Chemical and Morphological Characterization of Magnetron Sputtered at Different Bias Voltages Cr-Al-C Coatings
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
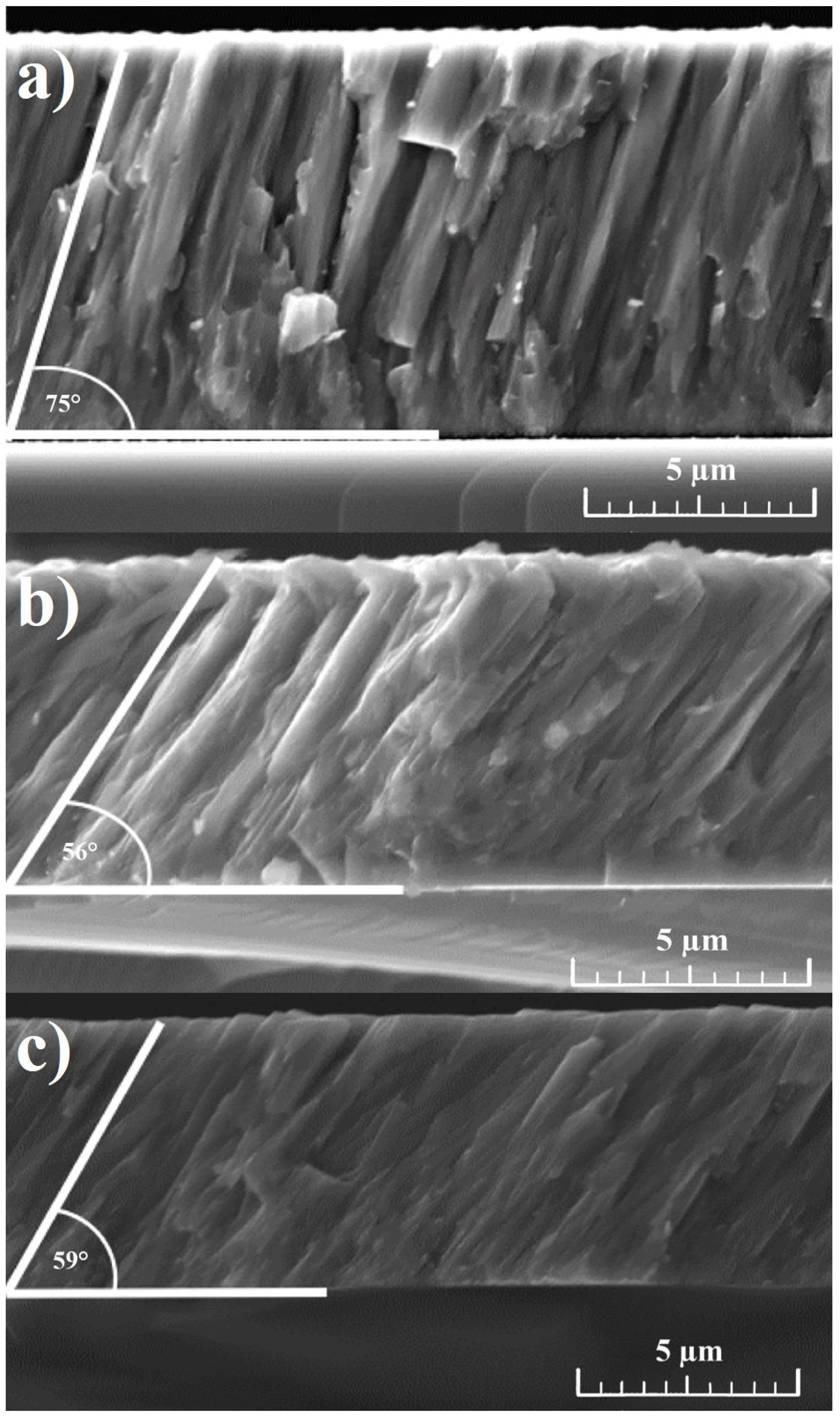
3.1. Scanning Electron Microscopy
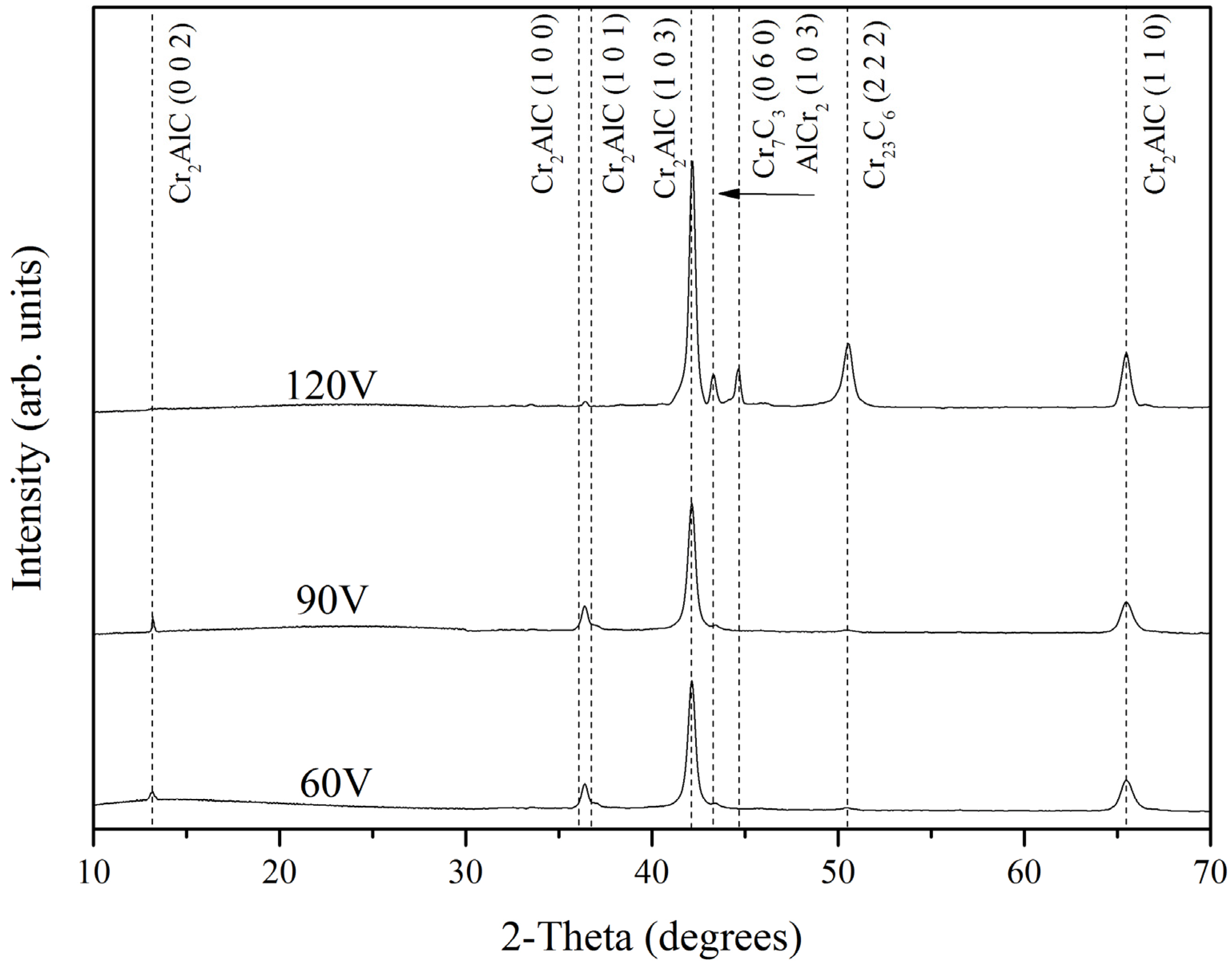
3.2. XRD
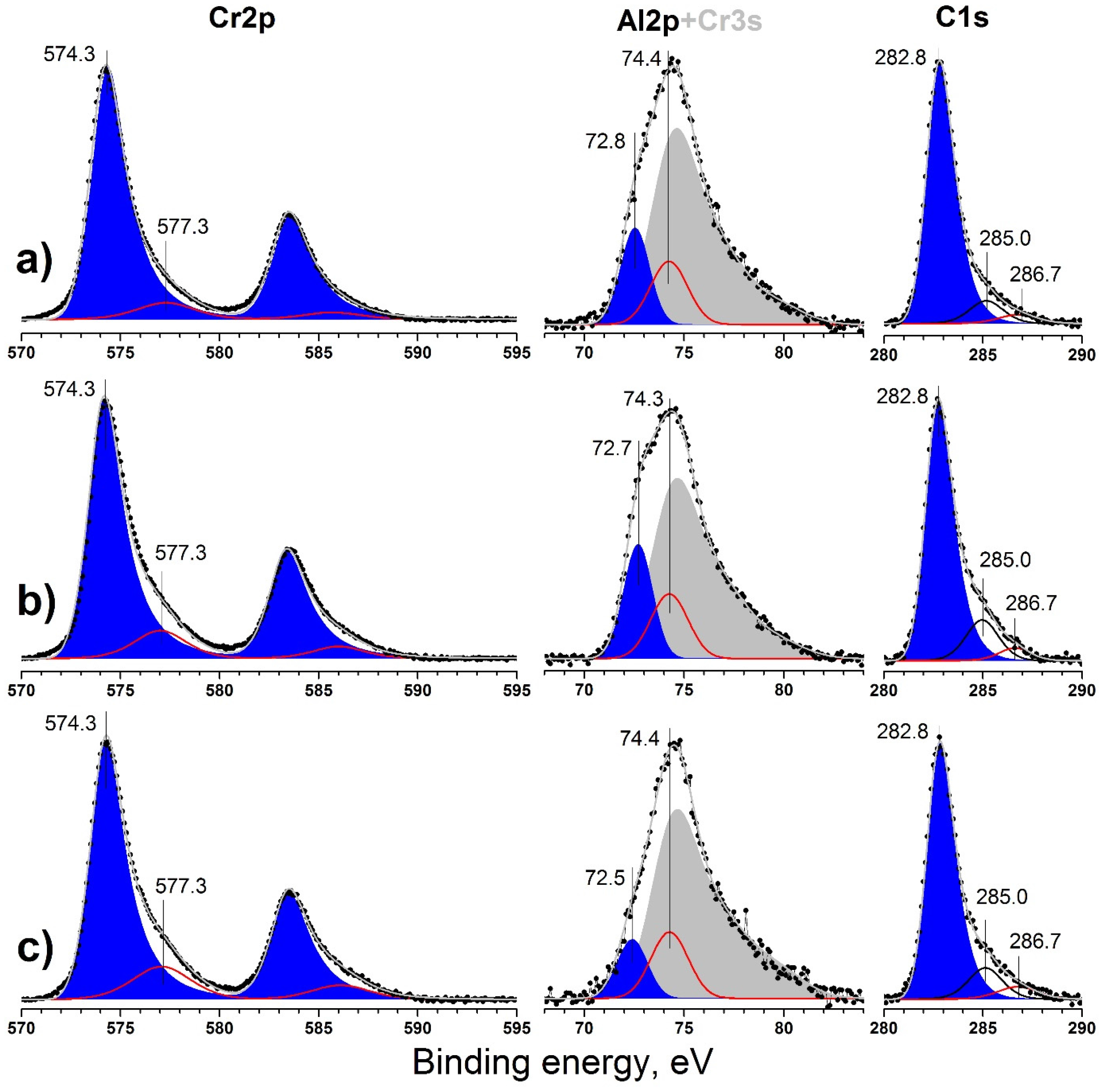
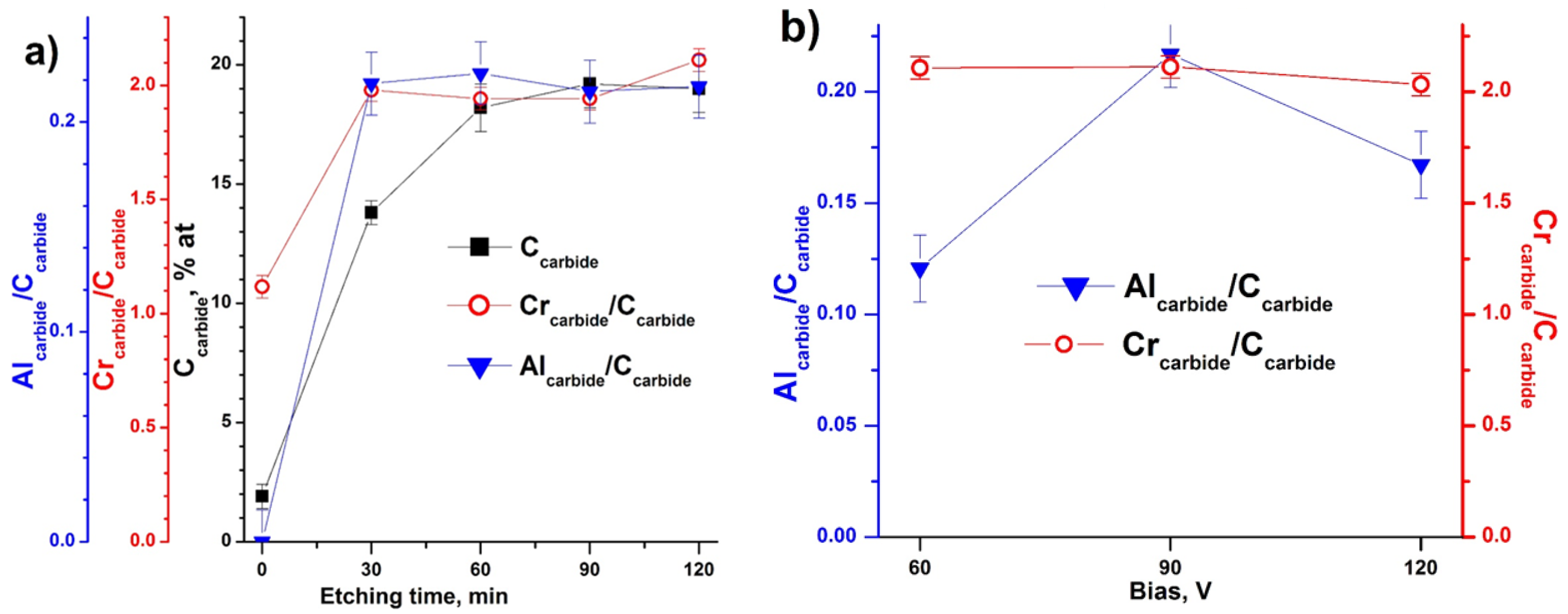
3.3. X-ray Photoelectron Spectroscopy
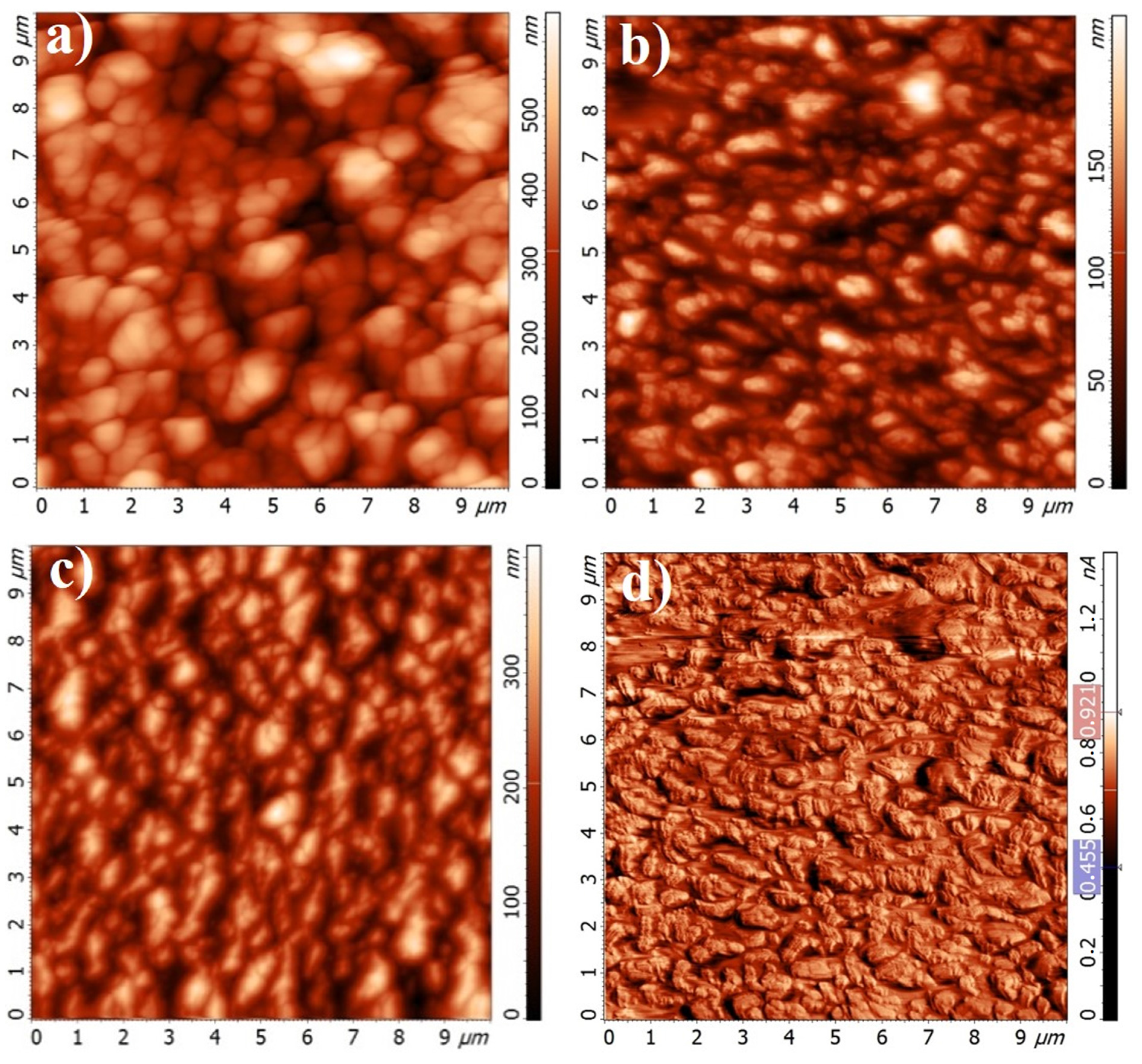
3.4. Atomic Force Microscopy
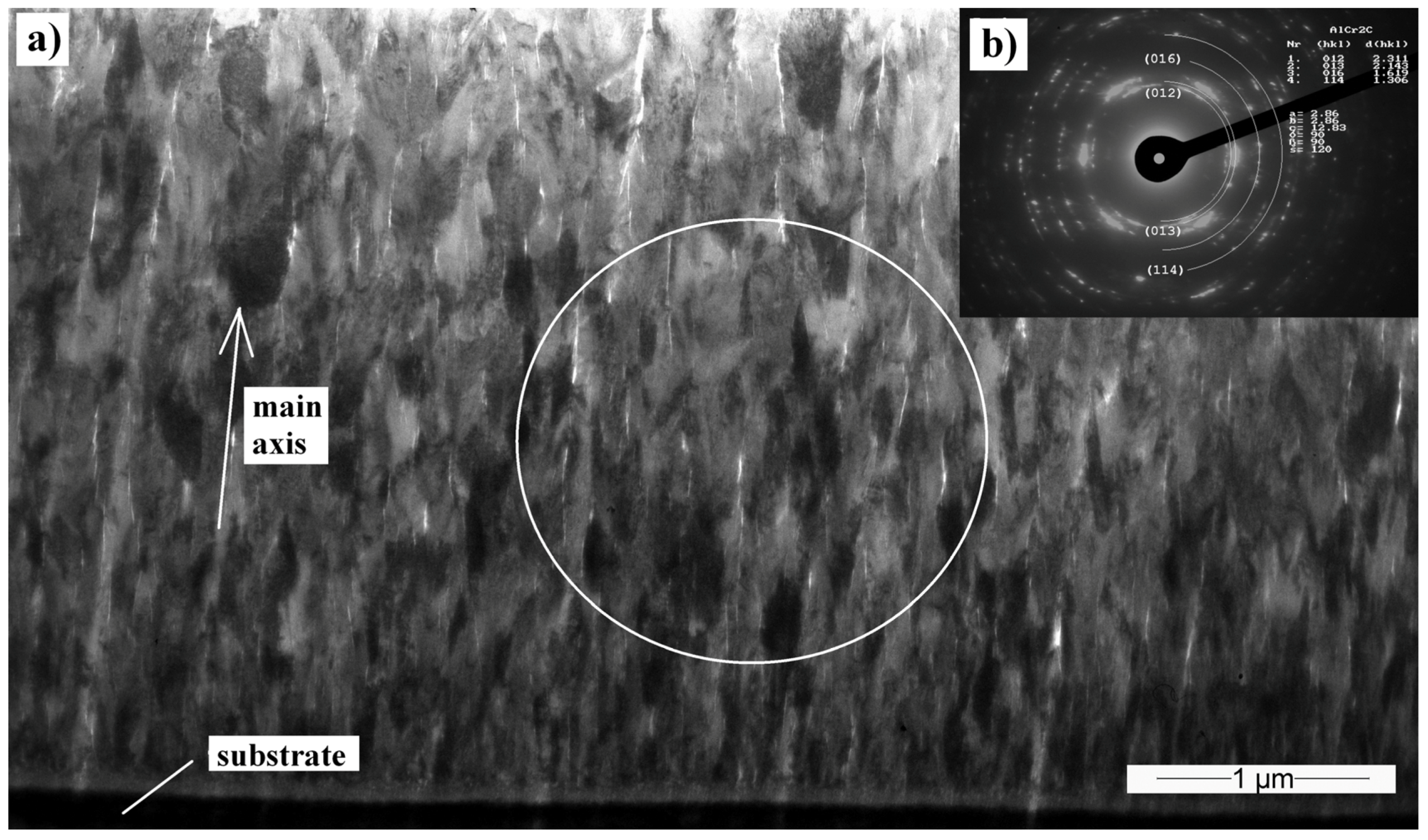
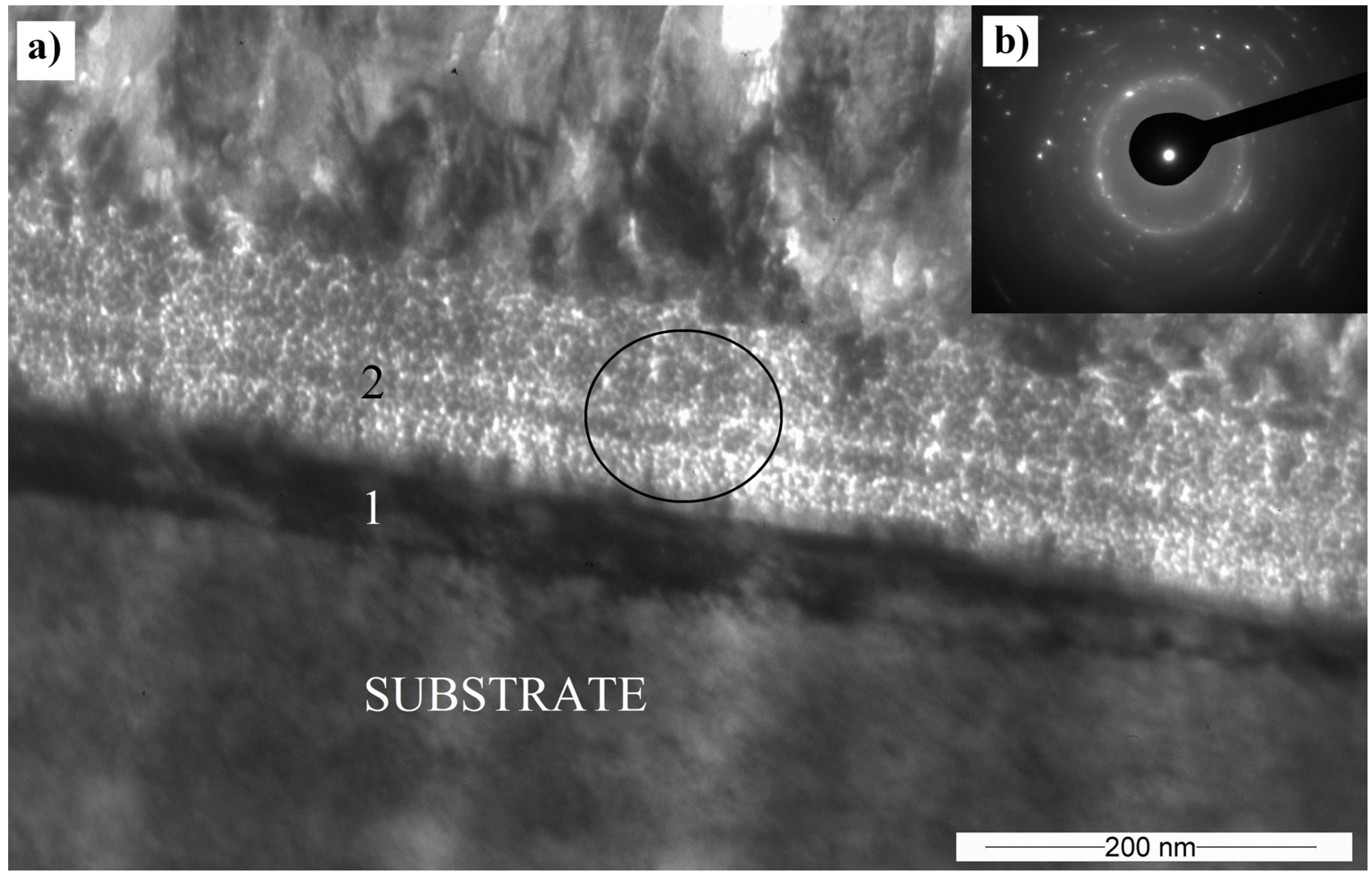
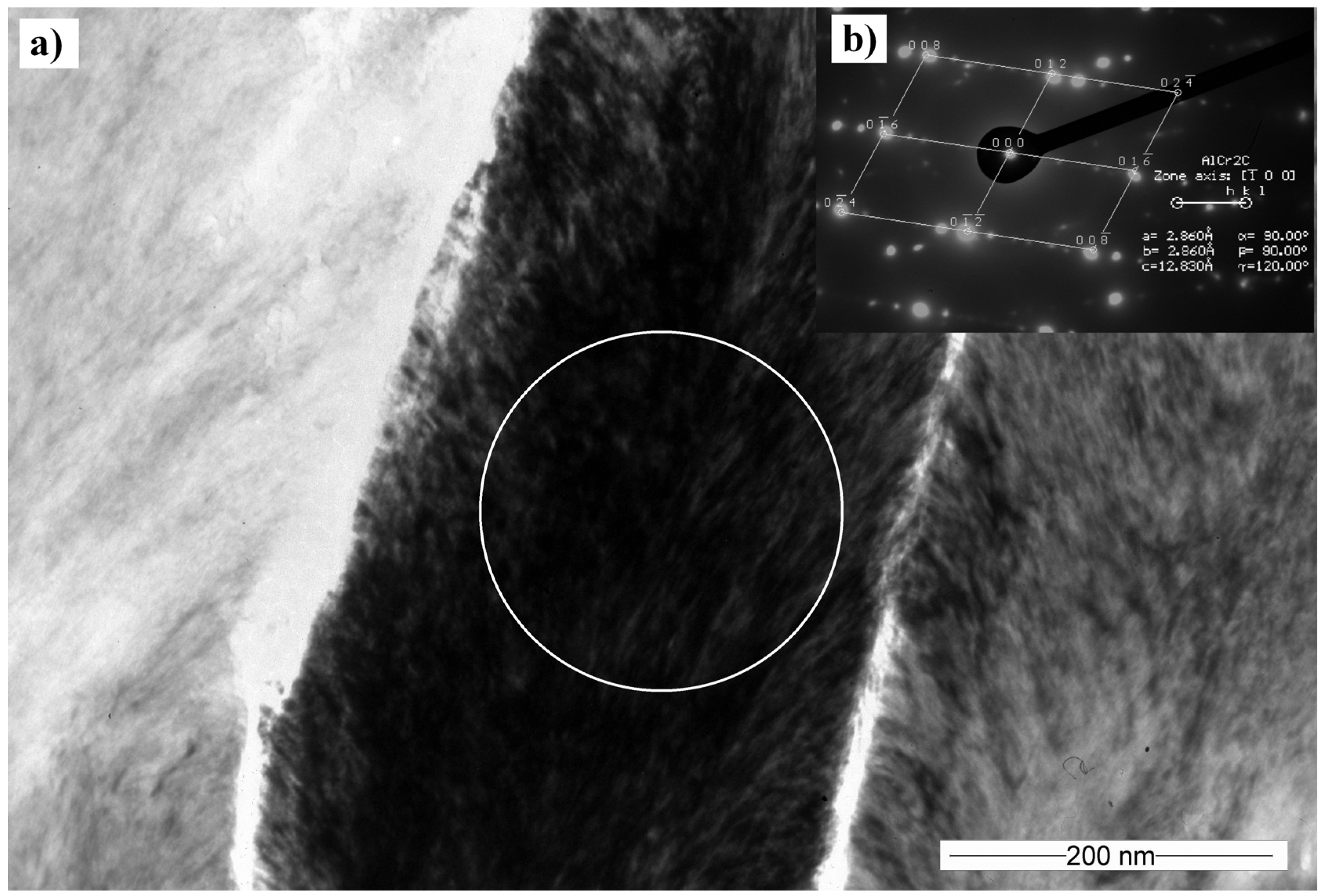
3.5. Transmission Electron Microscopy
3.6. Nanoindentation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barsoum, M.W. The Mn + 1AXn phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Jeitschko, W.; Nowotny, H.; Benesovsky, F. Ti2AlN, eine stickstoffhaltige H-Phase. Monatshefte Chem. Verwandte Teile And. Wiss. 1963, 94, 1198–1200. [Google Scholar] [CrossRef]
- Jeitschko, W.; Nowotny, H.; Benesovsky, F. Kohlenstoffhaltige ternäre Verbindungen (H-Phase). Monatshefte Chem. Verwandte Teile And. Wiss. 1963, 94, 672–676. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, Y.; Li, M. Synthesis, Microstructure, and Property of Cr2AlC. J. Mater. Sci. Technol. 2007, 23, 721–746. [Google Scholar]
- Eklund, P.; Beckers, M.; Jansson, U.; Högberg, H.; Hultman, L. The Mn + 1AXn phases: Materials science and thin-film processing. Thin Solid Films 2010, 518, 1851–1878. [Google Scholar] [CrossRef]
- Schneider, J.M.; Sigumonrong, D.; Music, D.; Walter, C.; Emmerlich, J.; Iskandar, R.; Mayer, J. Elastic properties of Cr2AlC thin films probed by nanoindentation and ab initio molecular dynamics. Scr. Mater. 2007, 57, 1137–1140. [Google Scholar] [CrossRef]
- Hettinger, J.D.; Lofland, S.E.; Finkel, P.; Meehan, T.; Palma, J.; Harrell, K.; Gupta, S.; Ganguly, A.; El-Raghy, T.; Barsoum, M.W. Electrical transport, thermal transport, and elastic properties of M2AlC (M = Ti, Cr, Nb, and V). Phys. Rev. B 2005, 72, 115120. [Google Scholar] [CrossRef]
- Song, G.M.; Pei, Y.T.; Sloof, W.G.; Li, S.B.; de Hosson, J.T.M.; van de Zwaag, S. Oxidation-induced crack healing in Ti3AlC2 ceramics. Scr. Mater. 2008, 58, 13–16. [Google Scholar] [CrossRef]
