A New Maraging Stainless Steel with Excellent Strength–Toughness–Corrosion Synergy
Abstract
:1. Introduction
2. Alloy Design
3. Experimental Details
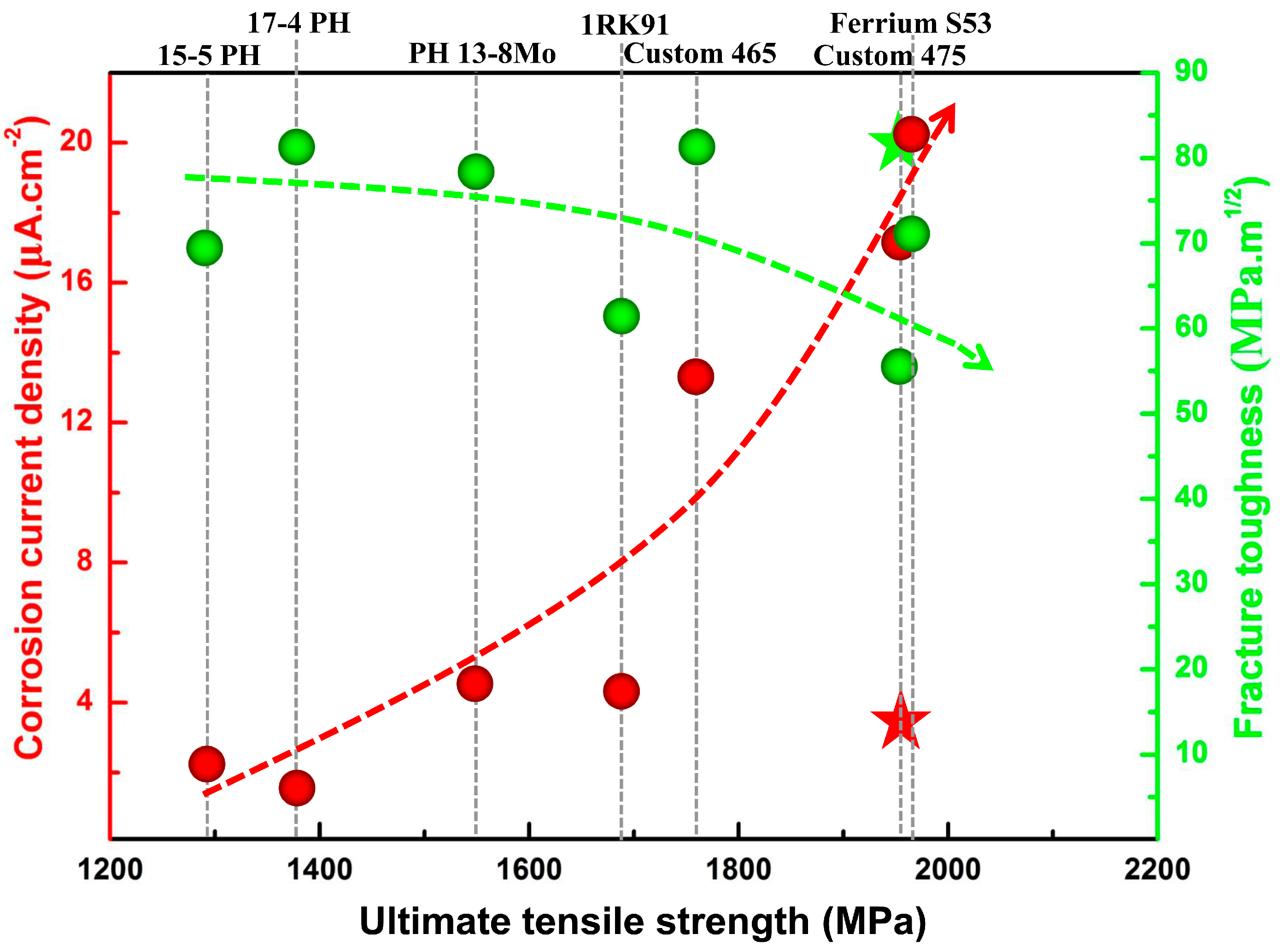
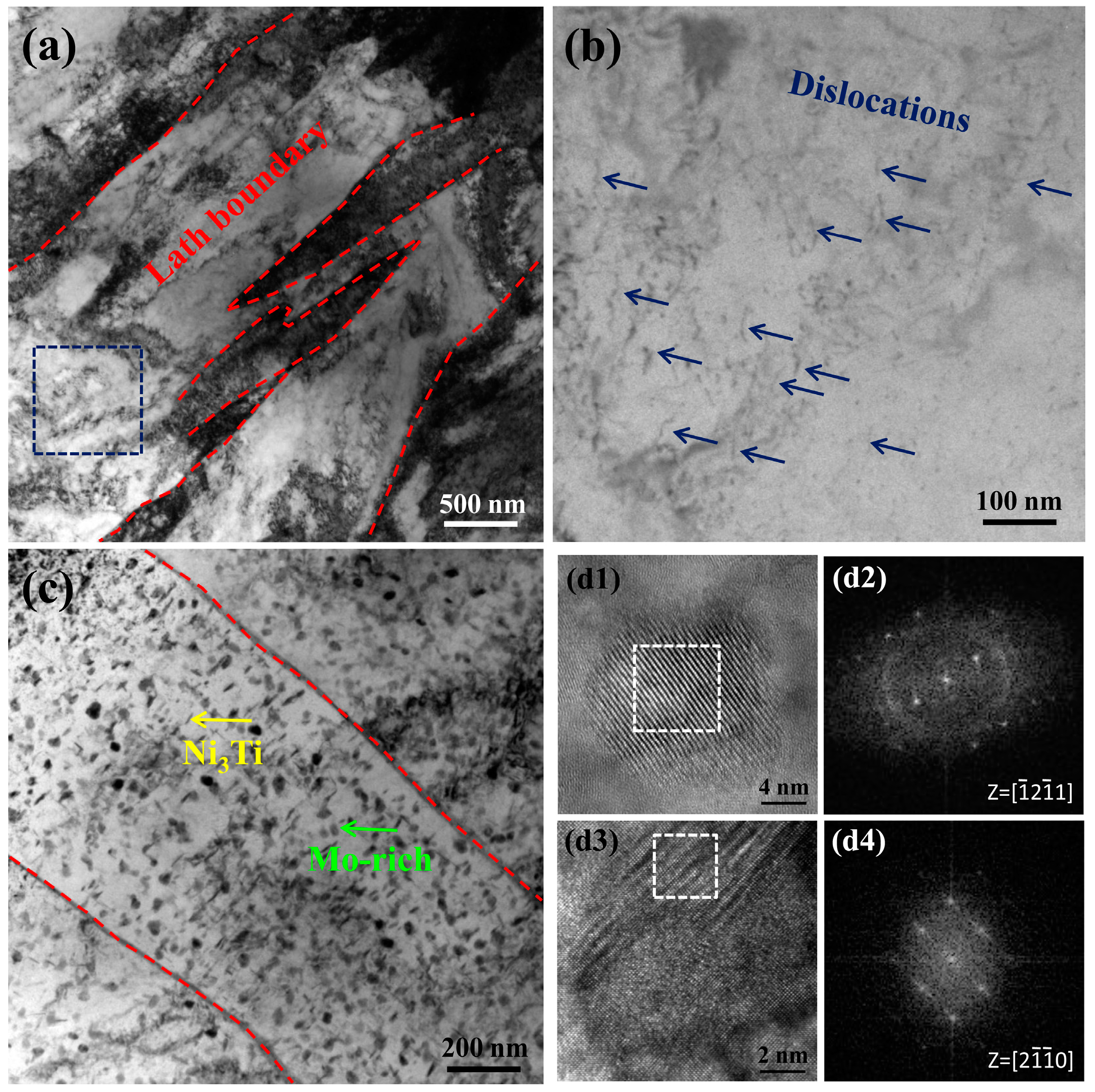
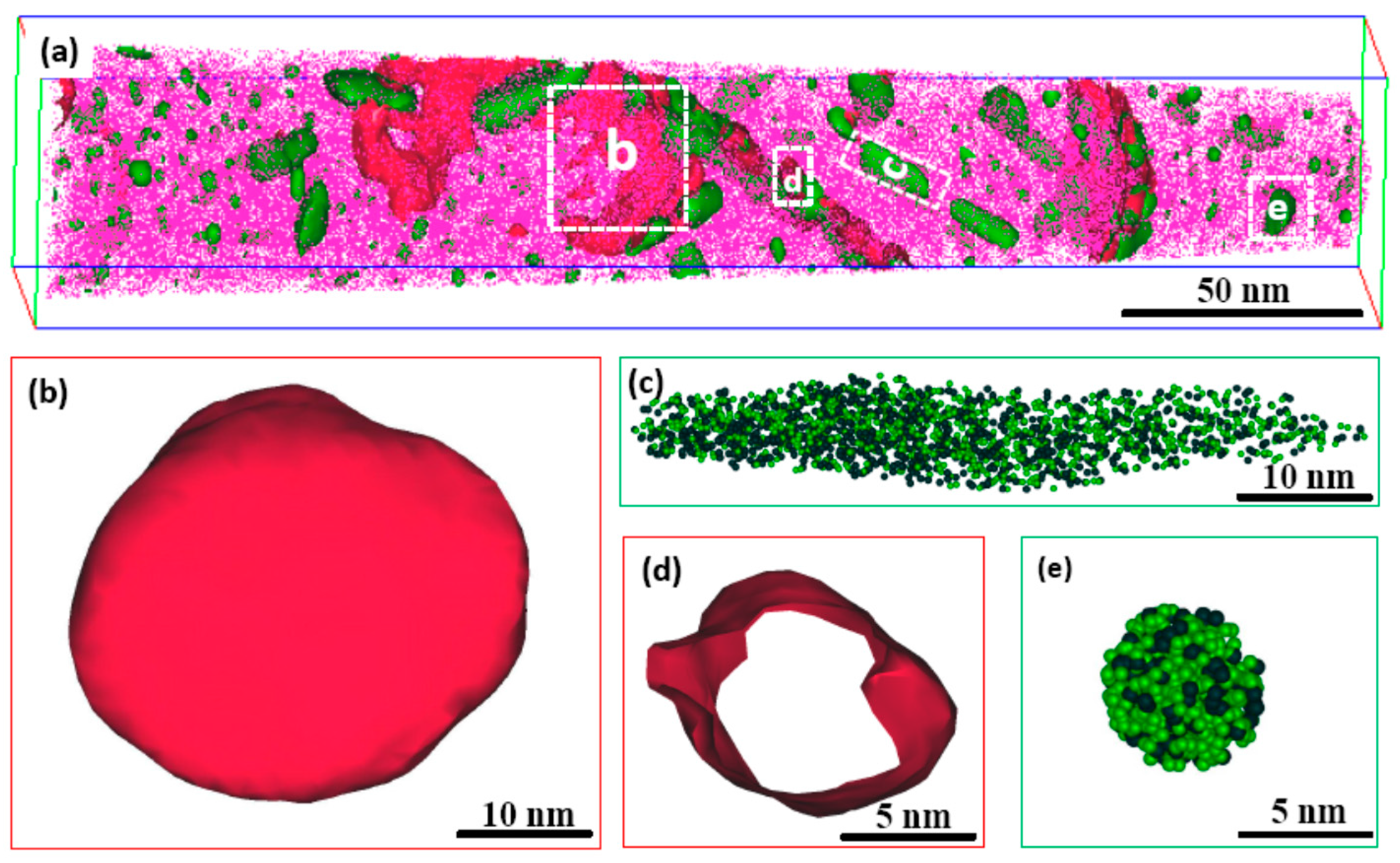
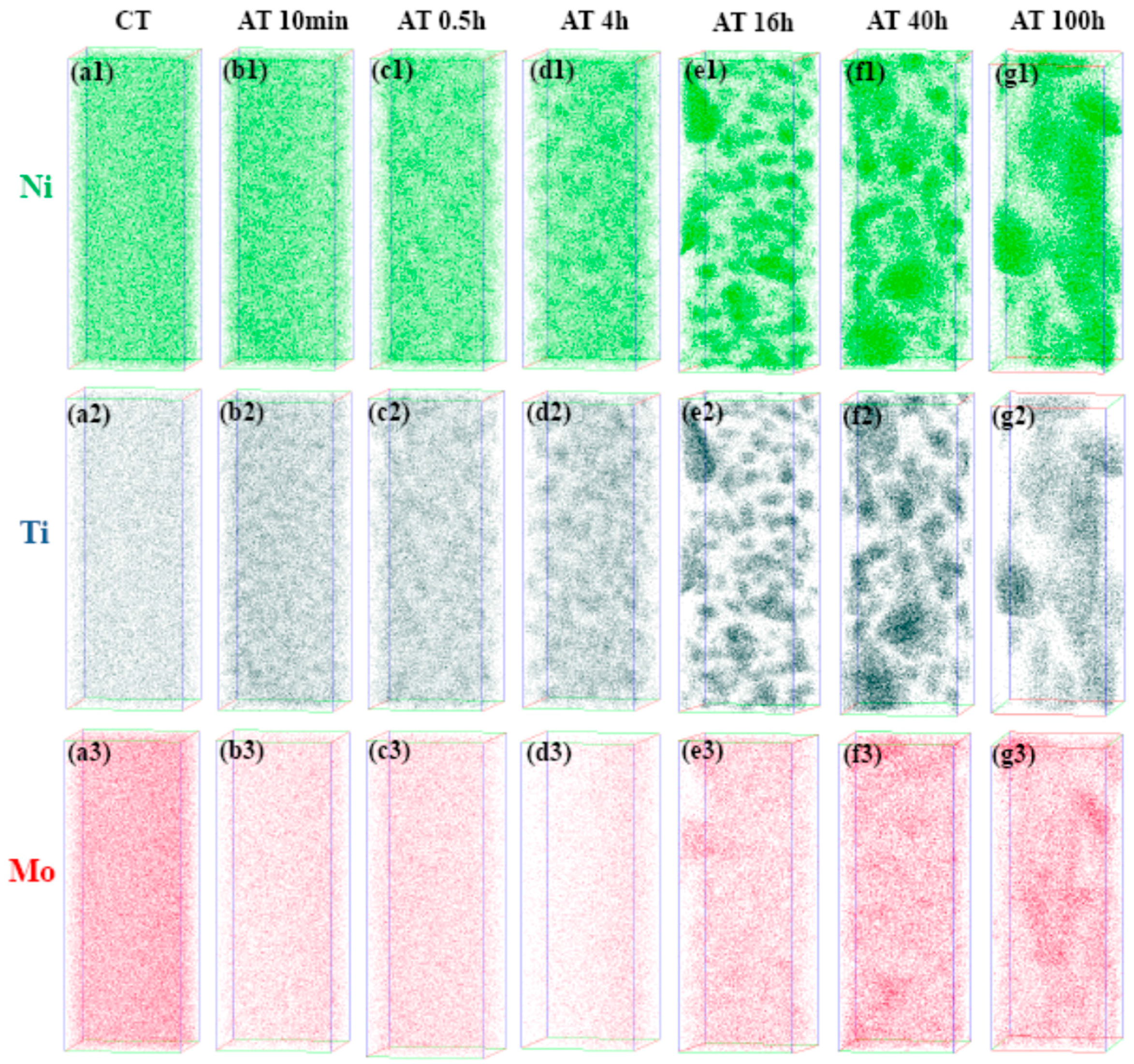
4. Results and Discussion
4.1. Microstructure and Properties
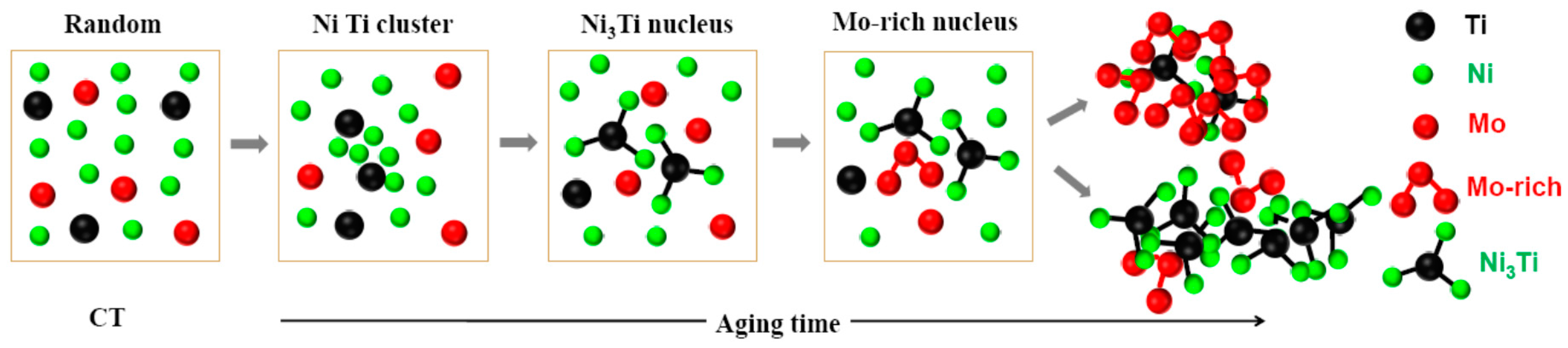
4.2. Precipitation Mechanism of Ni3Ti and Mo-Rich Precipitates
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, S.; Wang, H.; Wu, Y.; Liu, X.J.; Chen, H.G.; Yao, M.J.; Gault, B.; Ponge, D.; Raabe, D.; Hirata, A.; et al. Ultrastrong steel via minimal lattice misfit and high-density nanoprecipitation. Nature 2017, 544, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Yan, W.; Cotton, J.D.; Ryan, G.J.; Shen, Y.F.; Wang, W.; Shan, Y.Y.; Yang, K. A new 1.9 GPa maraging stainless steel strengthened by multiple precipitating species. Mater. Des. 2015, 82, 56–63. [Google Scholar] [CrossRef]
