Effect and Stability of Poly(Amido Amine)-Induced Biomineralization on Dentinal Tubule Occlusion
Abstract
:1. Introduction
2. Results
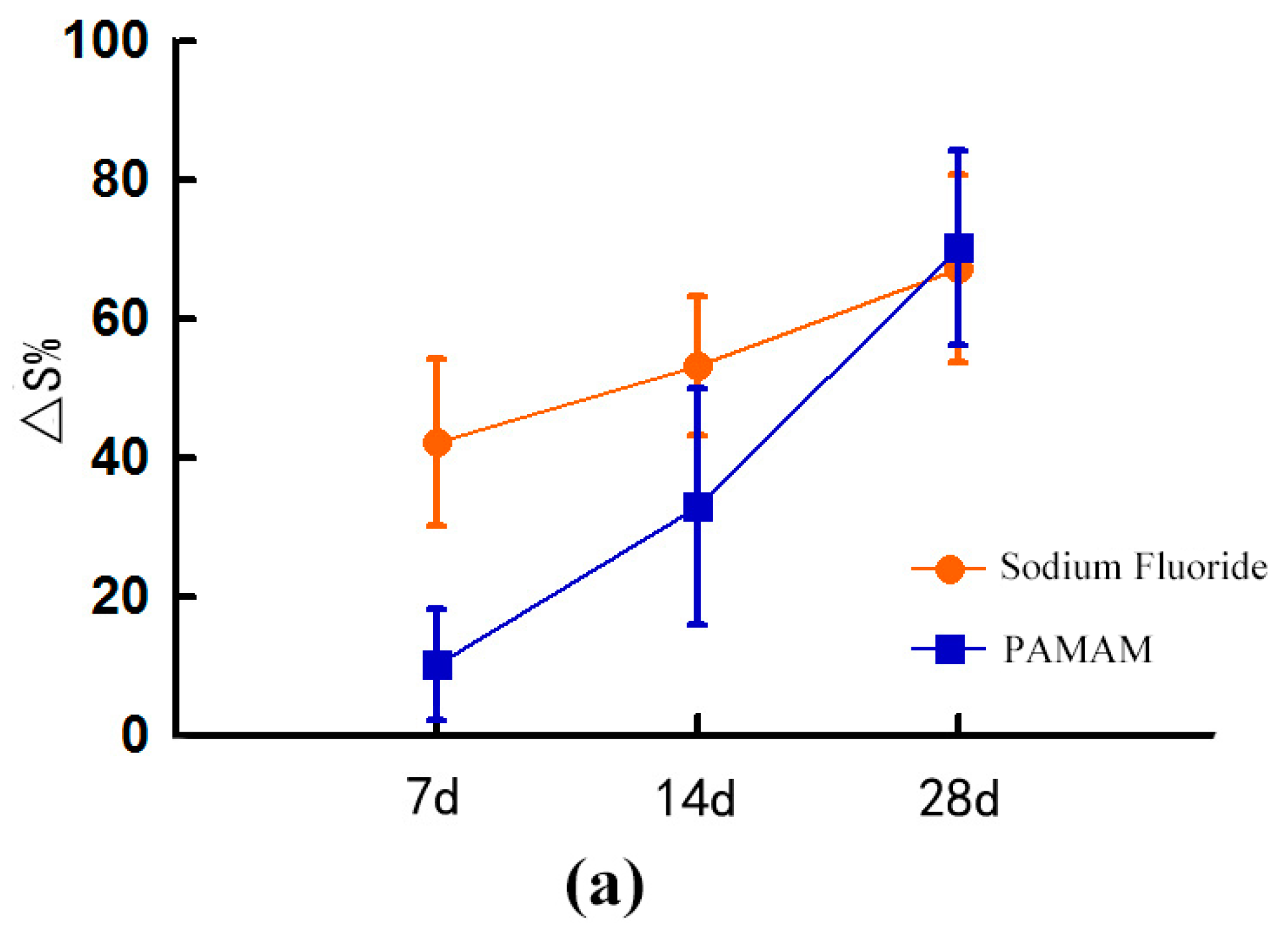
2.1. Dentinal Tubule Occlusion at Different Time Periods
2.1.1. Dentine Permeability Measurements
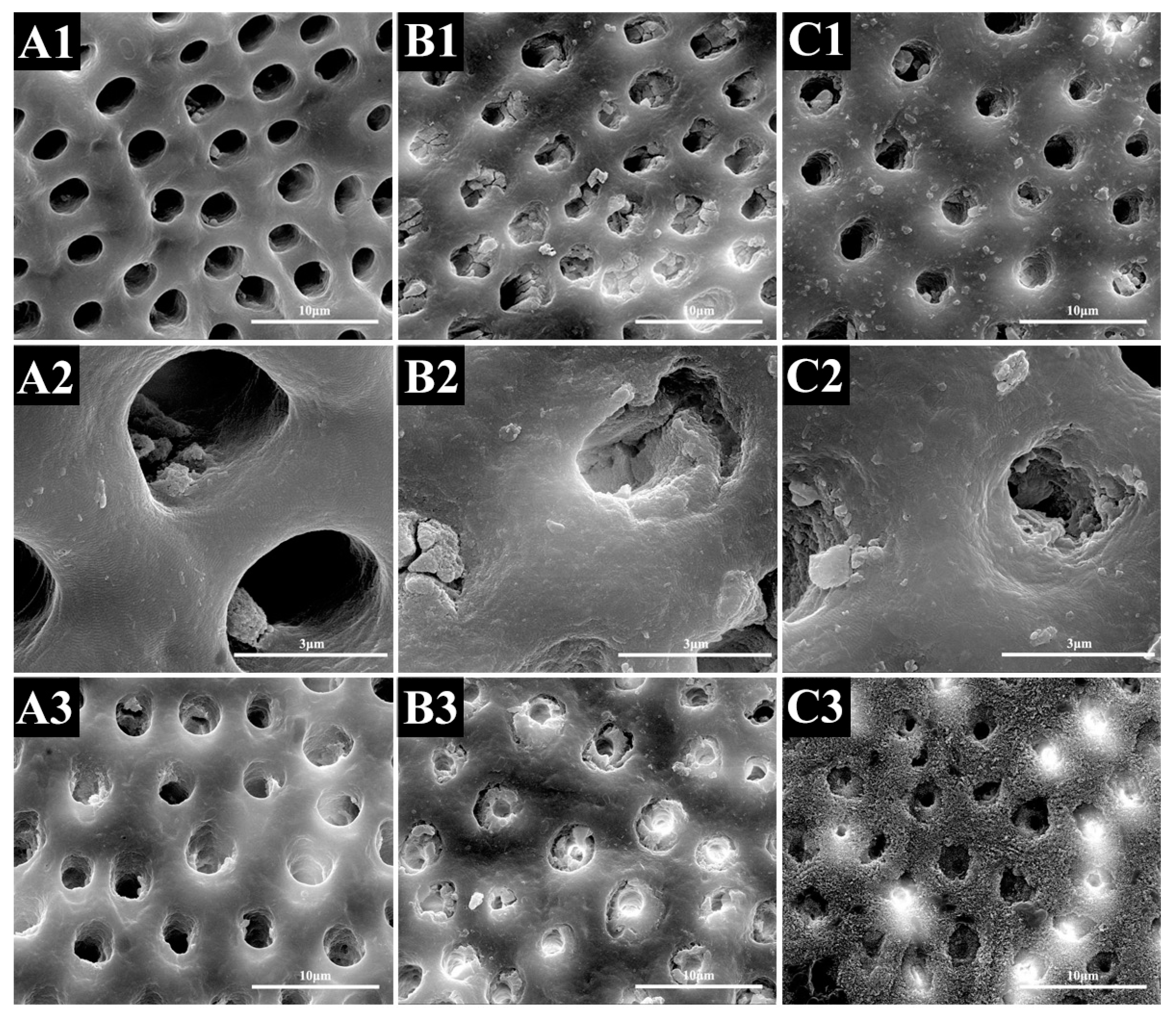
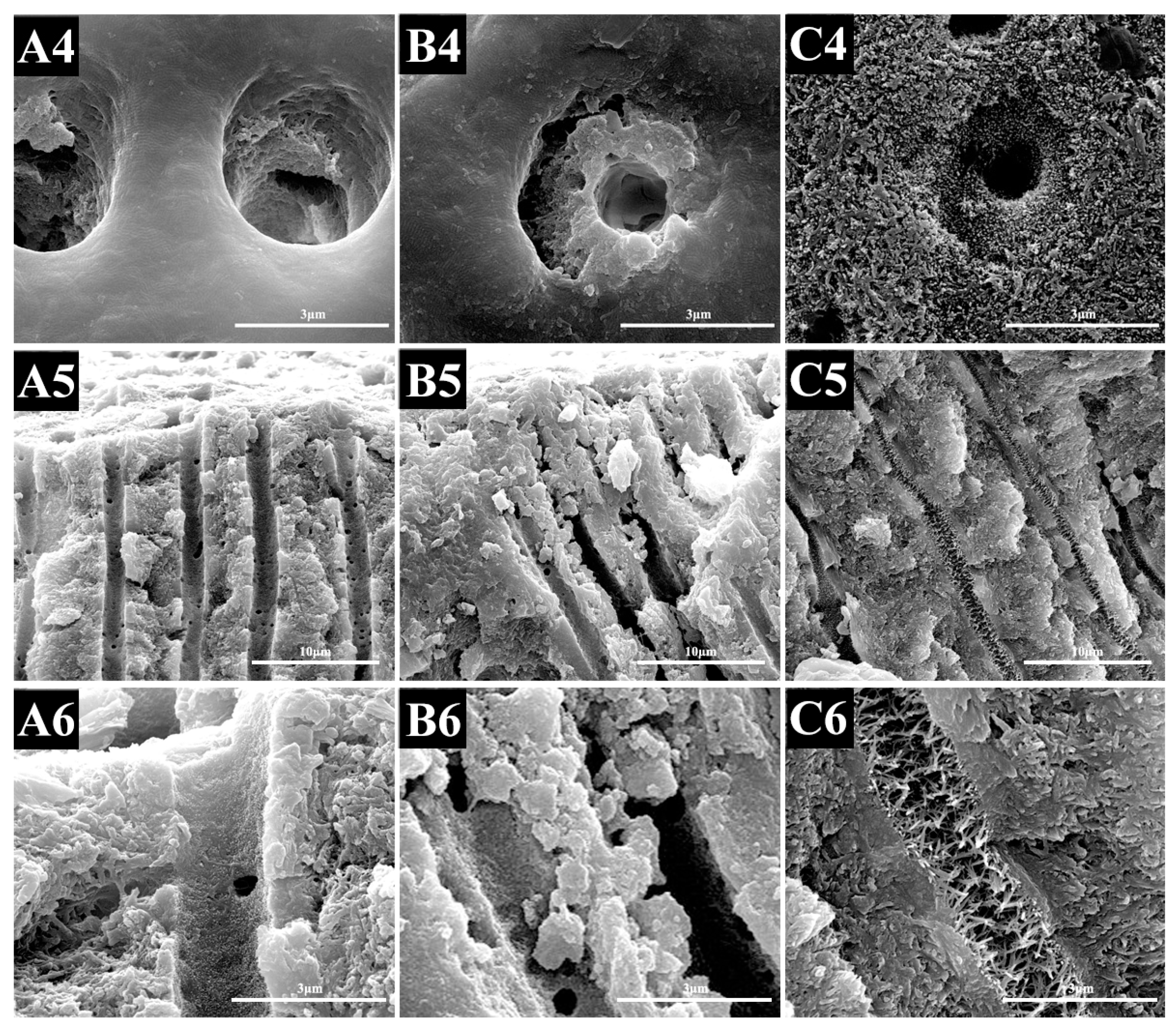
2.1.2. SEM Evaluation
2.1.3. SEM Image Analysis Combines with Image-Pro Plus
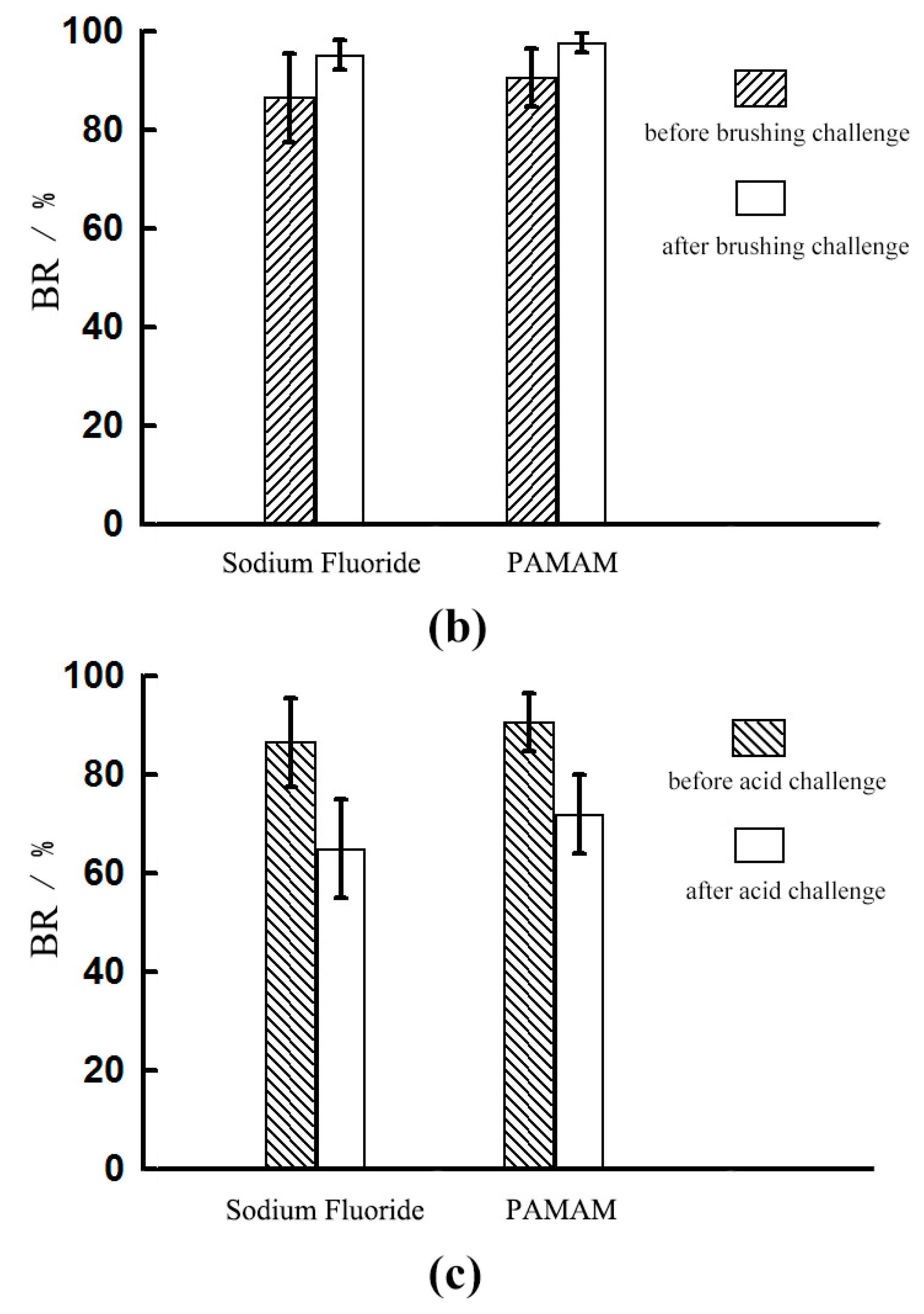
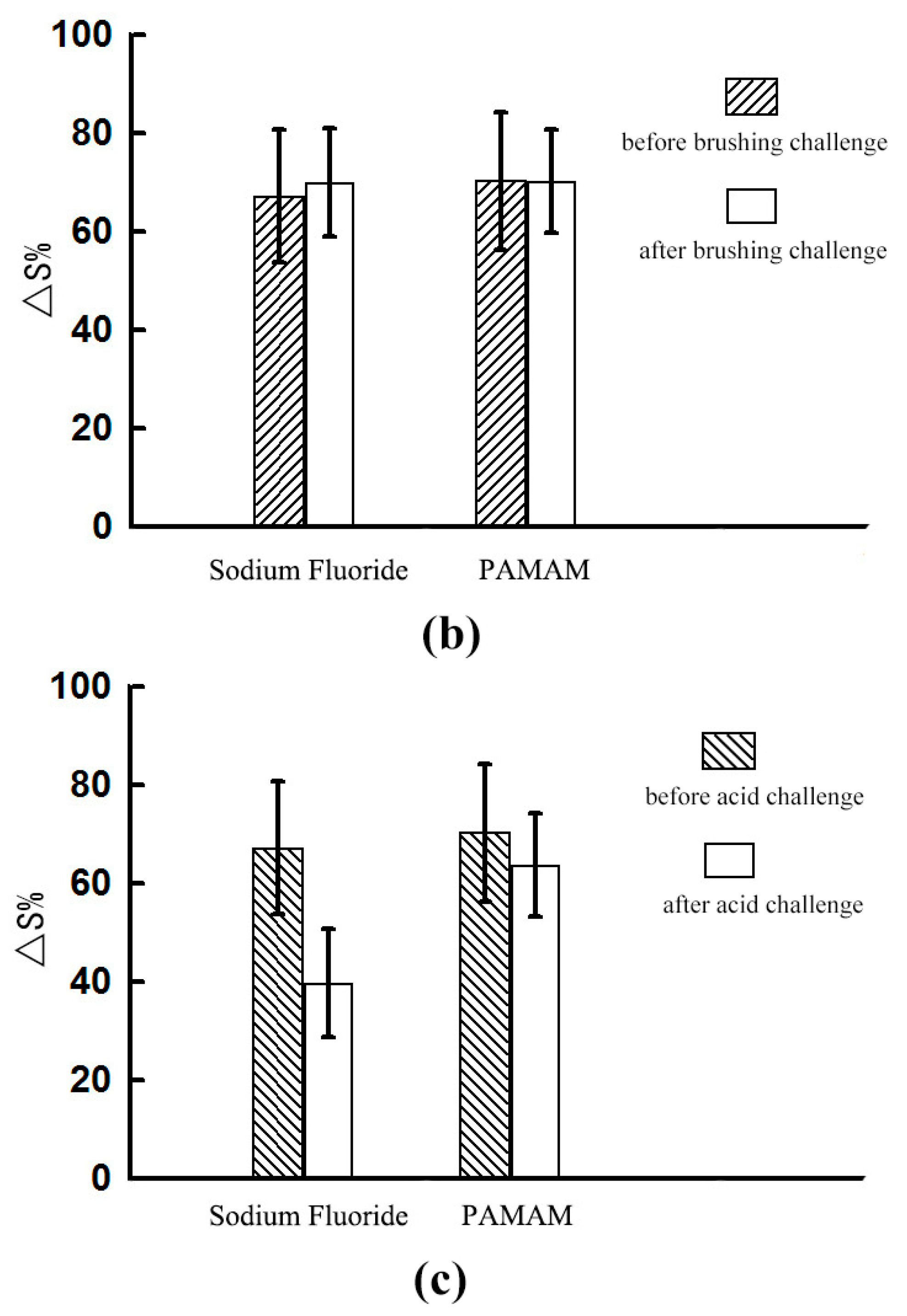
2.2. Dentinal Tubule Occlusion after Brushing Challenge
2.2.1. Dentine Permeability Measurements
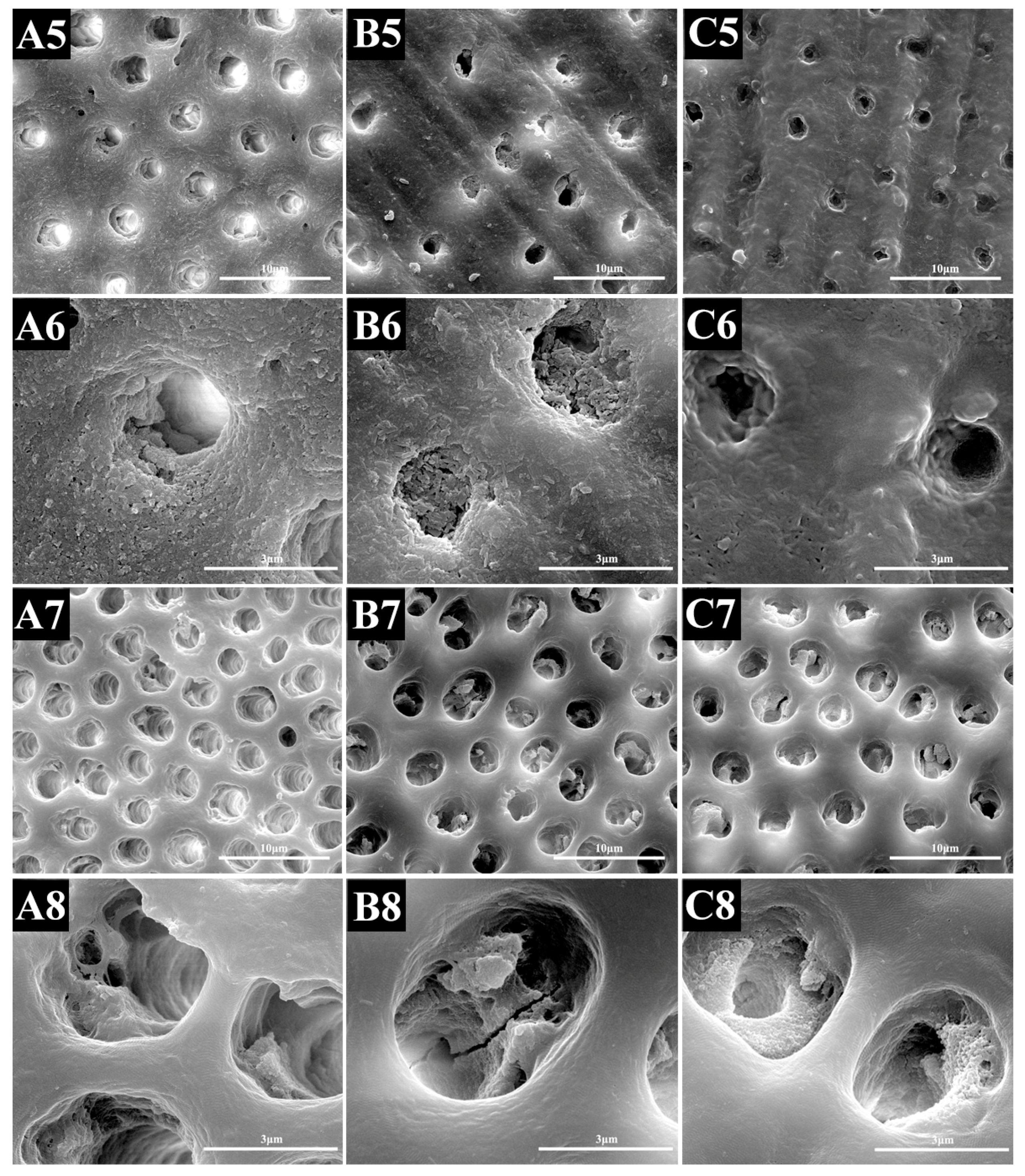
2.2.2. SEM Evaluation
2.2.3. SEM Image Analysis Combines with Image-Pro Plus
2.3. Dentinal Tubule Occlusion after Acid Challenge
2.3.1. Dentine Permeability Measurements
2.3.2. SEM Evaluation
2.3.3. SEM Image Analysis Combines with Image-Pro Plus
3. Discussion
4. Materials and Methods
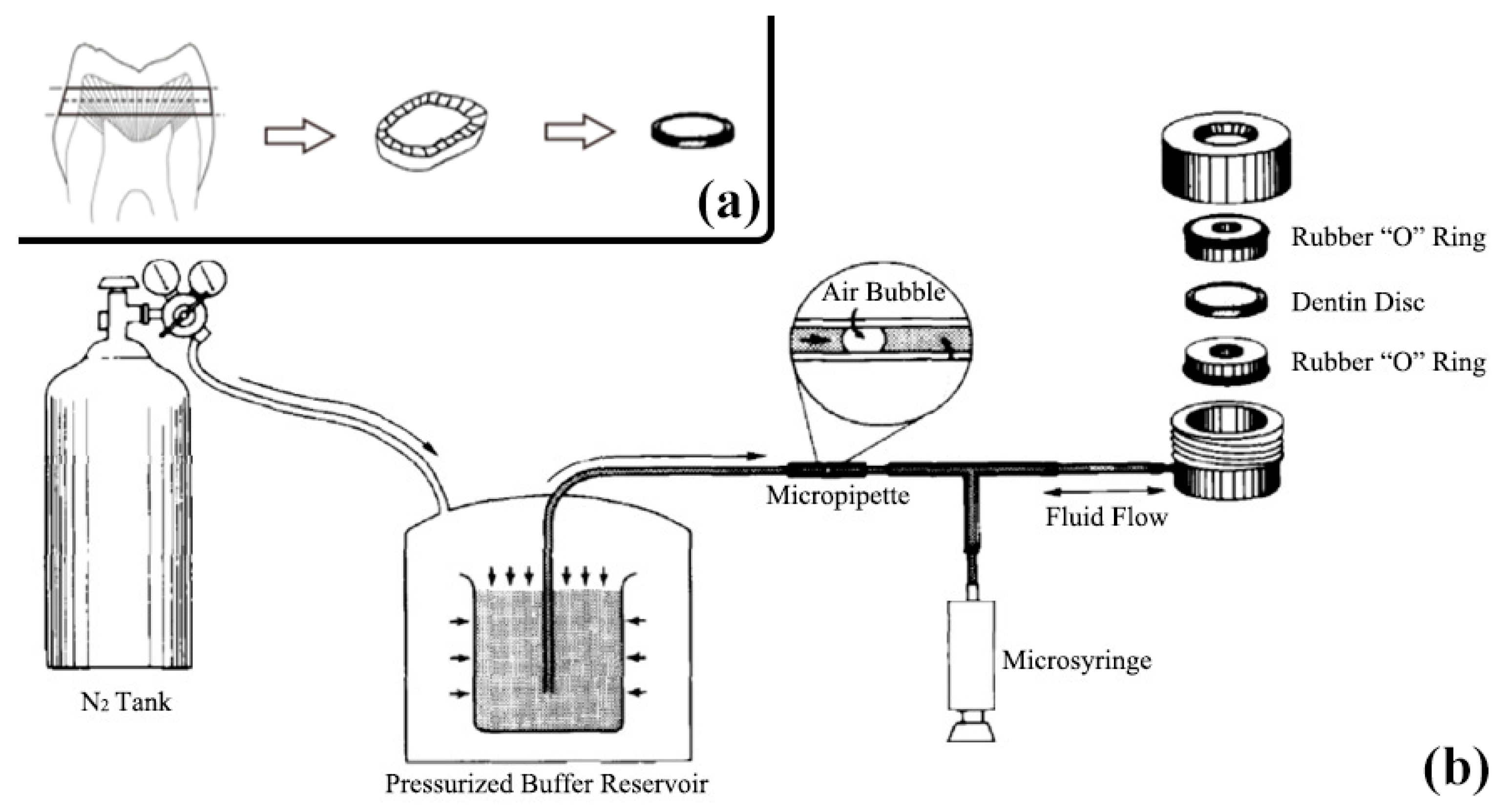
4.1. Dentine Sample Preparation
4.2. Dentine Permeability Measurement
4.3. SEM Analysis
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Orchardson, R.; Gillam, D.G. Managing dentin hypersensitivity. J. Am. Dent. Assoc. 2006, 137, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Dababneh, R.H.; Khouri, A.T.; Addy, M. Dentine hypersensitivity—An enigma? A review of terminology, mechanisms, aetiology and management. Br. Dent. J. 1999, 187, 606–611; discussion 603. [Google Scholar] [CrossRef] [PubMed]
