Figure 1.
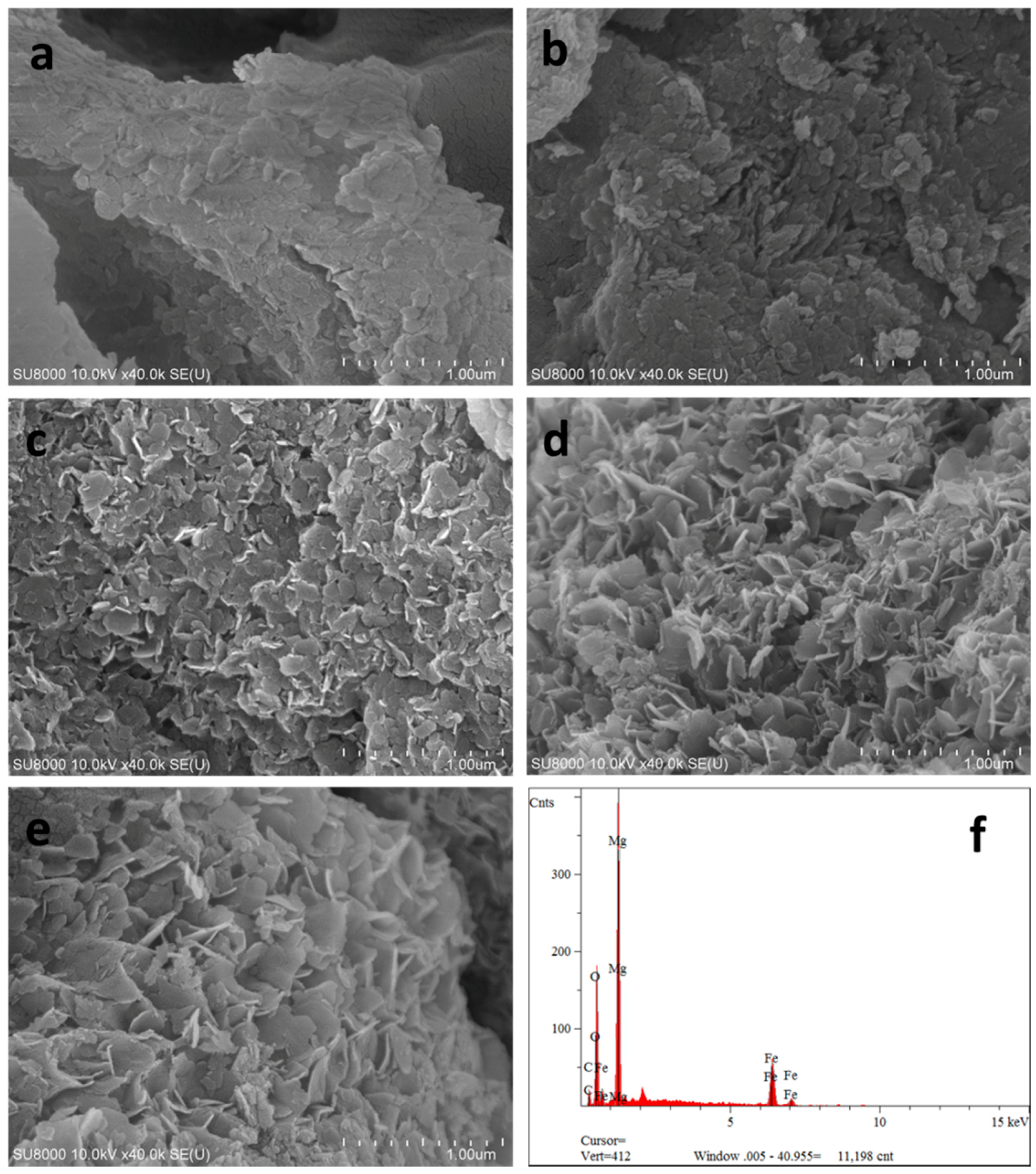
Scanning electron microscopic image of CSLDHs (a); LDO400 (b); CSLDHO300 (c); CSLDO400 (d); CSLDO500 (e); and the EDS of CSLDO400 (f).
Figure 1.
Scanning electron microscopic image of CSLDHs (a); LDO400 (b); CSLDHO300 (c); CSLDO400 (d); CSLDO500 (e); and the EDS of CSLDO400 (f).
Figure 2.