- Xu, W.; Rivera-Díaz-del-Castillo, P.E.J.; Yan, W.; Yang, K.; Martín, D.S.; Kestens, L.A.I.; van der Zwaag, S. A new ultrahigh-strength stainless steel strengthened by various coexisting nanoprecipitates. Acta Mater. 2010, 58, 4067–4075. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.B.; Liang, J.X.; Zhang, X.L.; Yang, Z.Y.; Sun, G.Q. The development and application of high-performance ultra-high strength stainless steel subject to marine environment. Mater. Sci. Forum 2014, 783–786, 867–874. [Google Scholar] [CrossRef]
- Guo, Z.; Sha, W.; Vaumousse, D. Microstructural evolution in a PH13-8 stainless steel after ageing. Acta Mater. 2003, 51, 101–116. [Google Scholar] [CrossRef]
- Hättestrand, M.; Nilsson, J.O.; Stiller, K.; Liu, P.; Andersson, M. Precipitation hardening in a 12%–9%Ni-4%Mo-2%Cu stainless steel. Acta Mater. 2004, 52, 1023–1037. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Luan, J.H.; Miller, M.K.; Liu, C.T. Precipitation mechanism and mechanical properties of an ultra-high strength steel hardened by nanoscale NiAl and Cu particles. Atca Mater. 2015, 97, 58–67. [Google Scholar] [CrossRef]