- Addy, M.; Edgar, W.M.; Embery, G.; Orchardson, R. Tooth Wear and Sensitivity: Clinical Advances in Restorative Dentistry; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Brännstrom, M.; Lindén, L.A.; Johnson, G. Movement of dentinal and pulpal fluid caused by clinical procedures. J. Dent. Res. 1968, 47, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, K.; Pashley, D.H. Personal reflections on a sensitive subject. J. Dent. Res. 2007, 86, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Brännström, M. Sensitivity of dentine. Oral Surg. Oral Med. Oral Pathol. 1966, 21, 517–526. [Google Scholar] [CrossRef]
- Pashley, D.H. Dentin permeability, dentin sensitivity, and treatment through tubule occlusion. J. Endod. 1986, 12, 465–474. [Google Scholar] [CrossRef]
- Yoshiyama, M.; Masada, J.; Uchida, A.; Ishida, H. Scanning electron microscopic characterization of sensitive vs. insensitive human radicular dentin. J. Dent. Res. 1989, 68, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, M.; Noiri, Y.; Ozaki, K.; Uchida, A.; Ishikawa, Y.; Ishida, H. Transmission electron microscopic characterization of hypersensitive human radicular dentin. J. Dent. Res. 1990, 69, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Absi, E.G.; Addy, M.; Adams, D. Dentine hypersensitivity: A study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J. Clin. Periodontol. 1987, 14, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J. Can. Dent. Assoc. 2003, 69, 221–226. [Google Scholar]
- Hoyt, W.H.; Bibby, B.G. Use of sodium fluoride for desensitizing dentin. J. Am. Dent. Assoc. 1943, 30, 1372–1376. [Google Scholar] [CrossRef]
- Femiano, F.; Femiano, R.; Lanza, A.; Festa, M.V.; Rullo, R.; Perillo, L. Efficacy of diode laser in association to sodium fluoride vs. Gluma desensitizer on treatment of cervical dentin hypersensitivity. A double blind controlled trial. Am. J. Dent. 2013, 26, 214–218. [Google Scholar] [PubMed]