- Xie, J.; Wang, X.; Li, A.; Li, F.; Zhou, Y. Corrosion behavior of selected Mn + 1AXn phases in hot concentrated HCl solution: Effect of A element and MX layer. Corros. Sci. 2012, 60, 129–135. [Google Scholar] [CrossRef]
- Lin, Z.J.; Li, M.S.; Wang, J.Y.; Zhou, Y.C. High-temperature oxidation and hot corrosion of Cr2AlC. Acta Mater. 2007, 55, 6182–6191. [Google Scholar] [CrossRef]
- Hajas, D.E.; Baben, M.; Hallstedt, B.; Iskandar, R.; Mayer, J.; Schneider, J.M. Oxidation of Cr2AlC coatings in the temperature range of 1230 to 1410 °C. Surf. Coat. Technol. 2011, 206, 591–598. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhou, Y.C. High-Temperature Oxidation Behavior of Ti2AlC in Air. Oxid. Met. 2003, 59, 303–320. [Google Scholar] [CrossRef]
- Abdulkadhim, A.; Baben, M.; Takahashi, T.; Schnabel, V.; Hans, M.; Polzer, C.; Polcik, P.; Schneider, J.M. Crystallization kinetics of amorphous Cr2AlC thin films. Surf. Coat. Technol. 2011, 206, 599–603. [Google Scholar] [CrossRef]
- Li, J.J.; Li, M.S.; Xiang, H.M.; Lu, X.P.; Zhou, Y.C. Short-term oxidation resistance and degradation of Cr2AlC coating on M38G superalloy at 900–1100 °C. Corros. Sci. 2011, 53, 3813–3820. [Google Scholar] [CrossRef]
- Palmquist, J.-P.; Jansson, U.; Seppänen, T.; Persson, P.U.A.; Birch, J.; Hultman, L.; Isberg, P. Magnetron sputtered epitaxial single-phase Ti3SiC2 thin films. Appl. Phys. Lett. 2002, 81, 835–837. [Google Scholar] [CrossRef]
- Tian, W.; Wang, P.; Kan, Y.; Zhang, G. Oxidation behavior of Cr2AlC ceramics at 1100 and 1250 °C. J. Mater. Sci. 2008, 43, 2785–2791. [Google Scholar] [CrossRef]
- Naveed, M.; Obrosov, A.; Zak, A.; Dudzinski, W.; Volinsky, A.A.; Weiß, S. Sputtering Power Effects on Growth and Mechanical Properties of Cr2AlC MAX Phase Coatings. Metals 2016, 6, 265. [Google Scholar] [CrossRef]
- Lee, D.B.; Nguyen, T.D. Cyclic oxidation of Cr2AlC between 1000 and 1300 °C in air. J. Alloys Compd. 2008, 464, 434–439. [Google Scholar] [CrossRef]
- Li, J.J.; Qian, Y.H.; Niu, D.; Zhang, M.M.; Liu, Z.M.; Li, M.S. Phase formation and microstructure evolution of arc ion deposited Cr2AlC coating after heat treatment. Appl. Surf. Sci. 2012, 263, 457–464. [Google Scholar] [CrossRef]