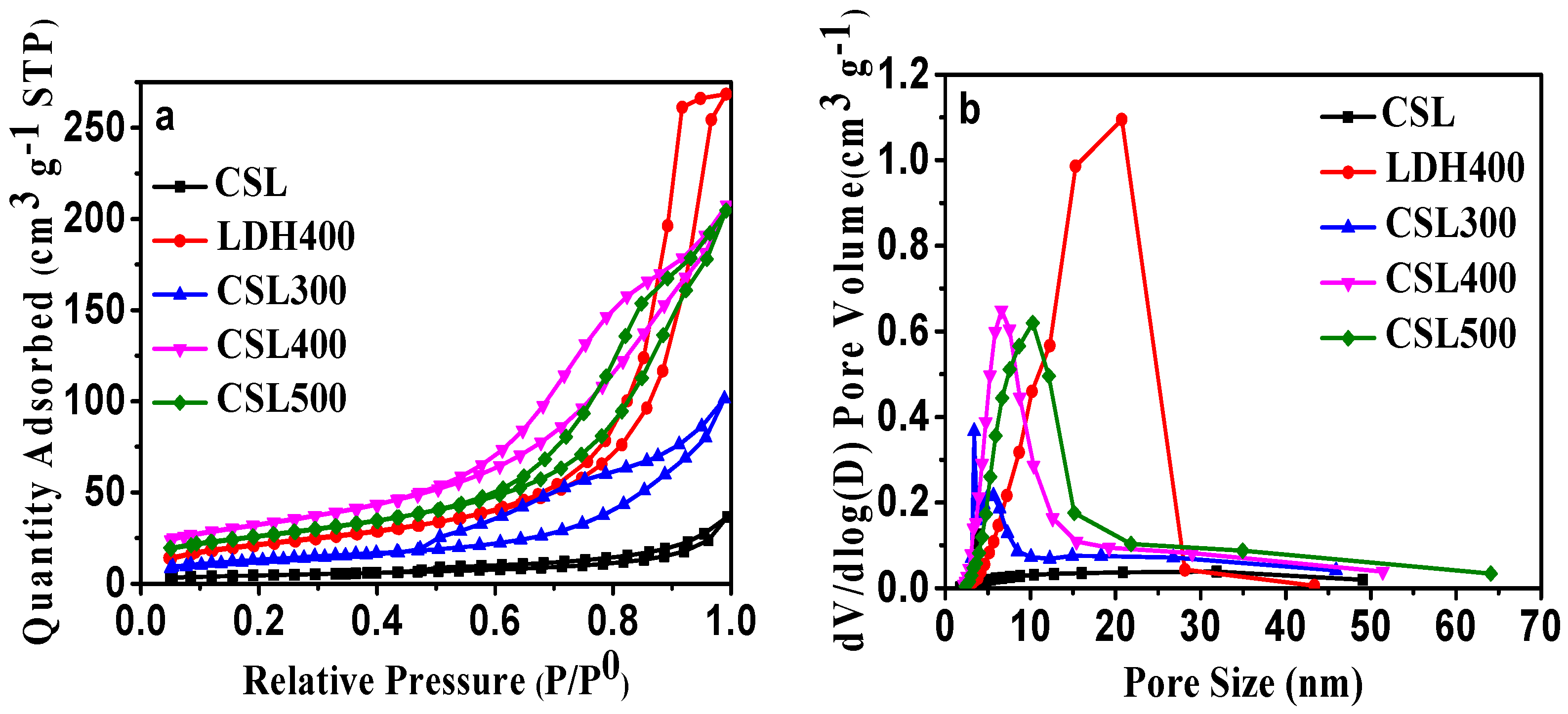
(a) N2 adsorption–desorption isotherms of CSLDHs, LDO400, CSLDO300, CSLDO400, and CSLDO500 at 77 K; and (b) pore size distribution.
Figure 2.
(a) N2 adsorption–desorption isotherms of CSLDHs, LDO400, CSLDO300, CSLDO400, and CSLDO500 at 77 K; and (b) pore size distribution.
Figure 3.
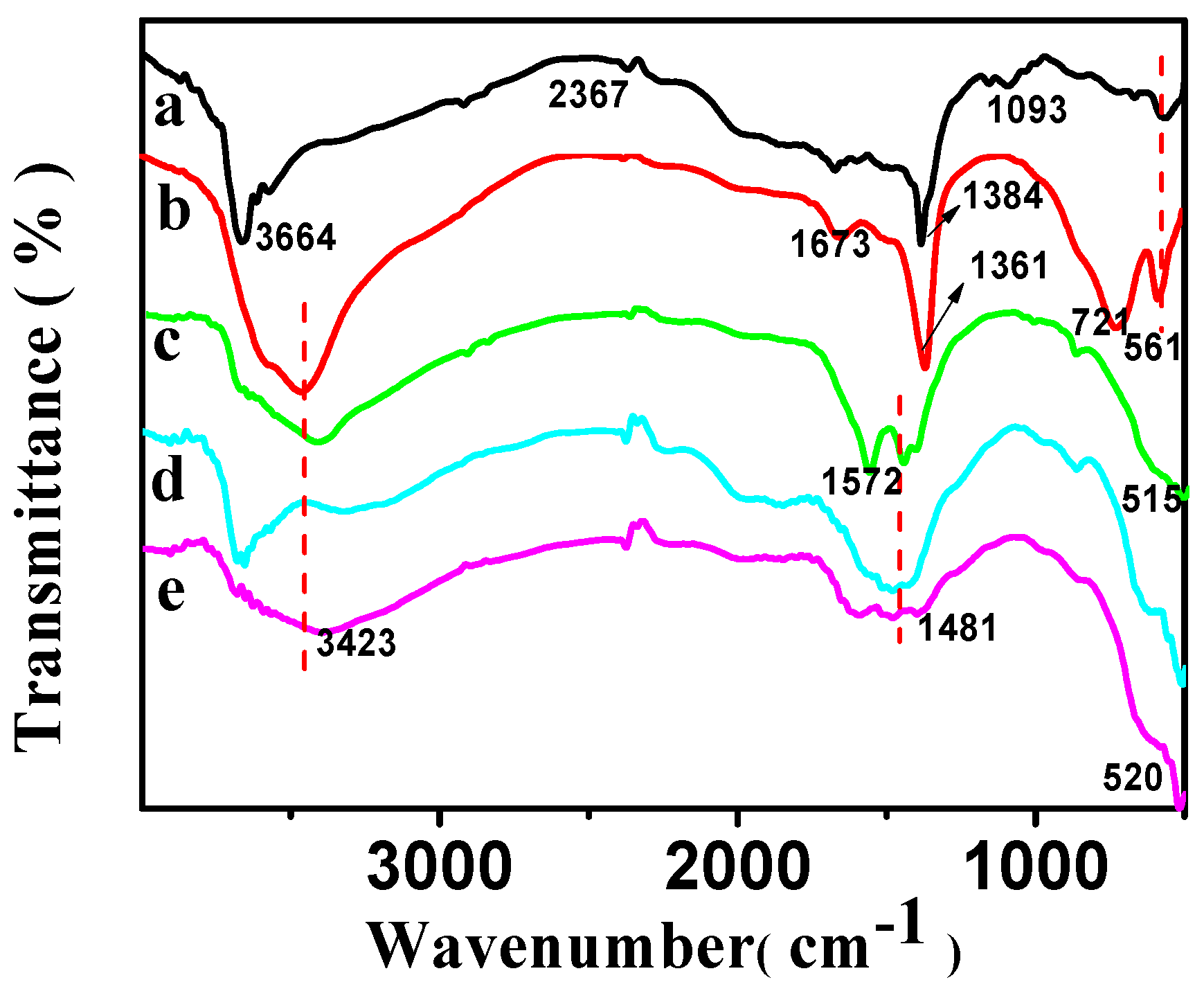
FT-IR spectra of CSLDHs (a); LDO400 (b); CSLDO300 (c); CSLDO400 (d); and CSLDO500 (e).
