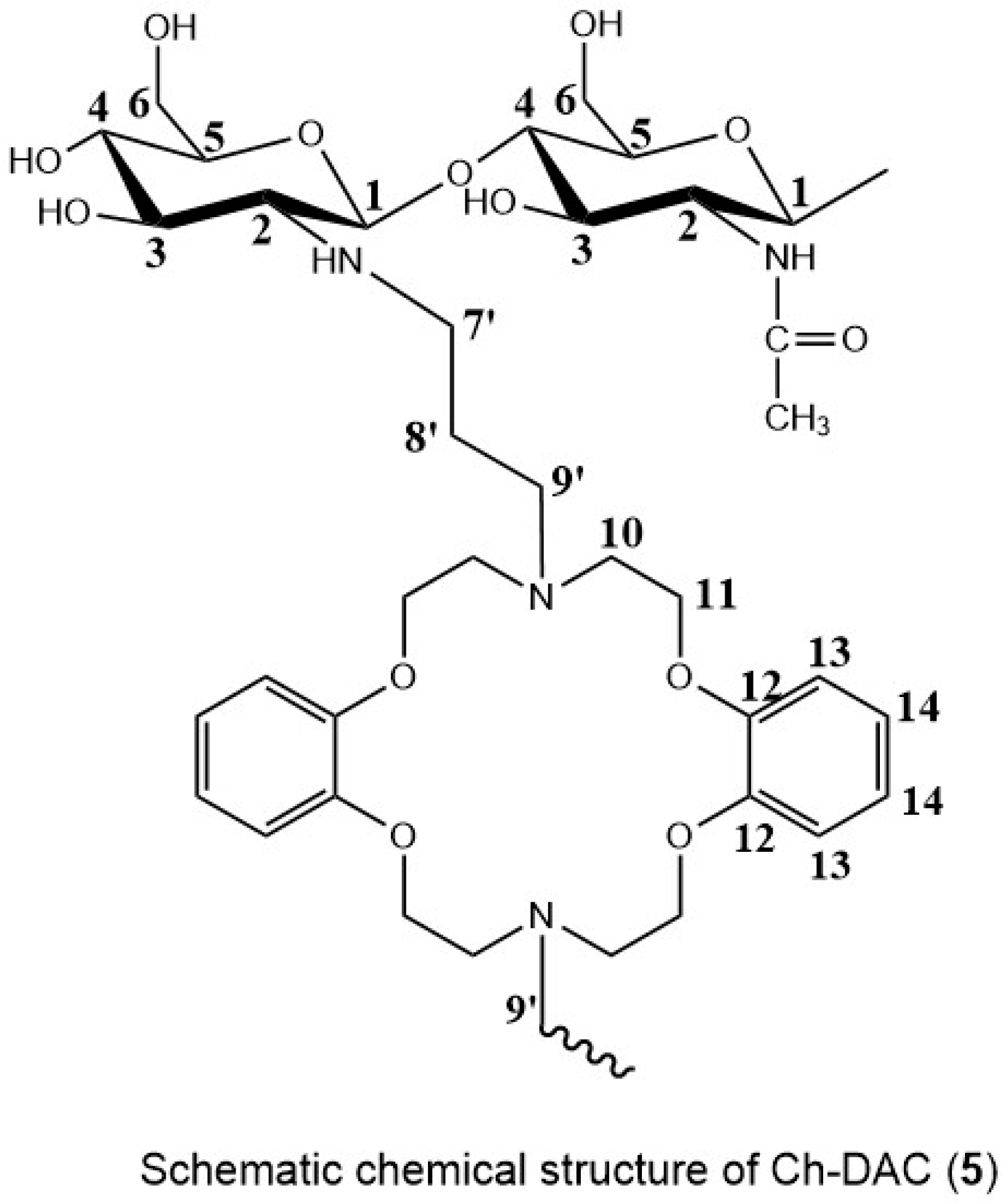
2.2. Composition and Crosslinking Chemistry of the Azacrown Ether/Chitosan Films
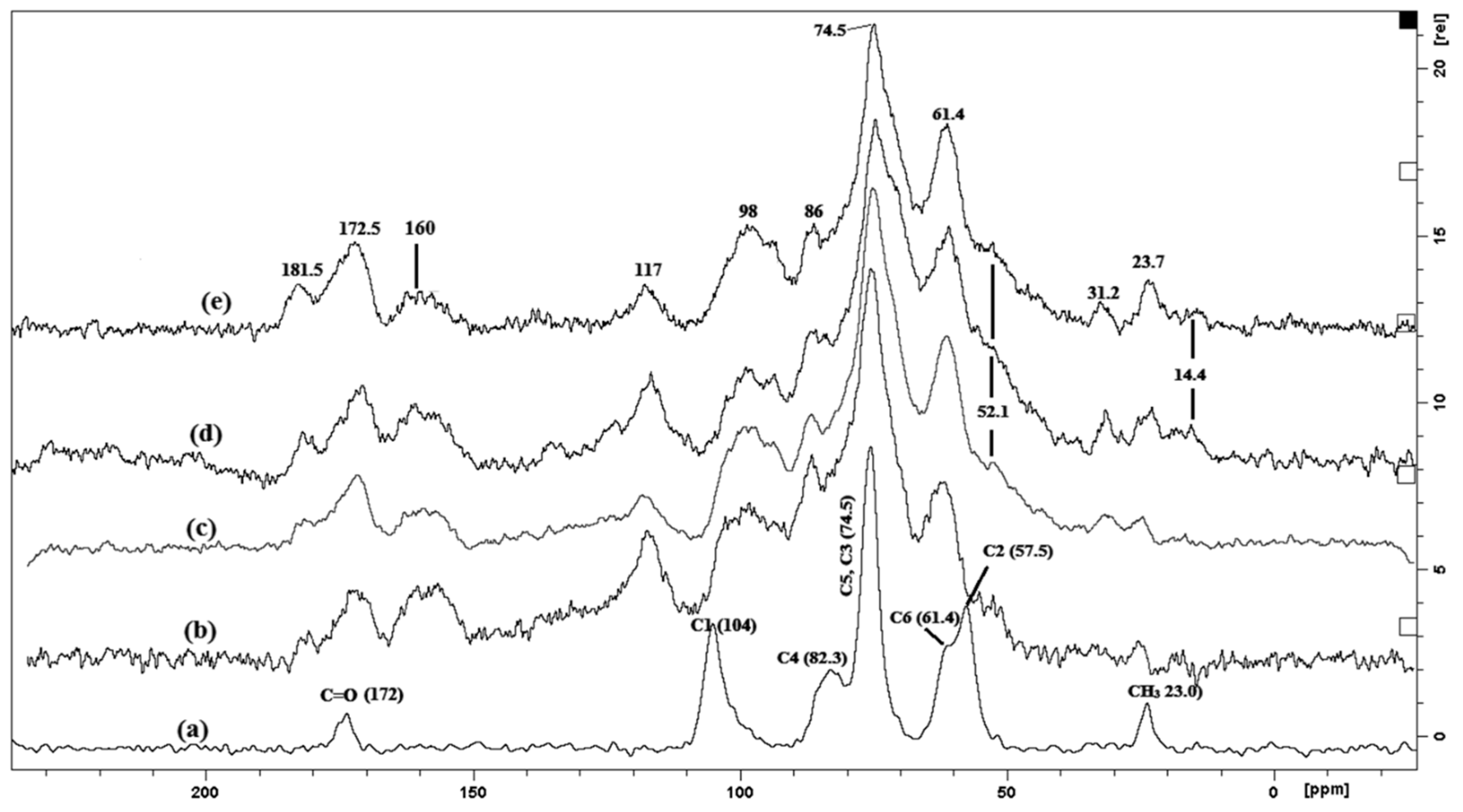
2.2.1. Carbon-NMR Analysis
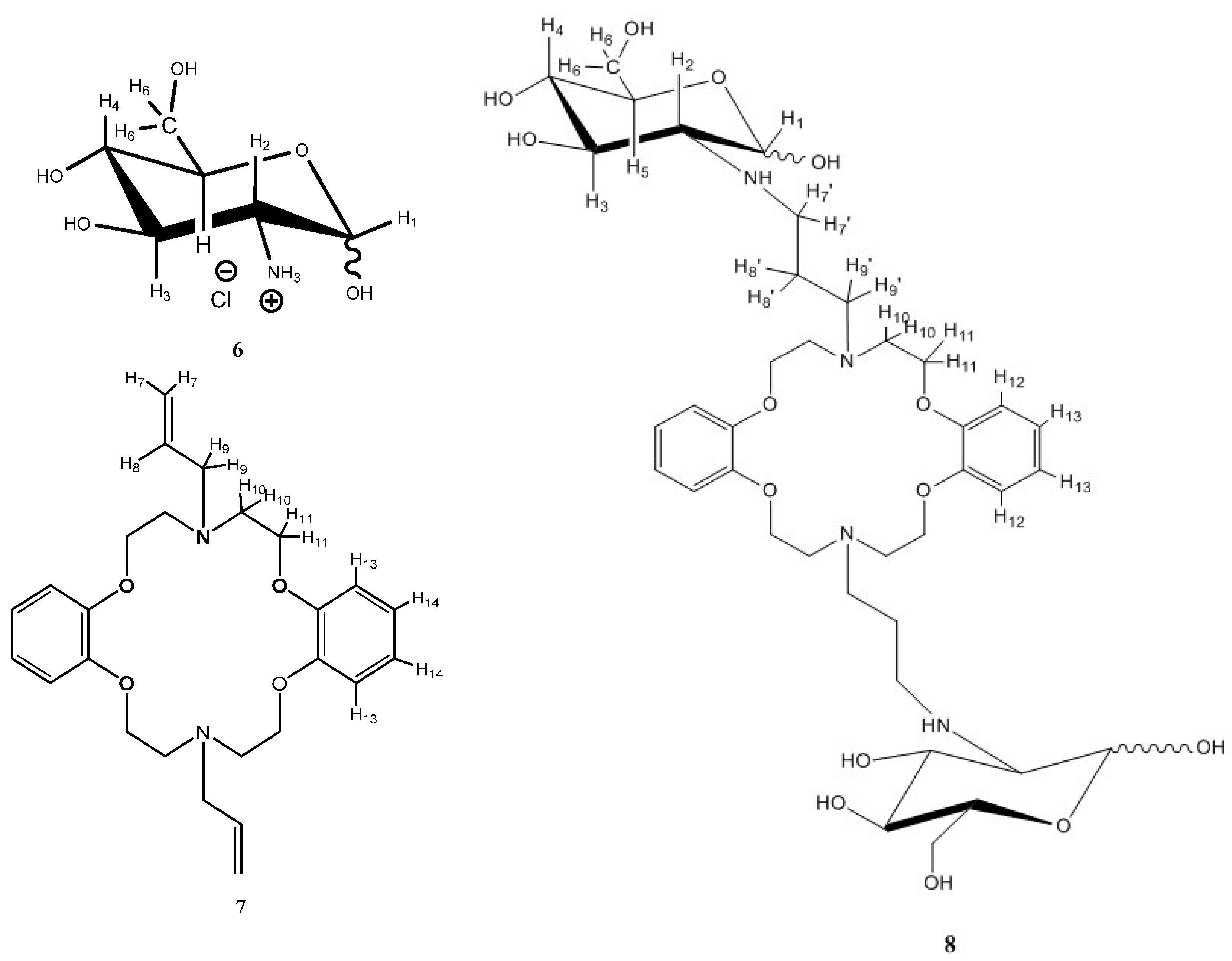
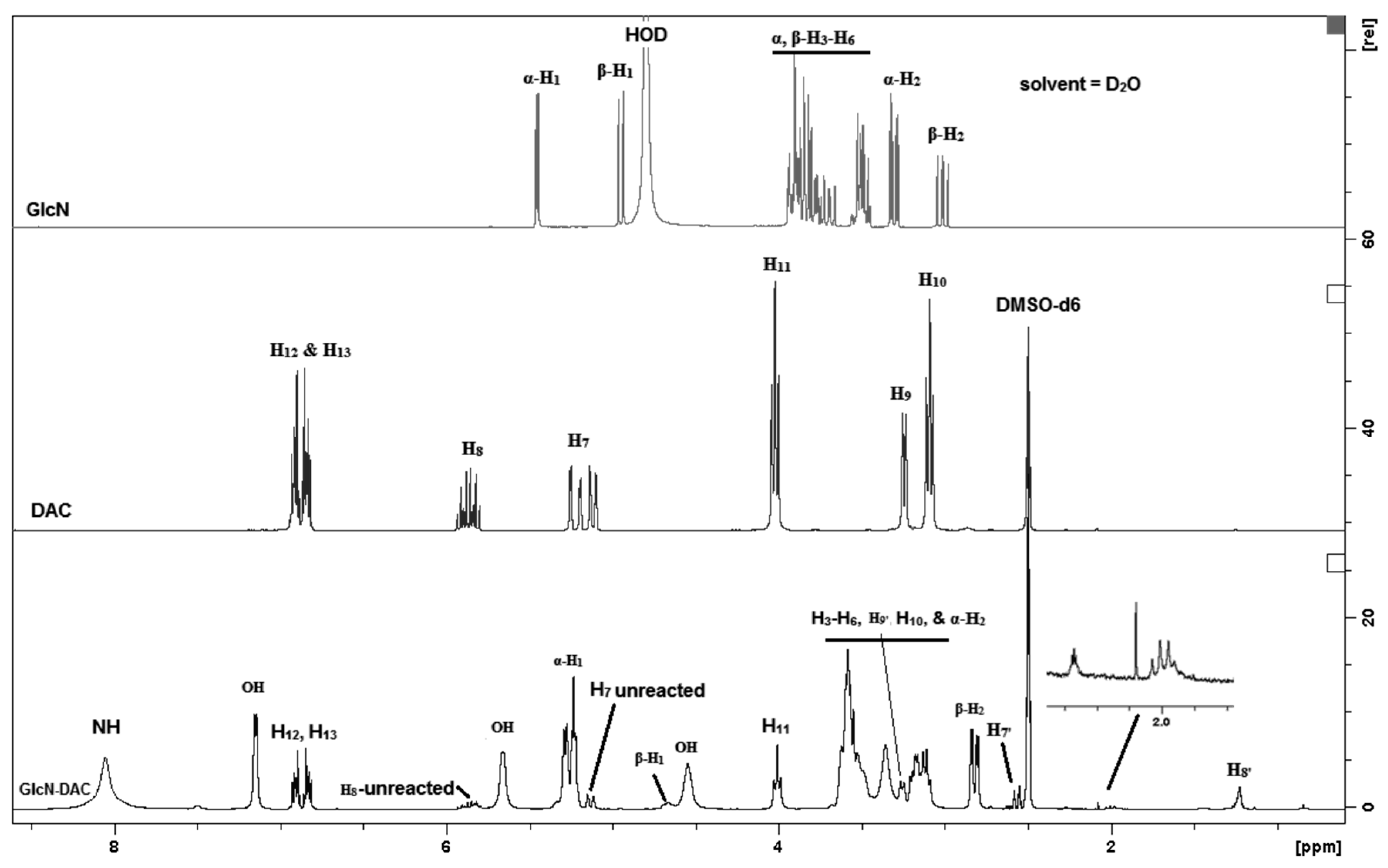
2.2.2. Model Studies to Verify the Chemical Reaction: Hydroamination of Azacrown Ether (DAC) by Glucosamine
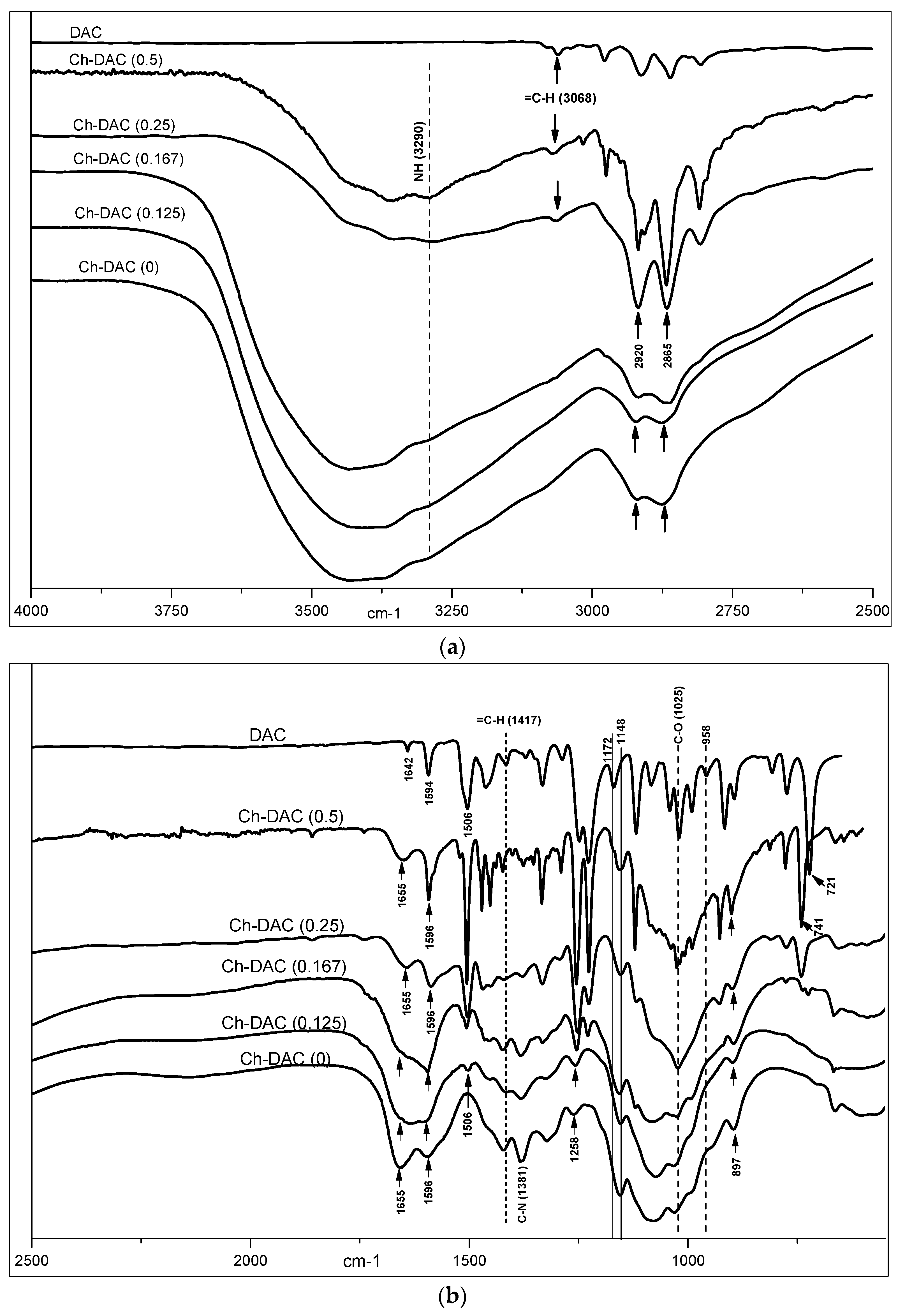
2.2.3. Fourier Transform Infrared (FTIR) Analysis
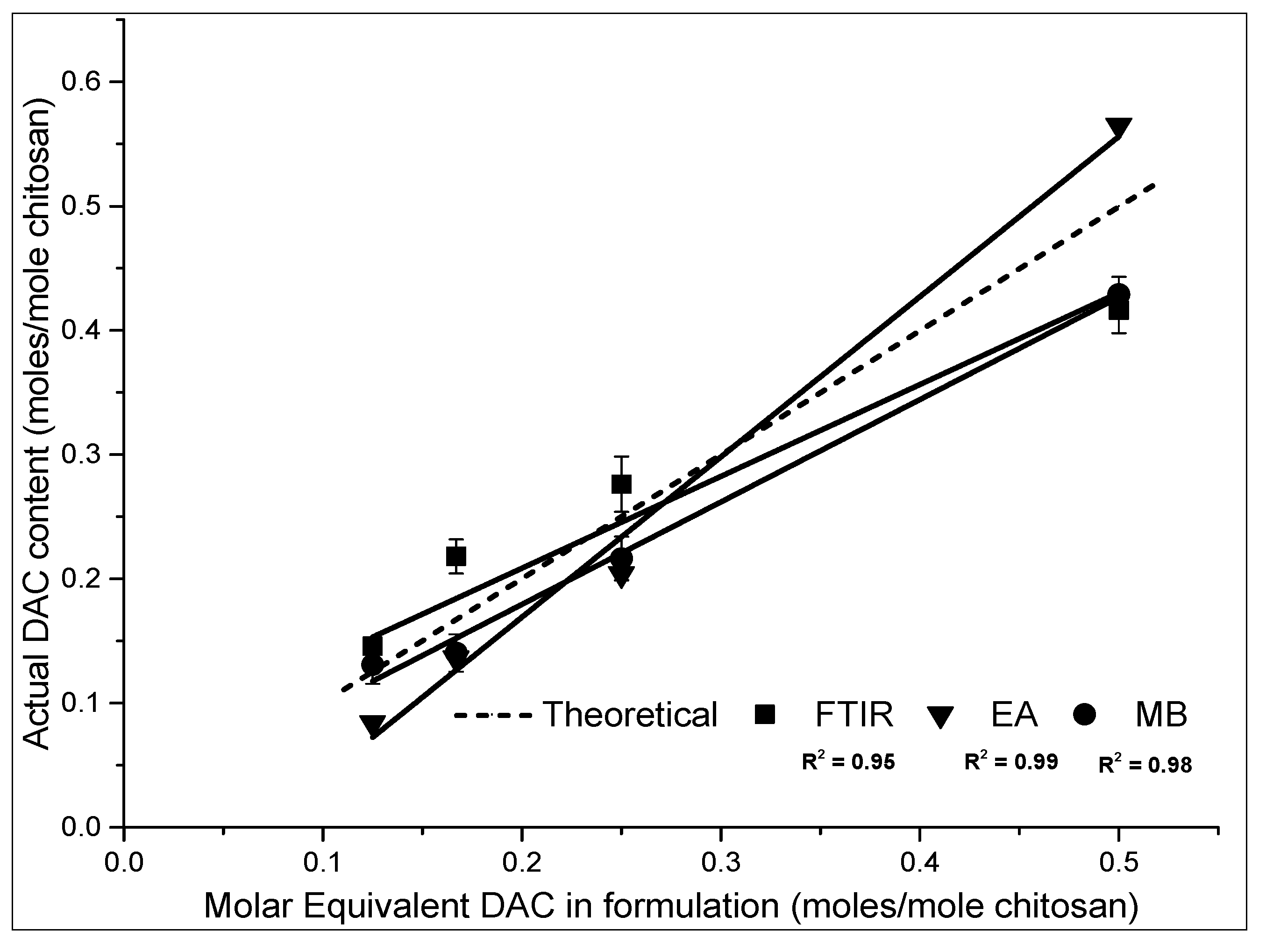
2.2.4. Stoichiometric Control of the Composition
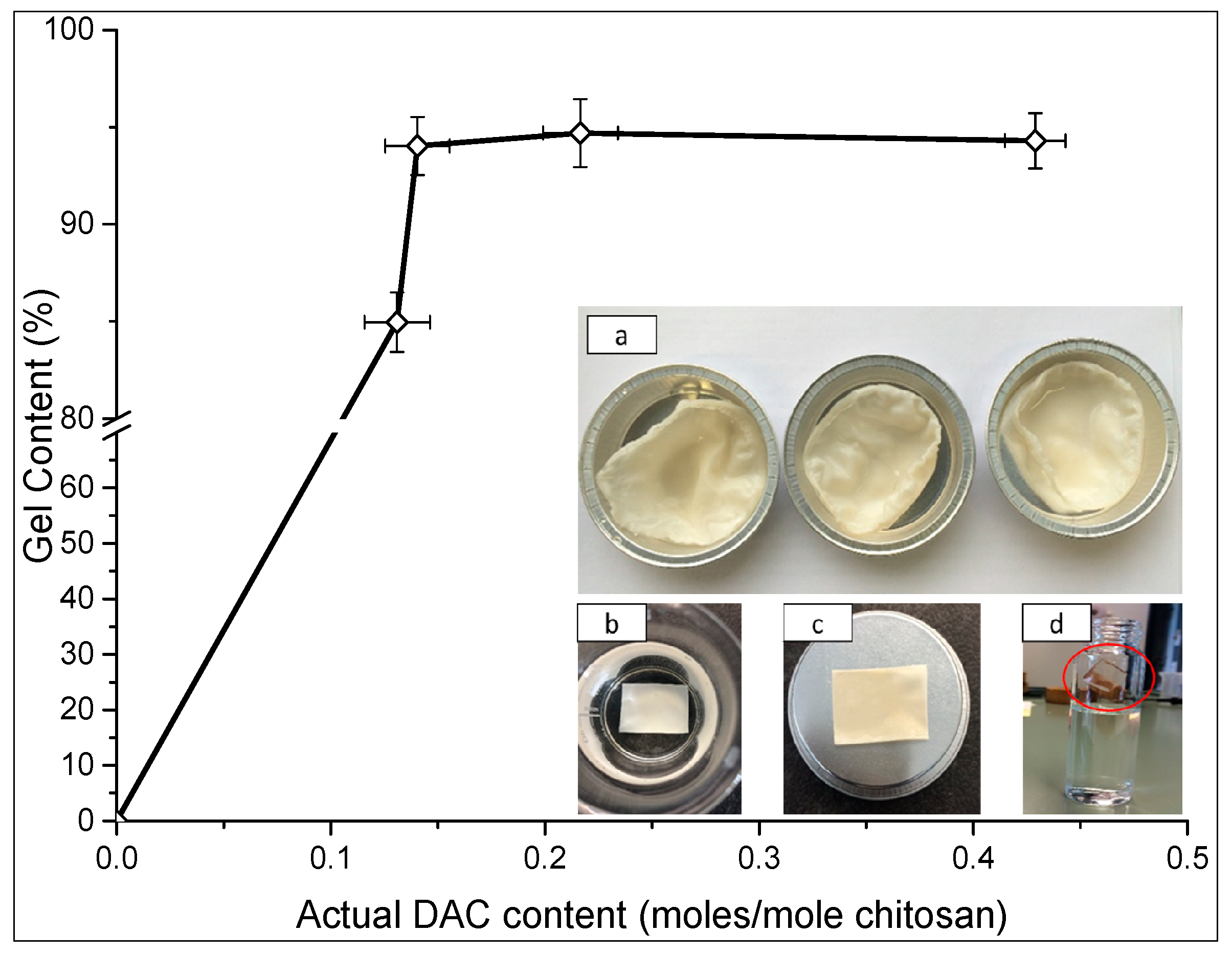
2.3. Gel Content
2.4. Degree of Substitution
2.5. Microstructural Characterization of the Azacrown Ether/Chitosan Films
2.5.1. Scanning Electron Microscopy (SEM) Images of the Synthesized Films
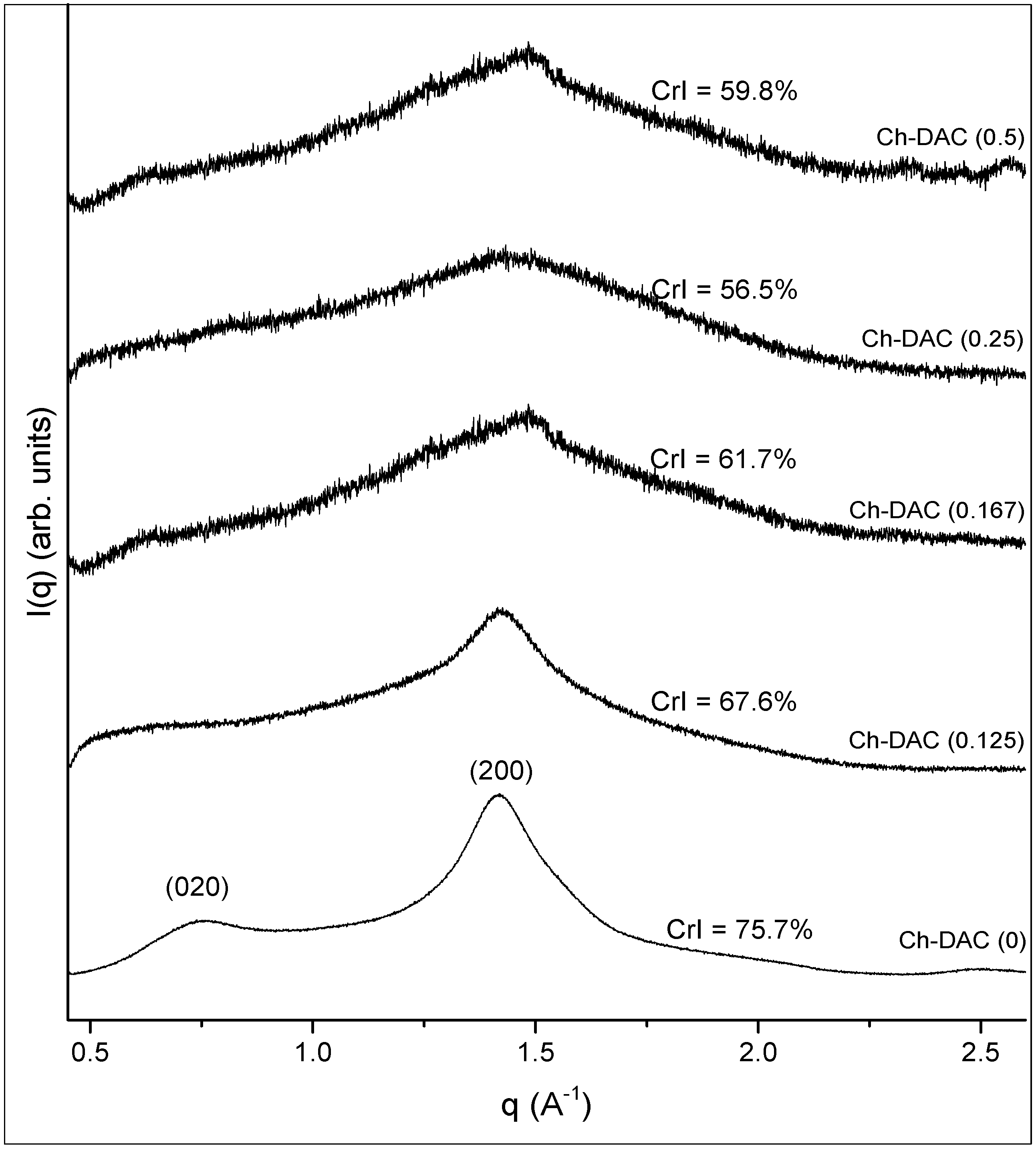
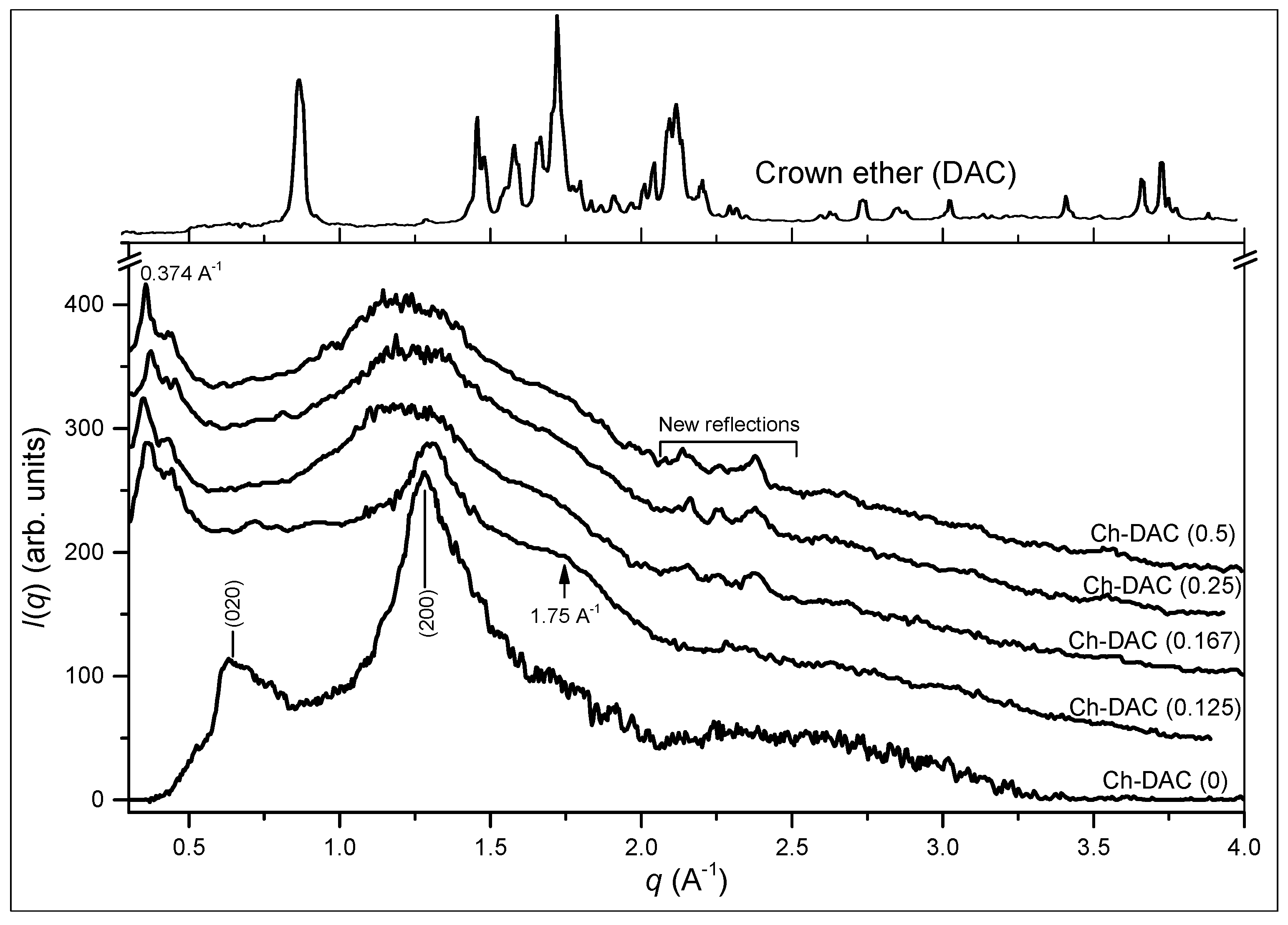
2.5.2. Crystalline Microstructure
3. Materials and Methods
3.1. Starting Materials
3.2. Preparation of Azacrown Ether/Chitosan Hydrogel Films
3.3. Model Studies to Verify the Chemical Reaction: Hydroamination of DAC by Glucosamine (GlcN-DAC)
3.4. Chemical Structure Analysis
3.5. Gel Content
3.6. Microstructural Characterization of the Azacrown Ether/Chitosan Films