- Schneider, J.M.; Sun, Z.; Mertens, R.; Uestel, F.; Ahuja, R. Ab initio calculations and experimental determination of the structure of Cr2AlC. Solid State Commun. 2004, 130, 445–449. [Google Scholar] [CrossRef]
- Bugnet, M.; Jaouen, M.; Mauchamp, V.; Cabioc’h, V.; Hug, G. Experimental and first-principles investigation of the electronic structure anisotropy of Cr2AlC. Phys. Rev. B 2014, 90, 195116. [Google Scholar] [CrossRef]
- Bugnet, M.; Mauchamp, V.; Oliviero, E.; Jaouen, M.; Cabioc’h, T. Chemically sensitive amorphization process in the nanolaminated Cr2AlC (A = Al or Ge) system from TEM in situ irradiation. J. Nucl. Mater. 2013, 441, 133–137. [Google Scholar] [CrossRef]
- Walter, C.; Sigumonrong, D.P.; El-Raghy, T.; Schneider, J.M. Towards large area deposition of Cr2AlC on steel. Thin Solid Films 2006, 515, 389–393. [Google Scholar] [CrossRef]
- Li, J.J.; Hu, L.F.; Li, F.Z.; Li, M.S.; Zhou, Y.C. Variation of microstructure and composition of the Cr2AlC coating prepared by sputtering at 370 and 500 °C. Surf. Coat. Technol. 2010, 204, 3838–3845. [Google Scholar] [CrossRef]
- Zamulaeva, E.I.; Levashov, E.A.; Sviridova, T.A.; Shvyndina, N.V.; Petrzhik, M.I. Pulsed electrospark deposition of MAX phase Cr2AlC based coatings on titanium alloy. Surf. Coat. Technol. 2013, 235, 454–460. [Google Scholar] [CrossRef]
- Mertens, R.; Sun, Z.; Music, D.; Schneider, J.M. Effect of the Composition on the Structure of Cr-Al-C Investigated by Combinatorial Thin Film Synthesis and ab Initio Calculations. Adv. Eng. Mater. 2004, 6, 903–907. [Google Scholar] [CrossRef]
- Field, M.R.; Carlsson, P.; Eklund, P.; Patridge, J.G.; McCulloch, D.G.; McKenzie, D.R.; Bilek, M.M.M. A combinatorial comparison of DC and high power impulse magnetron sputtered Cr2AlC. Surf. Coat. Technol. 2014, 259, 746–750. [Google Scholar] [CrossRef]
- Obrosov, A.; Naveed, M.; Volinsky, A.; Weiß, S. Substrate Frequency Effects on CrxN Coatings Deposited by DC Magnetron Sputtering. J. Mater. Eng. Perform. 2017, 26, 366–373. [Google Scholar] [CrossRef]
- Grieseler, R.; Hähnlein, B.; Stubenrauch, M.; Kups, T.; Wilke, M.; Hopfeld, M.; Pezoldt, J.; Schaaf, P. Nanostructured plasma etched, magnetron sputtered nanolaminar Cr2AlC MAX phase thin films. Appl. Surf. Sci. 2014, 292, 997–1001. [Google Scholar] [CrossRef]