Figure 3.
FT-IR spectra of CSLDHs (a); LDO400 (b); CSLDO300 (c); CSLDO400 (d); and CSLDO500 (e).
Figure 4.
X-ray diffraction (XRD) patterns of CSLDHs (a); LDO400 (b); CSLDO300 (c); CSLDO400 (d); CSLDO500 (e); and regenerated CSLDO400 (f) after five adsorption–desorption cycles.
Figure 4.
X-ray diffraction (XRD) patterns of CSLDHs (a); LDO400 (b); CSLDO300 (c); CSLDO400 (d); CSLDO500 (e); and regenerated CSLDO400 (f) after five adsorption–desorption cycles.
Figure 5.
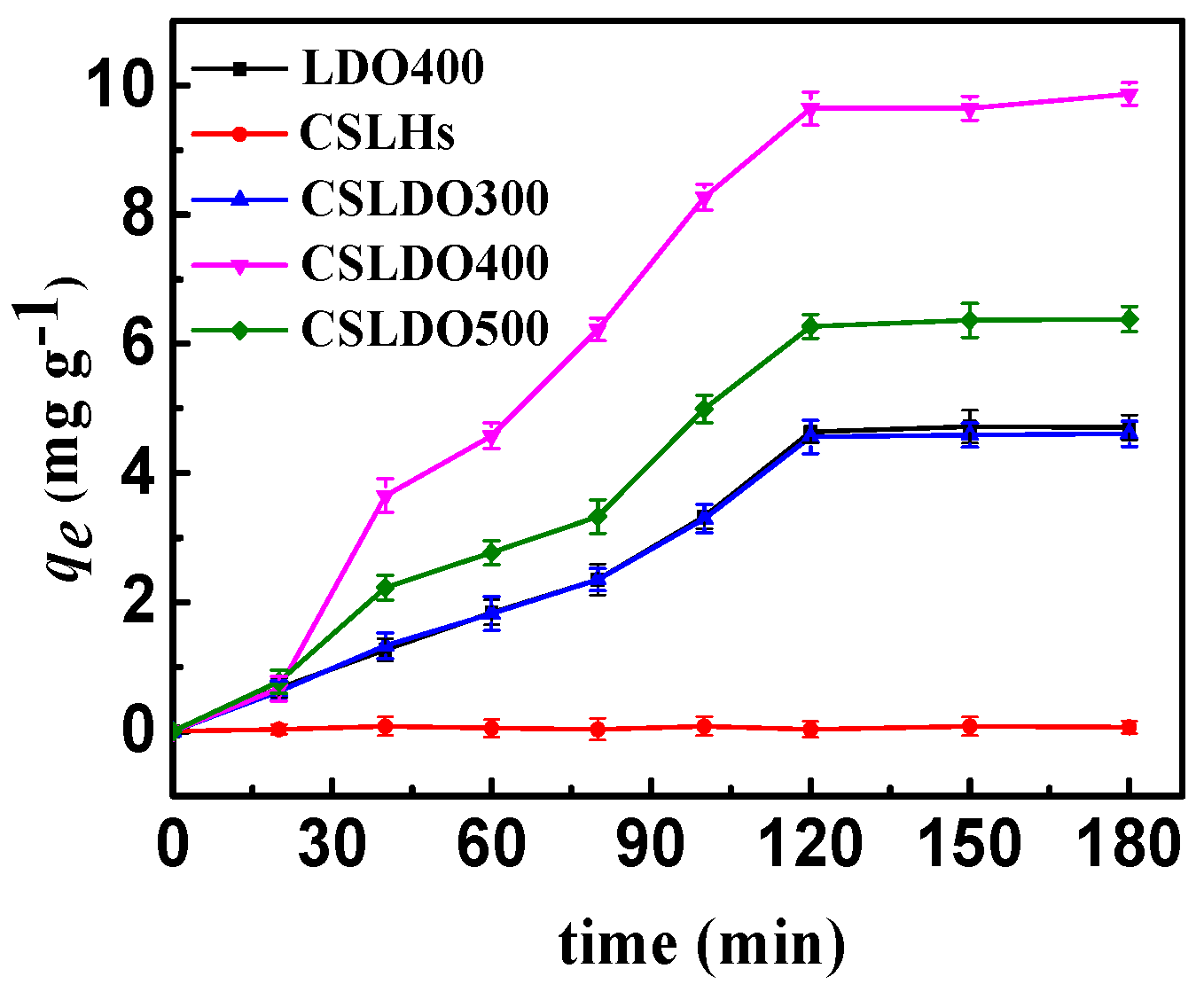
Adsorption capacities of CSLDHs, LDO400, CSLDO300, CSLDO400, and CSLDO500 to fluoride at different times (Adsorption dose, 0.625 g·L−1; solutions concentration, 10 mg·L−1; pH = 7; adsorption time, 3 h; temperature, 298 K).
Figure 5.
Adsorption capacities of CSLDHs, LDO400, CSLDO300, CSLDO400, and CSLDO500 to fluoride at different times (Adsorption dose, 0.625 g·L−1; solutions concentration, 10 mg·L−1; pH = 7; adsorption time, 3 h; temperature, 298 K).