- Morris, M.F.; Davis, R.D.; Richardson, B.W. Clinical efficacy of two dentin desensitizing agents. Am. J. Dent. 1999, 12, 72–76. [Google Scholar] [PubMed]
- Bartold, P.M. Dentinal hypersensitivity: A review. Aust. Dent. J. 2006, 51, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Suge, T.; Kawasaki, A.; Ishikawa, K.; Matsuo, T.; Ebisu, S. Effects of plaque control on the patency of dentinal tubules: An in vivo study in beagle dogs. J. Periodontol. 2006, 77, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Sesikeran, B.; Rao, S.H.; Krishnamurthi, D.; Reddy, D.R. Studies on sural nerve biopsies in endemic skeletal fluorosis. Fluoride 1994, 27, 189–193. [Google Scholar]
- Gandolfi, M.G.; Silvia, F.; Pd, H.; Gasparotto, G.; Carlo, P. Calcium silicate coating derived from Portland cement as treatment for hypersensitive dentine. J. Dent. 2008, 36, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Gandolfi, M.G.; Prati, C.; Mongiorgi, R. Oxalate-containing phytocomplexes as dentine desensitisers: An in vitro study. Arch. Oral Biol. 2006, 51, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Montebugnoli, L.; Suppa, P.; Valdrè, G.; Mongiorgi, R. Permeability and morphology of dentin after erosion induced by acidic drinks. J. Periodontol. 2003, 74, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.; Dedeus, G.; Leal, F.; Azevedo, E.; Coutinhofilho, T.; Paciornik, S. Strong effect on dentin after the use of high concentrations of citric acid: An assessment with co-site optical microscopy and ESEM. Dent. Mater. 