- Murthy, A.S.; Medvedeva, J.E.; Isheim, D.; Lekakh, S.L.; Richards, V.L.; Van Aken, D.C. Copper precipitation in cobalt-alloyed precipitation-hardened stainless steel. Scr. Mater. 2012, 66, 943–946. [Google Scholar] [CrossRef]
- Leitner, H.; Schnitzer, R.; Schober, M.; Zinner, S. Precipitate modification in PH13-8 Mo type maraging steel. Acta Mater. 2011, 59, 5012–5022. [Google Scholar] [CrossRef]
- Murayama, M.; Katayama, Y.; Hono, K. Microstructural evolution in a 17-4 PH stainless steel after aging at 400 °C. Metall. Mater. Trans. A 1999, 30A, 345–353. [Google Scholar] [CrossRef]
- Speich, G.R.; Dabkowski, D.S.; Porter, L.F. Strength and toughness of Fe-10Ni alloys containing C, Cr, Mo, and Co. Metall. Trans. 1973, 4, 303–315. [Google Scholar] [CrossRef]
- Squires, D.R.; Wilson, F.G.; Wilson, E.A. The influence of Mo and Co on the embrittlement of an Fe-Ni-Mn alloy. Metall. Trans. 1974, 5, 2569–2578. [Google Scholar] [CrossRef]
- Kwon, H.; Lee, J.H.; Lee, K.B.; Kwon, H.; Kim, C.M.; Yang, H.R. Effects of Co and Ni on secondary hardening and fracture behavior of martensitic steels bearing W and Cr. Metall. Mater. Trans. A 1998, 29, 397–401. [Google Scholar] [CrossRef]