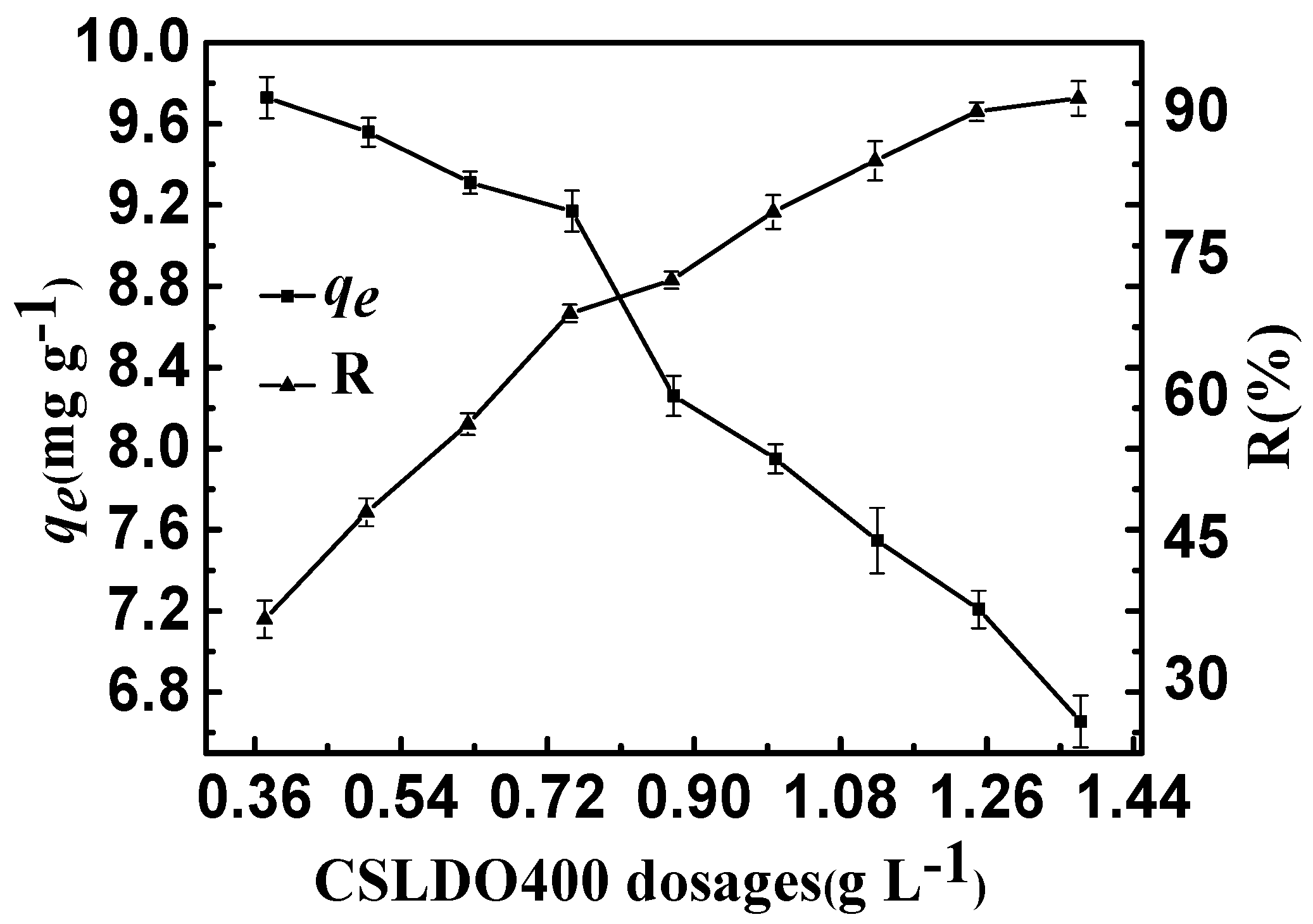
Figure 6.
Effect of CSLDO400 dosages on the adsorption of fluoride (Solution concentration, 10 mg·L−1; pH = 7; adsorption time, 3 h, temperature, 298 K).
Figure 6.
Effect of CSLDO400 dosages on the adsorption of fluoride (Solution concentration, 10 mg·L−1; pH = 7; adsorption time, 3 h, temperature, 298 K).
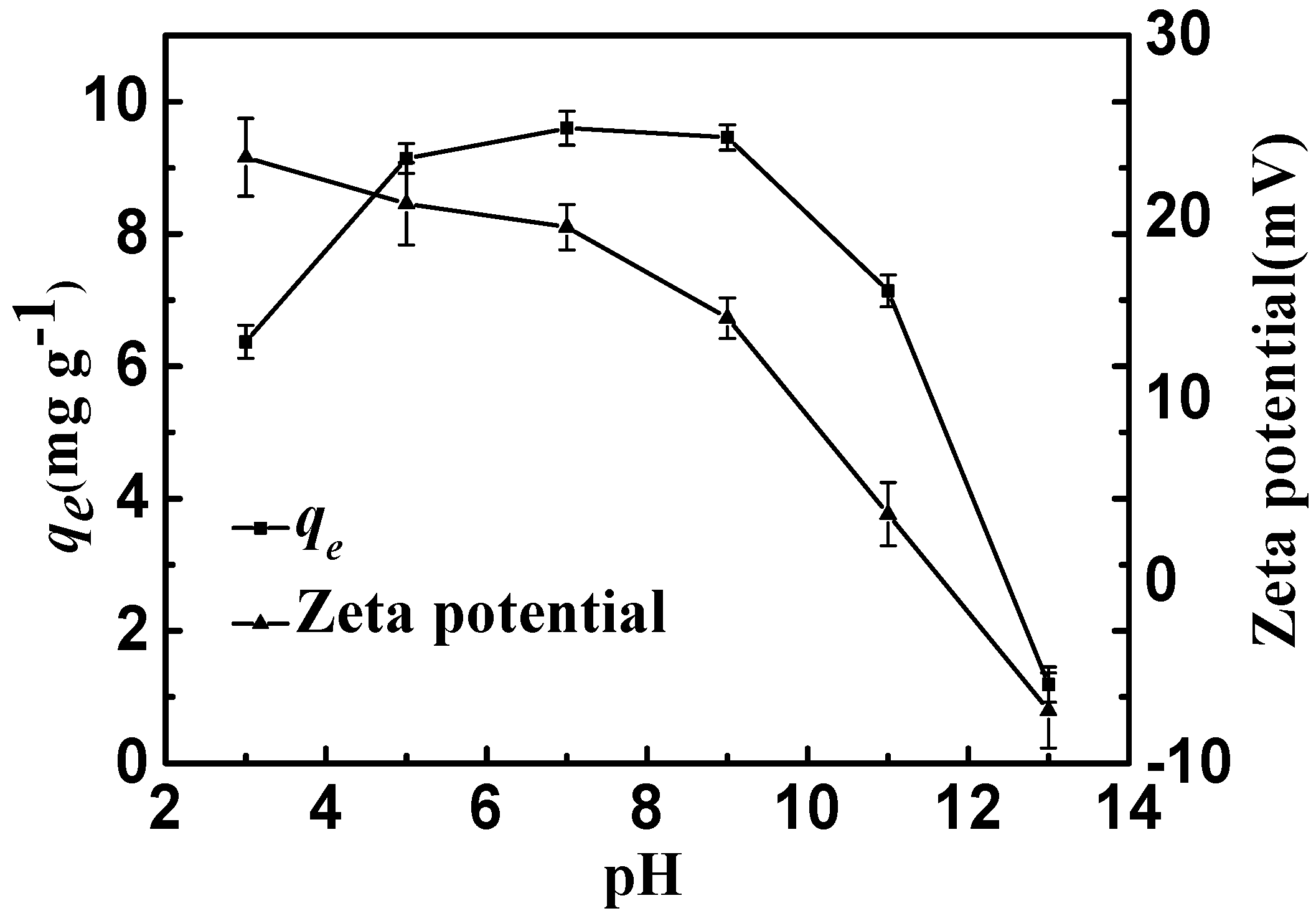
Figure 7.