2008, 24, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Mannocci, F.; Tay, F.R.; Pashley, D.H.; Cook, R.; Carpenter, G.H.; Watson, T.F. Deproteinization effects of NaOCl on acid-etched dentin in clinically-relevant vs prolonged periods of application. A confocal and environmental scanning electron microscopy study. Oper. Dent. 2009, 34, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D. Remineralization, the natural caries repair process—The need for new approaches. Adv. Dent. Res. 2009, 21, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Z.; Cao, Y.; Liu, W.; Chu, C.H.; Li, Q.L. Polydopamine-induced tooth remineralization. ACS Appl. Mater. Int. 2012, 4, 6901–6910. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials 2008, 29, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H. Biomimetic remineralization of resin-bonded acid-etched dentin. J. Dent. Res. 2009, 88, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Arola, D.D.; Gu, L.; Kim, Y.K.; Mai, S.; Liu, Y.; Pashley, D.H.; Tay, F.R. Functional biomimetic analogs help remineralize apatite-depleted demineralized resin-infiltrated dentin via a bottom–up approach. Acta Biomater. 2010, 6, 2740–2750. [Google Scholar] [CrossRef] [PubMed]
- Donners, J.J.J.M.; Nolte, R.J.M.; Sommerdijk, N.A.J.M. Dendrimer-based hydroxyapatite composites with remarkable materials properties. Adv. Mater. 2003, 15, 313–316. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Tsetsekou, A.; Kammenou, M.I.; Boukos, N. Controlling the formation of hydroxyapatite nanorods with dendrimers. J. Am. Ceram. Soc. 2011, 94, 2023–2029. [Google Scholar] [CrossRef]