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Varma, A.; Deshpande, S.; Kennedy, J. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Rijith, S. Glutaraldehyde cross-linked epoxyaminated chitosan as an adsorbent for the removal and recovery of copper(II) from aqueous media. Colloids Surf. Physicochem. Eng. Asp. 2009, 351, 52–59. [Google Scholar] [CrossRef]
- Chen, A.-H.; Yang, C.-Y.; Chen, C.-Y.; Chen, C.-Y.; Chen, C.-W. The chemically crosslinked metal-complexed chitosans for comparative adsorptions of Cu(II), Zn(II), Ni(II) and Pb(II) ions in aqueous medium. J. Hazard. Mater. 2009, 163, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.; Wilson, L.D.; Headley, J.V. Chitosan-glutaraldehyde copolymers and their sorption properties. Carbohydr. Polym. 2014, 109, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Laus, R.; Costa, T.G.; Szpoganicz, B.; Fávere, V.T. Adsorption and desorption of Cu(II), Cd(II) and Pb(II) ions using chitosan crosslinked with epichlorohydrin-triphosphate as the adsorbent. J. Hazard. Mater. 2010, 183, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.-H.; Liu, S.-C.; Chen, C.-Y.; Chen, C.-Y. Comparative adsorption of Cu(II), Zn(II), and Pb(II) ions in aqueous solution on the crosslinked chitosan with epichlorohydrin. J. Hazard. Mater. 