Adsorption capacities and zeta potential of CSLDO400 as a function of pH (Adsorption dose, 0.75 g·L−1; solutions concentration, 10 mg·L−1; adsorption time, 3 h; temperature: 298 K).
Figure 7.
Adsorption capacities and zeta potential of CSLDO400 as a function of pH (Adsorption dose, 0.75 g·L−1; solutions concentration, 10 mg·L−1; adsorption time, 3 h; temperature: 298 K).
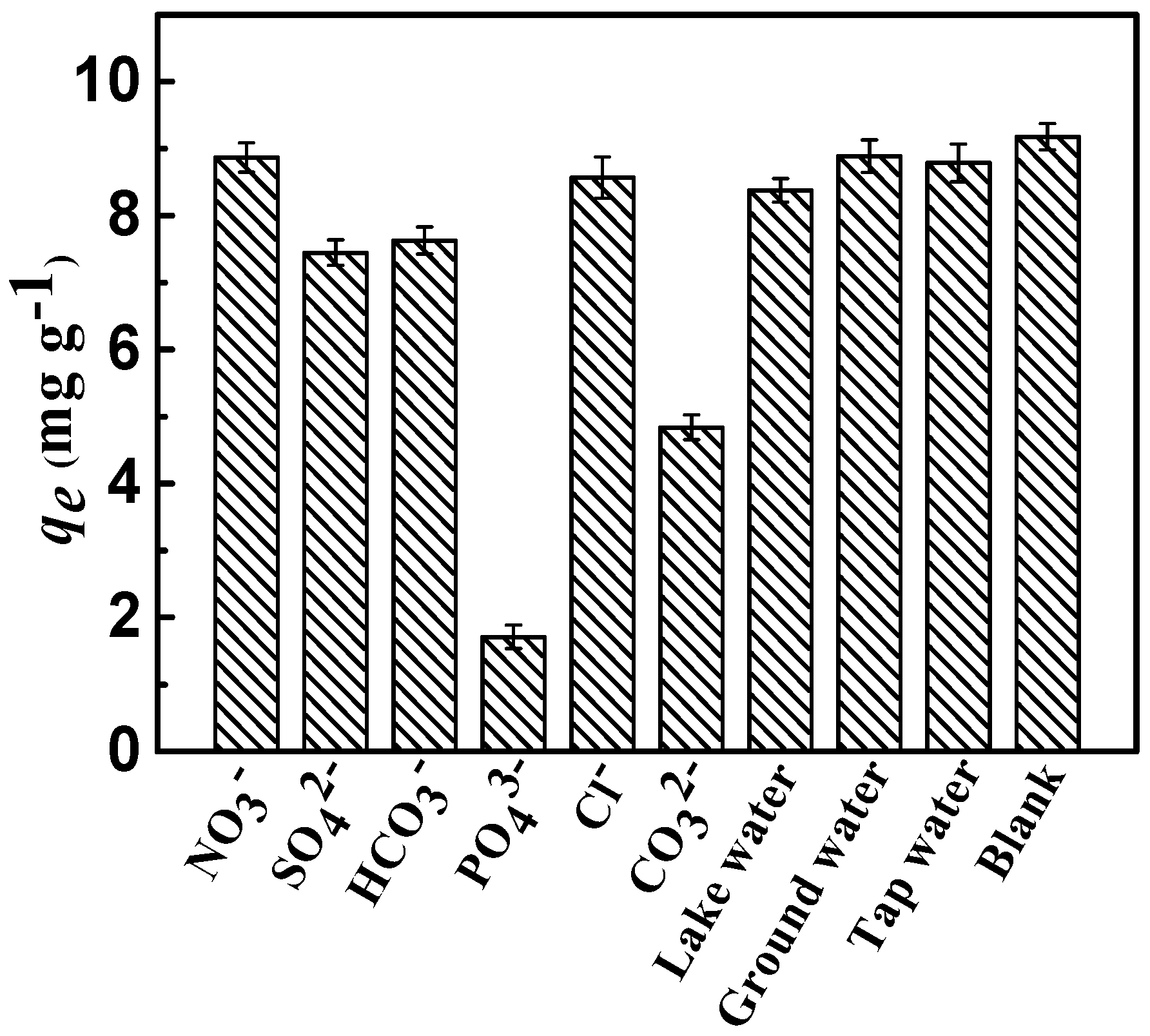
Figure 8.