- Wu, D.; Yang, J.; Li, J.; Chen, L.; Tang, B.; Chen, X.; Wu, W.; Li, J. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials 2013, 34, 5036–5047. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, J.J.; Li, J.; Chen, L.; Liang, K.; Wu, W.; Chen, X.; Li, J. Bioinspired intrafibrillar mineralization of human dentine by pamam dendrimer. Biomaterials 2013, 34, 6738–6747. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Gao, Y.; Li, J.; Liao, Y.; Xiao, S.; Lv, H.; He, L.; Cheng, L.; Zhou, X.; Li, J. Effective dentinal tubule occlusion induced by polyhydroxy-terminated PAMAM dendrimer in vitro. RSC Adv. 2014, 4, 43496–43503. [Google Scholar] [CrossRef]
- Jia, R.; Lu, Y.; Yang, C.W.; Luo, X.; Han, Y. Effect of generation 4.0 polyamidoamine dendrimer on the mineralization of demineralized dentinal tubules in vitro. Arch. Oral Biol. 2014, 59, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Yuan, H.; Li, J.; Yang, J.; Zhou, X.; He, L.; Cheng, L.; Gao, Y.; Xu, X.; Zhou, X. Remineralization of demineralized dentin induced by amine-terminated pamam dendrimer. Macromol. Mater. Eng. 2015, 300, 107–117. [Google Scholar] [CrossRef]
- Wang, T.; Yang, S.; Wang, L.; Feng, H. Use of multifunctional phosphorylated PAMAM dendrimers for dentin biomimetic remineralization and dentinal tubule occlusion. RSC Adv. 2015, 5, 11136–11144. [Google Scholar] [CrossRef]
- Liang, K.; Gao, Y.; Li, J.; Liao, Y.; Xiao, S.; Zhou, X.; Li, J. Biomimetic mineralization of collagen fibrils induced by amine-terminated PAMAM dendrimers—PAMAM dendrimers for remineralization. J. Biomater. Sci. Polym. Ed. 2015, 26, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, J.; Liang, K.; Li, J.; He, L.; Yang, X.; Peng, S.; Chen, X.; Ding, C.; Li, J. Effective dentin restorative material based on phosphate-terminated dendrimer as artificial protein. Colloids Surf. B Biointerfaces 2015, 128, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H. Dentin: A dynamic substrate—A review. Scanning Microsc. 1989, 3, 174–176. [Google Scholar]