- Kwon, H.; Lee, J.H.; Lee, K.B.; Kwon, H.; Kim, C.M.; Yang, H.R. Effect of alloying additions on secondary hardening behavior of mo-containing steels. Metall. Mater. Trans. A 1997, 28, 621–627. [Google Scholar] [CrossRef]
- Nakamichi, H.; Sato, K.; Miyata, Y.; Kimura, M.; Masamura, K. Quantitative analysis of Cr-depleted zone morphology in low carbon martensitic stainless steel using Fe-(S)TEM. Corros. Sci. 2008, 50, 309–315. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Subramanian, S.V.; Liu, C. Studies on Nb microalloying of 13Cr super martensitic stainless steel. Metall. Mater. Trans. A 2012, 43A, 4475–4486. [Google Scholar] [CrossRef]
- Tian, J.L.; Wang, W.; Yin, L.C.; Yan, W.; Shan, Y.Y.; Yang, K. Three dimensional atom probe and first-principles studies on spinodal decomposition of Cr in a Co-Alloyed maraging stainless steel. Scr. Mater. 2016, 121, 37–41. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Abdelshehid, M.; Mahmodieh, K.; Mori, K.; Chen, L.; Stoyanov, P.; Davlantes, D.; Foyos, J.; Ogren, J.; Clark, R., Jr.; Es-Said, O.S. On