Removal of fluoride as a function of the co-anions (Conditions: initial concentration = 10 mg·L−1, dose = 0.75 g·L−1, adsorption time = 3 h, adsorption temperature = 298 K, and pH = 7).
Figure 8.
Removal of fluoride as a function of the co-anions (Conditions: initial concentration = 10 mg·L−1, dose = 0.75 g·L−1, adsorption time = 3 h, adsorption temperature = 298 K, and pH = 7).
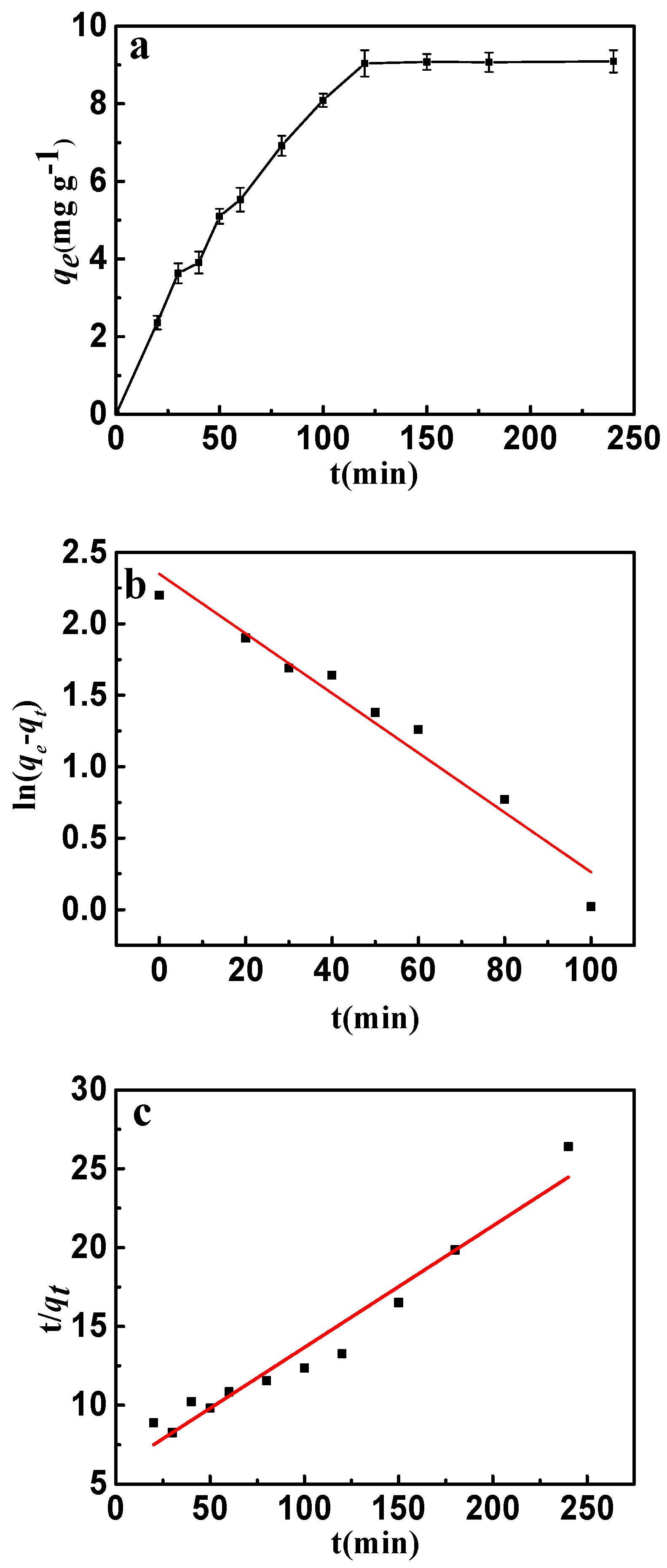
Figure 9.
(a) Effect of contact time on the adsorption of fluoride on to CSLDO400 (initial concentration, 10 mg·L−1; pH = 7; adsorption dose, 0.75 g·L−1; temperature, 298 K); (b) pseudo-first-order kinetic plots for adsorption of fluoride; (c) pseudo-second-order kinetic plots for adsorption of fluoride.
Figure 9.
(a) Effect of contact time on the adsorption of fluoride on to CSLDO400 (initial concentration, 10 mg·L−1; pH = 7; adsorption dose, 0.75 g·L−1; temperature, 298 K); (b) pseudo-first-order kinetic plots for adsorption of fluoride; (c) pseudo-second-order kinetic plots for adsorption of fluoride.