2008, 154, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Laus, R.; de Fávere, V.T. Competitive adsorption of Cu(II) and Cd(II) ions by chitosan crosslinked with epichlorohydrin–triphosphate. Bioresour. Technol. 2011, 102, 8769–8776. [Google Scholar] [CrossRef] [PubMed]
- Wan Ngah, W.; Endud, C.; Mayanar, R. Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar] [CrossRef]
- Izatt, R.M.; Bradshaw, J.S.; Nielsen, S.A.; Lamb, J.D.; Christensen, J.J.; Sen, D. Thermodynamic and kinetic data for cation-macrocycle interaction. Chem. Rev. 1985, 85, 271–339. [Google Scholar] [CrossRef]
- Izatt, R.M.; Pawlak, K.; Bradshaw, J.S.; Bruening, R.L. Thermodynamic and Kinetic Data for Macrocycle Interaction with Cations, Anions, and Neutral Molecules. Chem. Rev. 1995, 95, 2529–2586. [Google Scholar] [CrossRef]
- Guibal, E.; Von Offenberg Sweeney, N.; Vincent, T.; Tobin, J. Sulfur derivatives of chitosan for palladium sorption. React. Funct. Polym. 2002, 50, 149–163. [Google Scholar] [CrossRef]
- Gregor, H.P.; Taifer, M.; Citarel, L.; Becker, E.I. Chelate Ion Exchange Resins. Ind. Eng. Chem. 1952, 44, 2834–2839. [Google Scholar] [CrossRef]
- Tunca, U.; Yagci, Y. Crown ether-containing polymers. Prog. Polym. Sci. 1994, 19, 233–286. [Google Scholar] [CrossRef]