- Wang, Z.; Sa, Y.; Sauro, S.; Chen, H.; Xing, W.; Ma, X.; Jiang, T.; Wang, Y. Effect of desensitising toothpastes on dentinal tubule occlusion: A dentine permeability measurement and sem in vitro study. J. Dent. 2010, 38, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Barnes, V.; Devizio, W.; Yang, H.; Malmstrom, H.; Ren, Y. Effects of dentin tubule occlusion by dentifrice containing a PVM/MA bioadhesive copolymer in a silica base. J. Dent. 2011, 39, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Reinhardt, J.W.; Krell, K.V. Effect of dentin desensitizers and dentin bonding agents on dentin permeability. Am. J. Dent. 2000, 13, 21–27. [Google Scholar] [PubMed]
- Pereira, J.C.; Segala, A.D.; Gillam, D.G. Effect of desensitizing agents on the hydraulic conductance of human dentin subjected to different surface pre-treatments—An in vitro study. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2005, 21, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sesikeran, B.; Krishnamurthy, D.; Harinarayana, R.S.; Ramachandran, E.P.; Raja, R.D. Studies on skeletal muscle biopsies in endemic skeletal fluorosis. Neurol. India 2000, 48, 187–188. [Google Scholar] [PubMed]
- Xie, L.; Wang, L.; Jia, X.; Kuang, G.; Yang, S.; Feng, H. Effects of glutamic acid shelled PAMAM dendrimers on the crystallization of calcium phosphate in diffusion systems. Polym. Bull. 2011, 66, 119–132. [Google Scholar] [CrossRef]
- Yan, S.J.; Zhou, Z.H.; Zhang, F.; Yang, S.P.; Yang, L.Z.; Yu, X.B. Effect of anionic pamam with amido groups starburst dendrimers on the crystallization of Ca10(PO4)6(OH)2 by hydrothermal method. Mater. Chem. Phys. 2006, 99, 164–169. [Google Scholar] [CrossRef]