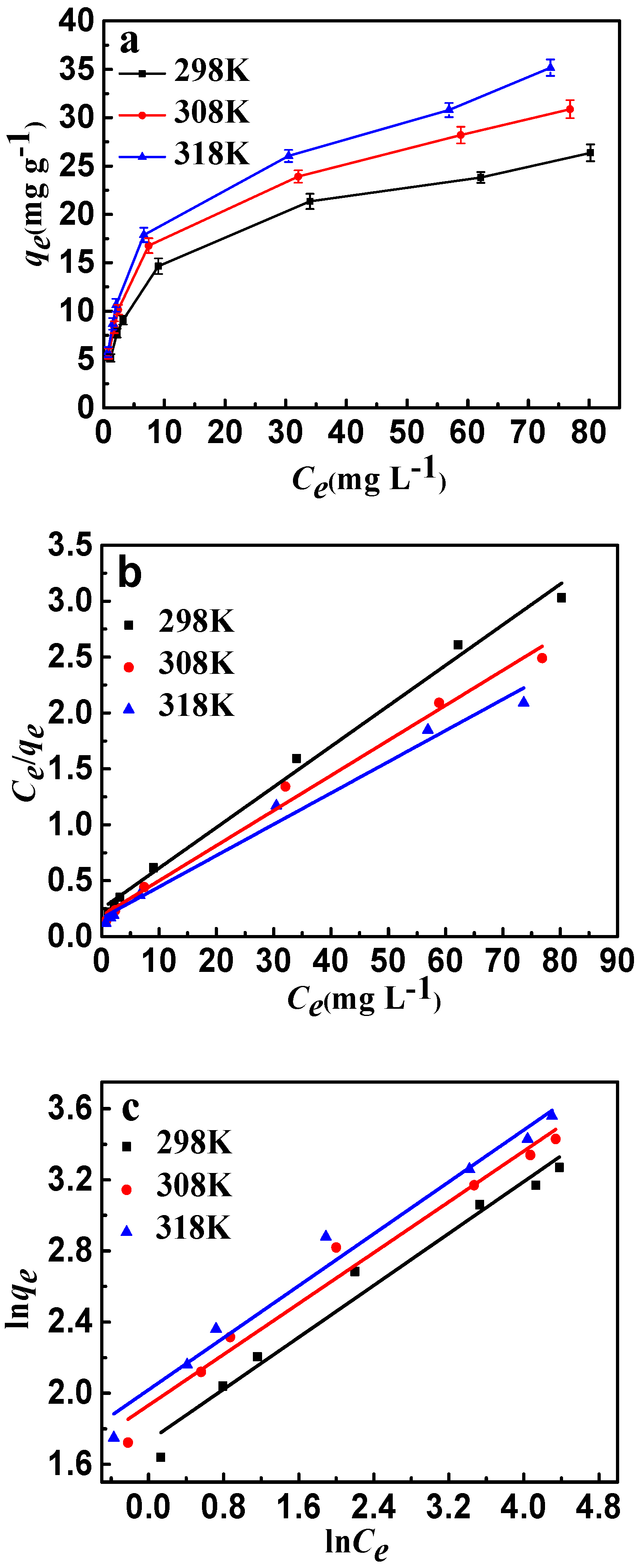
Figure 10.
(a) Adsorption isotherms of fluoride ion adsorption onto CSLDO400 (Conditions: concentration was 5, 8, 10, 20, 50, 80, 100 mg·L−1, pH = 7, dose = 0.75 g·L−1, adsorption time = 3 h); (b) Langmuir plots of the isotherms for fluoride; (c) Freundlich plots of the isotherms for fluoride.
Figure 10.
(a) Adsorption isotherms of fluoride ion adsorption onto CSLDO400 (Conditions: concentration was 5, 8, 10, 20, 50, 80, 100 mg·L−1, pH = 7, dose = 0.75 g·L−1, adsorption time = 3 h); (b) Langmuir plots of the isotherms for fluoride; (c) Freundlich plots of the isotherms for fluoride.
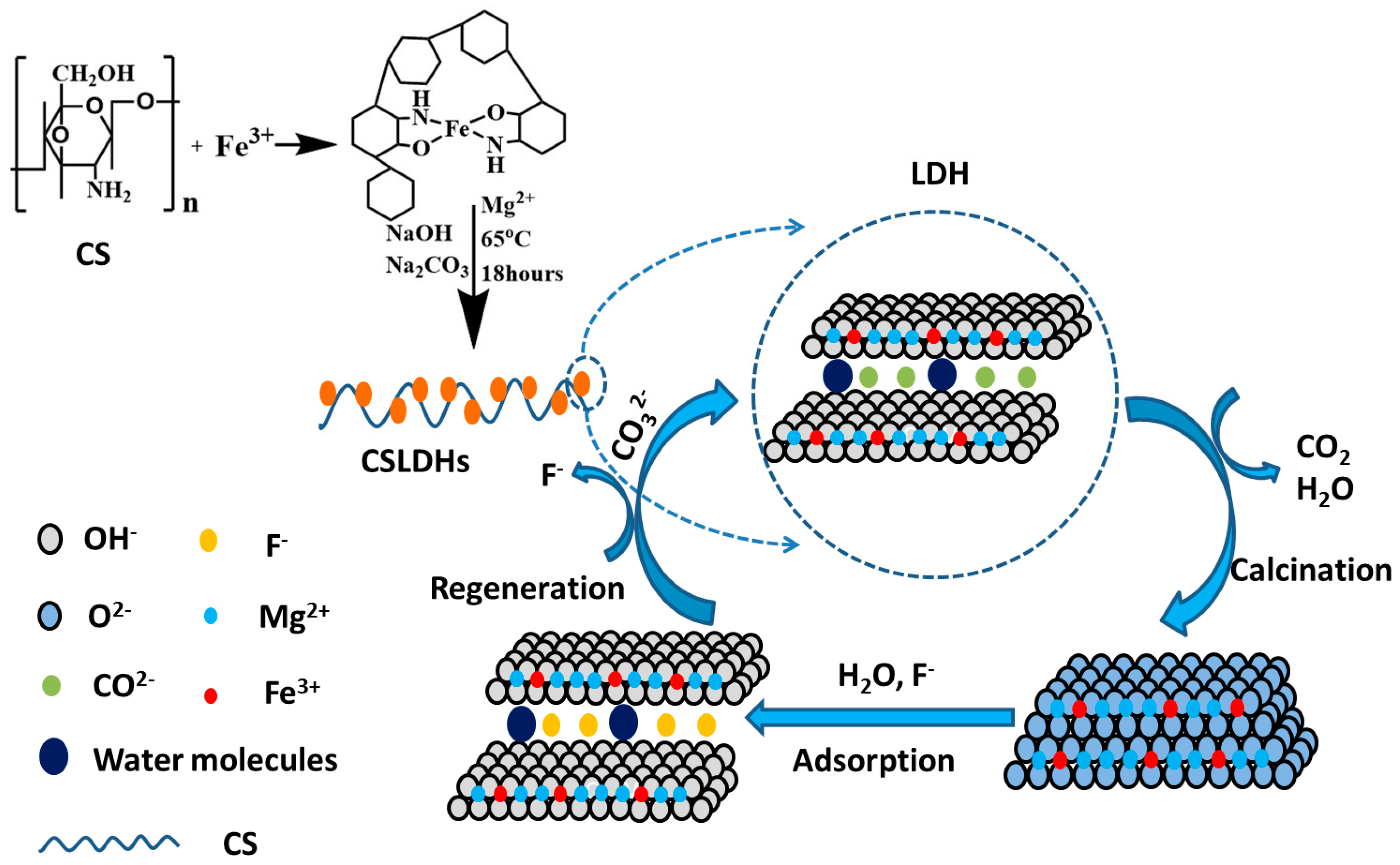
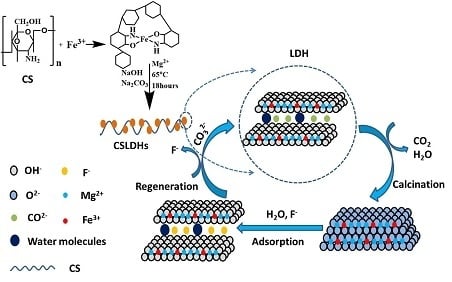
Figure 11.