- Wang, Q.M.; Flores, R.A.; Schroeter, O.; Mykhaylonka, R.; Leyens, C.; Garkas, W.; to Baben, M. Fabrication and oxidation behavior of Cr2AlC coating on Ti6242 alloy. Surf. Coat. Technol. 2010, 204, 2343–2352. [Google Scholar] [CrossRef]
- Baben, M.; Shang, L.; Emmerlich, J.; Schneider, J.M. Oxygen incorporation in M2AlC (M = Ti, V, Cr). Acta Mater. 2012, 60, 4810–4818. [Google Scholar] [CrossRef]
- Naveed, M.; Obrosov, A.; Weiß, S. Investigation of the Wear Resistance Properties of Cr/CrN Multilayer Coatings against Sand Erosion. Conf. Pap. Sci. 2015, 2015, 873543. [Google Scholar] [CrossRef]
- Gulyaev, R.V.; Koscheev, S.V.; Malykhin, S.E. An algorithm for removing charging effects from X-ray photoelectron spectra of nanoscaled non-conductive materials. J. Electron Spectrosc. Relat. Phenom. 2015, 202, 89–101. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Thornton, J.A. High Rate Thick Film Growth. Annu. Rev. Mater. Sci. 1977, 7, 239–260. [Google Scholar] [CrossRef]
- Berger, O.; Leyens, C.; Heinze, S.; Boucher, R.; Ruhnow, M. Characterization of Cr-Al-C and Cr-Al-C-Y films synthesized by High Power Impulse Magnetron Sputtering at a low deposition temperature. Thin Solid Films 2015, 580, 6–11. [Google Scholar] [CrossRef]
- Dahlqvist, M.; Alling, B.; Abrikosov, I.A.; Rosén, J. Phase stability of Ti2AlC upon oxygen incorporation: A first-principles investigation. Phys. Rev. B 2010, 81, 024111. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Kiryukhantsev-Korneev, P.V.; Sheveyko, A.N.; Mavrin, B.N.; Rojas, C.; Fernandez, A.; Levashov, E.A. Comparative investigation of TiAlC(N), TiCrAlC(N), and CrAlC(N) coatings deposited by sputtering of МАХ-phase Ti2 − хCrхAlC targets. Surf. Coat. Technol. 2009, 203, 3595–3609. [Google Scholar] [CrossRef]
- Hauert, R.; Patscheider, J.; Tobler, M.; Zehringer, R. XPS investigation of the a-C : H/Al interface. Surf. Sci. 1993, 292, 121–129. [Google Scholar] [CrossRef]
- Lewin, E.; Gorgoi, M.; Schäfers, F.; Svensson, F. Influence of sputter damage on the XPS analysis of metastable nanocomposite coatings. Surf. Coat. Technol. 2009, 204, 455–462. [Google Scholar] [CrossRef]
- Calderon, V.S.; Cavaleiro, A.; Carvalho, S. Chemical and structural characterization of ZrCNAg coatings: XPS, XRD and Raman spectroscopy. Appl. Surf. Sci. 2015, 346, 240–247. [Google Scholar] [CrossRef]