- Liang, K.; Weir, M.D.; Xie, X.; Wang, L.; Reynolds, M.A.; Li, J.; Xu, H.H. Dentin remineralization in acid challenge environment via PAMAM and calcium phosphate composite. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2016, 32, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liang, K.; Li, J.; Wu, D.; Zhou, X.; Li, J. Regeneration of biomimetic hydroxyapatite on etched human enamel by anionic PAMAM template in vitro. Arch. Oral Biol. 2013, 58, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.Y.; Gillam, D.G.; Barber, P.M.; Mordan, N.J.; Critchell, J. An investigation of potential desensitizing agents in the dentine disc model: A scanning electron microscopy study. J. Oral Rehabil. 1997, 24, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Hong, C.X.; Heipp, P.S. A novel potassium oxalate-containing tooth-desensitising mouthrinse: A comparative in vitro study. J. Dent. 2013, 41, S18–S27. [Google Scholar] [CrossRef]
- Sauro, S.; Pashley, D.H.; Montanari, M.; Chersoni, S.; Carvalho, R.M.; Toledano, M.; Osorio, R.; Tay, F.R.; Prati, C. Effect of simulated pulpal pressure on dentin permeability and adhesion of self-etch adhesives. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2007, 23, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Ten Cate, J.M.; Duijsters, P.P. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982, 16, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Galloway, S.E. The effects of oxalate treatment on the smear layer of ground surfaces of human dentine. Arch. Oral Biol. 1985, 30, 731–737. [Google Scholar] [CrossRef]