the correlation between fracture toughness and precipitation hardening heat treatment in 15-5PH stainless steel. Eng. Fail. Anal. 2007, 14, 626–631. [Google Scholar] [CrossRef]
- Rack, H.J.; Kalish, D. The strength, fracture toughness, and low cycle fatigue behavior of 17-4 PH stainless steel. Metall. Trans. 1974, 5, 1595–1605. [Google Scholar] [CrossRef]
- Rossi, D.J.; Rossi, J.D. PH stainless steel is tougher than you thought. Adv. Mater. Process. 1987, 131, 45–47. [Google Scholar]
- Wert, D.E.; Disabella, R.P. Strong, corrosion-resistance stainless steel. Adv. Mater. Process. 2006, 164, 34–36. [Google Scholar]
- Kuehmann, C.; Tufts, B.; Trester, P. Computational design for ultra high-strength alloy. Adv. Mater. Process. 2008, 166, 37–40. [Google Scholar]
- Cahn, J.W. Nucleation on dislocations. Acta Metall. 1957, 5, 169–172. [Google Scholar] [CrossRef]
- Druzhkov, A.P.; Perminov, D.A. Positron annihilation studies of microstructural changes in cold-worked Fe-Ni-base aging alloys. Mater. Sci. Eng. A 2010, 527, 3877–3885. [Google Scholar] [CrossRef]