- Vyas, A.; Shen, Y.G.; Zhou, Z.F.; Li, K.Y. Nano-structured CrN/CNx multilayer films deposited by magnetron sputtering. Compos. Sci. Technol. 2008, 68, 2922–2929. [Google Scholar] [CrossRef]
- Ikemoto, I.; Kikujiro, I.; Shuichi, K.; Haruo, K.; Franco, A.; Thomas, J.M. X-ray photoelectron spectroscopic studies of CrO2 and some related chromium compounds. J. Solid State Chem. 1976, 17, 425–430. [Google Scholar] [CrossRef]
- Healy, M.D.; Smith, D.C.; Rubiano, R.R.; Elliott, N.E.; Springer, R.W. Use of Tetraneopentylchromium as a Precursor for the Organometallic Chemical Vapor Deposition of Chromium Carbide: A Reinvestigation. Chem. Mater. 1994, 6, 448–453. [Google Scholar] [CrossRef]
- Zamulaeva, E.I.; Levashov, E.A.; Skryleva, E.A.; Sviridova, T.A.; Kiryukhantsev-Korneev, P.V. Conditions for formation of MAX phase Cr2AlC in electrospark coatings deposited onto titanium alloy. Surf. Coat. Technol. 2016, 298, 15–23. [Google Scholar] [CrossRef]
- Scofield, J.H. Theoretical Photoionization cross Sections from 1 to 1500 keV; Lawrence Livermore National Laboratory Report UCRL-51326; Lawrence Livermore National Laboratory: Livermore, CA, USA, 1973. [Google Scholar]
- Agostinelli, E.; Battistoni, C.; Fiorani, D.; Nogues, M. An XPS study of the electronic structure of the ZnxCd1 − xCr2(X = S, Se) spinel system. J. Phys. Chem. Solids 1989, 50, 269–272. [Google Scholar] [CrossRef]
- Allen, G.C.; Tucker, P.M.; Wild, R.K. X-ray photoelectron/Auger electron spectroscopic study of the initial oxidation of chromium metal. J. Chem. Soc. Faraday Trans. 2 1978, 74, 1126–1140. [Google Scholar] [CrossRef]
- Dejun, K.; Haoyuan, G. Friction-wear behaviors of cathodic arc ion plating AlTiN coatings at high temperatures. Tribol. Int. 2015, 88, 31–39. [Google Scholar] [CrossRef]
- Slavinskaya, E.M.; Stonkus, O.; Gulyaev, R.V.; Boronin, A.I. Structural and chemical states of palladium in Pd/Al2O3 catalysts under self-sustained oscillations in reaction of CO oxidation. Appl. Catal. A Gen. 2011, 401, 83–97. [Google Scholar] [CrossRef]