- Ahmed, T.R.; Mordan, N.J.; Gilthorpe, M.S.; Gillam, D.G. In vitro quantification of changes in human dentine tubule parameters using SEM and digital analysis. J. Oral Rehabil. 2005, 32, 589–597. [Google Scholar] [CrossRef] [PubMed]


| Groups | Immediate | 7 d | 14 d | 28 d |
|---|---|---|---|---|
| DIW | 98.5 ± 4.5 | 91.2 ± 3.3 * | 88.3 ± 3.0 * | 82.6 ± 3.4 * |
| NaF | 71.6 ± 6.5 * | 40.9 ± 7.2 * | 23.3 ± 4.2 * | 20.7 ± 3.1 |
| PAMAM | 90.1 ± 7.4 | 77.3 ± 8.1 * | 57.6 ± 8.5 * | 25.1 ± 6.3 |
| Groups | Abrasive Test | Acid Challenge |
|---|---|---|
| DIW | 58.3 ± 8.8 *,** | 105.8 ± 9.2 *,** |
| NaF | 17.3 ± 9.1 | 61.2 ± 8.7 *,** |
| PAMAM | 18.6 ± 9.4 | 33.1 ± 9.0 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Liang, K.; Li, J.; Yuan, H.; Liu, H.; Duan, X.; Li, J. Effect and Stability of Poly(Amido Amine)-Induced Biomineralization on Dentinal Tubule Occlusion. Materials 2017, 10, 384. https://doi.org/10.3390/ma10040384
Gao Y, Liang K, Li J, Yuan H, Liu H, Duan X, Li J. Effect and Stability of Poly(Amido Amine)-Induced Biomineralization on Dentinal Tubule Occlusion. Materials. 2017; 10(4):384. https://doi.org/10.3390/ma10040384
Chicago/Turabian StyleGao, Yuan, Kunneng Liang, Jianshu Li, He Yuan, Hongling Liu, Xiaolei Duan, and Jiyao Li. 2017. "Effect and Stability of Poly(Amido Amine)-Induced Biomineralization on Dentinal Tubule Occlusion" Materials 10, no. 4: 384. https://doi.org/10.3390/ma10040384
APA StyleGao, Y., Liang, K., Li, J., Yuan, H., Liu, H., Duan, X., & Li, J. (2017). Effect and Stability of Poly(Amido Amine)-Induced Biomineralization on Dentinal Tubule Occlusion. Materials, 10(4), 384. https://doi.org/10.3390/ma10040384