- Alexandratos, S.D.; Stine, C.L. Synthesis of ion-selective polymer-supported crown ethers: A review. React. Funct. Polym. 2004, 60, 3–16. [Google Scholar] [CrossRef]
- Wan, L.; Wang, Y.; Qian, S. Study on the adsorption properties of novel crown ether crosslinked chitosan for metal ions. J. Appl. Polym. Sci. 2002, 84, 29–34. [Google Scholar] [CrossRef]
- Changhong, P.; Weijun, Y.; Motang, T. Chemical modification of chitosan: Synthesis and characterization of chitosan-crown ethers. J. Appl. Polym. Sci. 2003, 87, 2221–2225. [Google Scholar] [CrossRef]
- Malkondu, S.; Kocak, A.; Yilmaz, M. Immobilization of Two Azacrown Ethers on Chitosan: Evaluation of Selective Extraction Ability Toward Cu(II) and Ni(II). J. Macromol. Sci. Part A 2009, 46, 745–750. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Y.; Tang, Y. Synthesis of crosslinked chitosan-crown ethers and evaluation of these products as adsorbents for metal ions. J. Appl. Polym. Sci. 1998, 70, 501–506. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, S.; Wang, Y.; Feng, X.; Peng, Q.; Ma, S. Synthesis and adsorption properties of metal ions of novel azacrown ether crosslinked chitosan. J. Appl. Polym. Sci. 2006, 100, 2705–2709. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Y. Synthesis, characterization, and adsorption properties of chitosan azacrown ethers bearing hydroxyl group. J. Appl. Polym. Sci. 2001, 81, 1793–1798. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, X.; Feng, X.; Wang, Y.; Ma, S.; Peng, Q.; Zhang, W. Synthesis of N,N′-diallyl dibenzo 18-crown-6 crown ether crosslinked chitosan and their adsorption properties for metal ions. React. Funct. Polym. 2006, 66, 357–363. [Google Scholar] [CrossRef]
- Senn, H.M.; Blöchl, P.E.; Togni, A. Toward an Alkene Hydroamination Catalyst: Static and Dynamic ab Initio DFT Studies. J. Am. Chem. Soc. 2000, 122, 4098–4107. [Google Scholar] [CrossRef]
- Müller, T.E.; Hultzsch, K.C.; Yus, M.; Foubelo, F.; Tada, M. Hydroamination: Direct Addition of Amines to Alkenes and Alkynes. Chem. Rev. 2008, 108, 3795–3892. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.E.; Beller, M. Metal-Initiated Amination of Alkenes and Alkynes. Chem. Rev. 1998, 98, 675–704. [Google Scholar] [CrossRef] [PubMed]
- Kadib, A. Chitosan as a Sustainable Organocatalyst: A Concise Overview. Chemsuschem Rev. 2015, 8, 217–244. [Google Scholar] [CrossRef] [PubMed]
- Macquarrie, D.; Hardy, J. Applications of Functionalized Chitosan in Catalysis. Ind. Eng. Chem. Res. 2005, 44, 8499–8520. [Google Scholar] [CrossRef]