- Zhang, Z.; Nie, Y.; Shen, L.; Chai, J.; Pan, J.; Wong, L.M.; Sullivan, M.B.; Jin, H.; Wang, S.J. Charge Distribution in the Single Crystalline Ti2AlN Thin Films Grown on MgO(111) Substrates. J. Phys. Chem. C 2013, 117, 11656–11662. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, H.; Chai, J.; Pan, J.; Seng, H.L.; Goh, G.T.W.; Wong, L.M.; Sullivan, M.B.; Wang, S.J. Temperature-dependent microstructural evolution of Ti2AlN thin films deposited by reactive magnetron sputtering. Appl. Surf. Sci. 2016, 368, 88–96. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Acharya, R.; Chattopadhyay, A.K.; Paul, S. Effect of substrate bias voltage on structural and mechanical properties of pulsed DC magnetron sputtered TiN–MoSx composite coatings. Vacuum 2010, 84, 843–850. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, H.J.; Bao, S.N.; Shen, Y.G.; Zhou, Z.F. XPS study for reactively sputtered titanium nitride thin films deposited under different substrate bias. Phys. B Condens. Matter 2004, 352, 118–126. [Google Scholar] [CrossRef]
- Kong, Q.; Ji, L.; Li, H.; Liu, X.; Wang, Y.; Chen, J.; Zhou, H. Influence of substrate bias voltage on the microstructure and residual stress of CrN films deposited by medium frequency magnetron sputtering. Mater. Sci. Eng. B 2011, 176, 850–854. [Google Scholar] [CrossRef]
- Benegra, M.; Lamas, D.G.; de Rapp, M.E.F.; Mingolo, N.; Kunrath, A.O.; Souza, R.M. Residual stresses in titanium nitride thin films deposited by direct current and pulsed direct current unbalanced magnetron sputtering. Thin Solid Films 2006, 494, 146–150. [Google Scholar] [CrossRef]
- Forniés, E.; Escobar, G.R.; Sánchez, O.; Albella, J.M. Growth of CrNx films by DC reactive magnetron sputtering at constant N2/Ar gas flow. Surf. Coat. Technol. 2006, 200, 6047–6053. [Google Scholar] [CrossRef]
- Heo, S.J.; Kim, S.W.; Yeo, I.W.; Park, S.J.; Oh, Y.S. Effect of bias voltage on microstructure and phase evolution of Cr-Mo-N coatings by an arc bonded sputter system. Ceram. Int. 2016, 42, 5231–5237. [Google Scholar]
- Devia, D.M.; Restrepo-Parra, E.; Arango, P.J.; Tschiptschin, A.P.; Velez, J.M. TiAlN coatings deposited by triode magnetron sputtering varying the bias voltage. Appl. Surf. Sci. 2011, 257, 6181–6185. [Google Scholar] [CrossRef]
- Olaya, J.J.; Rodil, S.E.; Muhl, S.; Sánchez, E. Comparative study of chromium nitride coatings deposited by unbalanced and balanced magnetron sputtering. Thin Solid Films 2005, 474, 119–126. [Google Scholar] [CrossRef]