- Sha, W.; Cerezo, A.; Smith, G.D.W. Phase chemistry and precipitation reactions in maraging steels: Part IV. Discussion and conclusions. Metall. Trans. A 1993, 24, 1251–1256. [Google Scholar] [CrossRef]
- Nitta, H.; Yamamoto, T.; Kanno, R.; Takasawa, K.; Iida, T.; Yamazaki, Y.; Ogu, S.; Iijima, Y. Diffusion of molybdenum in α-iron. Acta Mater. 2002, 50, 4117–4125. [Google Scholar] [CrossRef]
- Shapovalov, V.P.; Kurasov, A.N. Diffusion of titanium in iron. Met. Sci. Heat Treat. 1975, 17, 803–805. [Google Scholar] [CrossRef]
- Hettich, G.; Mehrer, H.; Maier, K. Self-Diffusion in ferromagnetic α-iron. Scr. Metall. 1977, 11, 795–802. [Google Scholar] [CrossRef]



| Cr | Ni | Co | Mo | Ti | C | O | N | Fe |
|---|---|---|---|---|---|---|---|---|
| 12.53 | 7.45 | 7.16 | 3.14 | 1.75 | 0.0024 | 0.0028 | 0.0026 | Bal. |
| Items | CT | AT 10 min | AT 0.5 h | AT 4 h | AT 16 h | AT 40 h | AT 100 h |
|---|---|---|---|---|---|---|---|
| Volume of analyzed body (nm3) | 638,160 | 745,996 | 554,895 | 757,120 | 772,475 | 874,380 | 799,779 |
| Ni + Ti cluster density (10−5 nm−3) | 0 | 59.92 | 116.78 | 114.51 | 79.74 | 56.04 | 15.63 |