- Toeri, J.; Laborie, M.-P. Synthesis and Characterization of Macrocyclic Polyether N,N′-Diallyl-7,16-diaza-1,4,10,13-tetraoxa-dibenzo-18-crown-6. Molecules 2016, 21, 171. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.; Milas, M.; Dung, P.L. Characterization of chitosan. Influence of ionic strength and degree of acetylation on chain expansion. Int. J. Biol. Macromol. 1993, 15, 281–285. [Google Scholar] [CrossRef]
- Organikum: Organisch-Chemisches Grundpraktikum; Becker, H.G.O. (Ed.) Deutscher Verlag der Wissenschaften: Berlin, Germany, 1993. [Google Scholar]
- Krishnapriya, K.R.; Kandaswamy, M. Synthesis and characterization of a crosslinked chitosan derivative with a complexing agent and its adsorption studies toward metal(II) ions. Carbohydr. Res. 2009, 344, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Wang, Y.; Liu, H. Preparation of new crosslinked chitosan with crown ether and their adsorption for silver ion for antibacterial activities. Carbohydr. Polym. 2003, 53, 425–430. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Mathias, L.J.; Hankins, M.G.; Bertolucci, C.M.; Grubb, T.L.; Muthiah, J. Quantitative analysis by FTIR. Thin films of copolymers of ethylene and vinyl acetate. J. Chem. Educ. 1992, 69, A217. [Google Scholar] [CrossRef]