Schematic diagram of the adsorption of fluoride on CSLDO400.
Figure 11.
Schematic diagram of the adsorption of fluoride on CSLDO400.
Figure 12.
Effect of recycling times on the concentration of fluoride in desorbing solution (a); and the removal rate of fluoride (b).
Figure 12.
Effect of recycling times on the concentration of fluoride in desorbing solution (a); and the removal rate of fluoride (b).
Table 1.
Specific surface area and pore volume parameters of CSLDHs, LDO400, CSLDO300, CSLDO400, and CSLDO500.
Table 1.
Specific surface area and pore volume parameters of CSLDHs, LDO400, CSLDO300, CSLDO400, and CSLDO500.
| Materials | SBET a/m2·g−1 | Smic b/m2·g−1 | Vmic c/cm3·g−1 | Vmeso d/cm3·g−1 | Vt e/cm3·g−1 | Dp f/nm |
|---|
| CSLDHs | 16.38 | 2.03 | 0.0003 | 0.0557 | 0.056 | 13.28 |
| LDO400 | 80.73 | 7.59 | 0.0056 | 0.3174 | 0.323 | 15.83 |
| CSLDO300 | 47.55 | 4.05 | 0.0012 | 0.1538 | 0.155 | 12.01 |
| CSLDO400 | 116.98 | 4.37 | 0.0014 | 0.4106 | 0.412 | 8.84 |
| CSLDO500 | 94.35 | 2.21 | 0.0005 | 0.3175 | 0.318 | 10.99 |
Table 2.
Parameters for fluoride adsorption by CSLDO400 according to different kinetic models.
Table 2.
Parameters for fluoride adsorption by CSLDO400 according to different kinetic models.
| qe(exp) (mg·g−1) | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model |
|---|
| k1 (min−1) | qe1(cal) (mg·g−1) | R2 | k2 (×10−4) (g·mg−1·min−1) | qe2(cal) (mg·g−1) | R2 |
|---|
| 9.58 | 0.02 | 10.47 | 0.9514 | 9.99 | 12.97 | 0.9483 |
Table 3.
Isotherm model constants and correlation coefficients for adsorption of fluoride onto CSLDO400 at different temperature.
Table 3.
Isotherm model constants and correlation coefficients for adsorption of fluoride onto CSLDO400 at different temperature.
| T (K) | Langmuir Model | Freundlich Model |
|---|
| b (L·mg−1) | qmax (mg·g−1) | R2 | n | Kf | R2 |
|---|
| 298 | 0.1457 | 27.56 | 0.9930 | 2.740 | 5.6423 | 0.9724 |
| 308 | 0.1689 | 31.88 | 0.9909 | 2.797 | 6.9023 | 0.9735 |
| 318 | 0.1695 | 35.77 | 0.9842 | 2.7372 | 7.5334 | 0.9756 |
Table 4.
Thermodynamic parameters for the adsorption of fluoride.
Table 4.
Thermodynamic parameters for the adsorption of fluoride.
| T (K) | ∆S (J·mol−1·K−1) | ∆H (kJ·mol−1) | ∆G (kJ·mol−1) | R2 |
|---|
| 298 | 60.52 | 5.706 | −12.33 | 0.9576 |
| 308 | - | - | −12.93 | - |
| 318 | - | - | −13.54 | - |
Table 5.
Comparison of adsorption capacity of CSLDO400 with different adsorbents.
Table 5.
Comparison of adsorption capacity of CSLDO400 with different adsorbents.
| Adsorbents | qmax (mg·g−1) | pH | References |
|---|
| CSLDO400 | 27.56 | 5~9 | Present study |
| Iron–aluminum mixed oxide | 17.73 | 5.5~5.7 | [39] |
| Quick lime | 16.67 | - | [40] |
| CSLDH-75 | 13.8 | - | [13] |
| Granular ceramic | 12.12 | 5~8 | [41] |
| Ceramic adsorbent | 2.16 | 5.8 ± 0.2 | [42] |
Table 6.
Major anion contents in real water samples.
Table 6.
Major anion contents in real water samples.
| Samples | NO3− (mg·L−1) | SO42− (mg·L−1) | HCO3− (mg·L−1) | PO43− (mg·L−1) | Cl− (mg·L−1) | CO32− (mg·L−1) |
|---|
| Lake water | 110.97 | 62.65 | 312.78 | detection limit | 84.41 | 35.62 |
| Tap water | 5.87 | 53.71 | 225.37 | detection limit | 68.16 | 10.23 |
| Groundwater | 15.49 | 38.59 | 301.74 | detection limit | 71.36 | 27.38 |