- Hultman, L.; Münz, W.-D.; Musil, J.; Kadlec, S.; Petrov, I.; Greene, J.E. Low-energy (~100 eV) ion irradiation during growth of TiN deposited by reactive magnetron sputtering: Effects of ion flux on film microstructure. J. Vac. Sci. Technol. A 1991, 9, 434–438. [Google Scholar] [CrossRef]
- Caicedo, J.C.; Yate, L.; Cabrera, G.; Pietro, P. Effect of negative bias voltage on mechanical and electrochemical nature in Ti-W-N coatings. J. Mater. Sci. 2010, 46, 1244–1252. [Google Scholar] [CrossRef]
- Lee, C.-T.; Cho, W.-H.; Shiao, M.-H.; Hsiao, C.-N.; Tang, K.-S.; Jaing, C.-C. Effects of DC Bias on the Microstructure, Residual Stress and Hardness Properties of TiVCrZrTaN Films by Reactive RF Magnetron Sputtering. Procedia Eng. 2012, 36, 316–321. [Google Scholar] [CrossRef]
- Banerjee, R.; Chandra, R.; Ayyub, P. Influence of the sputtering gas on the preferred orientation of nanocrystalline titanium nitride thin films. Thin Solid Films 2002, 405, 64–72. [Google Scholar] [CrossRef]
- Zhang, T.F.; Gan, B.; Park, S.; Wang, Q.M.; Kim, K.H. Influence of negative bias voltage and deposition temperature on microstructure and properties of superhard TiB2 coatings deposited by high power impulse magnetron sputtering. Surf. Coat. Technol. 2014, 253, 115–122. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Anderson, P.M.; Li, C. Hall-Petch relations for multilayered materials. Nanostruct. Mater. 1995, 5, 349–362. [Google Scholar] [CrossRef]
- Mounier, E.; Pauleau, Y. Mechanisms of intrinsic stress generation in amorphous carbon thin films prepared by magnetron sputtering. Diam. Relat. Mater. 1997, 6, 1182–1191. [Google Scholar] [CrossRef]
- Carrasco, C.A.; Vergara, S.; Benavente, G.; Mingolo, N.; Ros, J.C. The relationship between residual stress and process parameters in TiN coatings on copper alloy substrates. Mater. Charact. 2002, 48, 81–88. [Google Scholar] [CrossRef]
- Tang, C.; Klimenkov, M.; Jaentsch, U.; Leiste, H.; Ulrich, S.; Steinbrück, M.; Seifert, H.J.; Stueber, M. Synthesis and characterization of Ti2AlC coatings by magnetron sputtering from three elemental targets and ex-situ annealing. Surf. Coat. Technol. 2017, 309, 445–455. [Google Scholar] [CrossRef]