- Sen, M.; Yakar, A.; Güven, O. Determination of average molecular weight between cross-links (Mc) from swelling behaviours of diprotic acid-containing hydrogels. Polymer 1999, 40, 2969–2974. [Google Scholar] [CrossRef]
- Hayes, R.A. A New Look at Measurements of Network Density. Rubber Chem. Technol. 1986, 59, 138–141. [Google Scholar] [CrossRef]
- Antony, R. Structural, Surface, Thermal and Catalytic Properties of Chitosan Supported Cu(II) Mixed Ligand Complex Materials. J. Surf. Eng. Mater. Adv. Technol. 2012, 2, 284–291. [Google Scholar] [CrossRef]
- Mohamed, N.A.; El-Ghany, N.A.A.; Fahmy, M.M. Novel antimicrobial superporous cross-linked chitosan/pyromellitimide benzoyl thiourea hydrogels. Int. J. Biol. Macromol. 2016, 82, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Noguchi, K.; Miyazawa, T.; Yui, T.; Ogawa, K. Molecular and Crystal Structure of Hydrated Chitosan. Macromolecules 1997, 30, 5849–5855. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Peniche-Covas, C.; Rochas, C.; Putaux, J.-L.; Trombotto, S.; Alcouffe, P.; Domard, A. Fine microstructure of processed chitosan nanofibril networks preserving directional packing and high molecular weight. Carbohydr. Polym. 