| Mo cluster density (10−5 nm−3) | 0 | 0 | 0 | 0.26 | 51.52 | 3.09 | 9.38 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Wang, W.; Babar Shahzad, M.; Yan, W.; Shan, Y.; Jiang, Z.; Yang, K. A New Maraging Stainless Steel with Excellent Strength–Toughness–Corrosion Synergy. Materials 2017, 10, 1293. https://doi.org/10.3390/ma10111293
Tian J, Wang W, Babar Shahzad M, Yan W, Shan Y, Jiang Z, Yang K. A New Maraging Stainless Steel with Excellent Strength–Toughness–Corrosion Synergy. Materials. 2017; 10(11):1293. https://doi.org/10.3390/ma10111293
Chicago/Turabian StyleTian, Jialong, Wei Wang, M. Babar Shahzad, Wei Yan, Yiyin Shan, Zhouhua Jiang, and Ke Yang. 2017. "A New Maraging Stainless Steel with Excellent Strength–Toughness–Corrosion Synergy" Materials 10, no. 11: 1293. https://doi.org/10.3390/ma10111293
APA StyleTian, J., Wang, W., Babar Shahzad, M., Yan, W., Shan, Y., Jiang, Z., & Yang, K. (2017). A New Maraging Stainless Steel with Excellent Strength–Toughness–Corrosion Synergy. Materials, 10(11), 1293. https://doi.org/10.3390/ma10111293