- Wilhelmsson, O.; Palmquist, J.-P.; Nyberg, T.; Jansson, U. Deposition of Ti2AlC and Ti3AlC2 epitaxial films by magnetron sputtering. Appl. Phys. Lett. 2004, 85, 1066–1068. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Zhou, J.; Liu, P.; Huang, Q.; Ke, P.; Wang, A. Microstructure evolution of V-Al-C coatings synthesized from a V2AlC compound target after vacuum annealing treatment. J. Alloys Compd. 2016, 661, 476–482. [Google Scholar] [CrossRef]
- Siumonrong, D.P.; Zhang, J.; Zhou, Y.; Music, D.; Schneider, J.M. Synthesis and elastic properties of V2AlC thin films by magnetron sputtering from elemental targets. J. Phys. D Appl. Phys. 2009, 42, 185408. [Google Scholar] [CrossRef]
- Hall, E.O. The Deformation and Ageing of Mild Steel: III Discussion of Results. Proc. Phys. Soc. Sect. B 1951, 64, 747. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Ar Pressure | 600 MPa |
| Cathode Power | 2.5 KW |
| Deposition Temperature | 560 °C |
| Bias Voltage | −60 V/−90 V/−120 V |
| Deposition Time | 1 h |
| Bias Voltage (V) | Thickness (µm) | RMS (nm) | Granular Size (nm) |
|---|---|---|---|
| 60 | 8.95 | 100 | 179 |
| 90 | 7.16 | 33 | 76 |
| 120 | 6.98 | 60 | 134 |
| Bias (V) | Hardness (GPa) | E-Modulus (GPa) | H/E | H3/E2 |
|---|---|---|---|---|
| 60 | 8.8 ± 0.9 | 223 ± 20 | 0.014 | 0.039 |
| 90 | 15.9 ± 3.2 | 308 ± 73 | 0.042 | 0.052 |
| 120 | 10.3 ± 0.5 | 257 ± 14 | 0.017 | 0.040 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obrosov, A.; Gulyaev, R.; Zak, A.; Ratzke, M.; Naveed, M.; Dudzinski, W.; Weiß, S. Chemical and Morphological Characterization of Magnetron Sputtered at Different Bias Voltages Cr-Al-C Coatings. Materials 2017, 10, 156. https://doi.org/10.3390/ma10020156
Obrosov A, Gulyaev R, Zak A, Ratzke M, Naveed M, Dudzinski W, Weiß S. Chemical and Morphological Characterization of Magnetron Sputtered at Different Bias Voltages Cr-Al-C Coatings. Materials. 2017; 10(2):156. https://doi.org/10.3390/ma10020156
Chicago/Turabian StyleObrosov, Aleksei, Roman Gulyaev, Andrzej Zak, Markus Ratzke, Muhammad Naveed, Wlodzimierz Dudzinski, and Sabine Weiß. 2017. "Chemical and Morphological Characterization of Magnetron Sputtered at Different Bias Voltages Cr-Al-C Coatings" Materials 10, no. 2: 156. https://doi.org/10.3390/ma10020156