2015, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Peniche-Covas, C.; Domard, A. Kinetics study of the solid-state acid hydrolysis of chitosan: Evolution of the crystallinity and macromolecular structure. Biomacromolecules 2010, 11, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Goetz, L.; Mathew, A.; Oksman, K.; Gatenholm, P.; Ragauskas, A.J. A novel nanocomposite film prepared from crosslinked cellulosic whiskers. Carbohydr. Polym. 2009, 75, 85–89. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toeri, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation and Chemical/Microstructural Characterization of Azacrown Ether-Crosslinked Chitosan Films. Materials 2017, 10, 400. https://doi.org/10.3390/ma10040400
Toeri J, Osorio-Madrazo A, Laborie M-P. Preparation and Chemical/Microstructural Characterization of Azacrown Ether-Crosslinked Chitosan Films. Materials. 2017; 10(4):400. https://doi.org/10.3390/ma10040400
Chicago/Turabian StyleToeri, Julius, Anayancy Osorio-Madrazo, and Marie-Pierre Laborie. 2017. "Preparation and Chemical/Microstructural Characterization of Azacrown Ether-Crosslinked Chitosan Films" Materials 10, no. 4: 400. https://doi.org/10.3390/ma10040400
APA StyleToeri, J., Osorio-Madrazo, A., & Laborie, M.-P. (2017). Preparation and Chemical/Microstructural Characterization of Azacrown Ether-Crosslinked Chitosan Films. Materials, 10(4), 400. https://doi.org/10.3390/ma10040400







