A New Ion-Imprinted Chitosan-Based Membrane with an Azo-Derivative Ligand for the Efficient Removal of Pd(II)
Abstract
:1. Introduction
2. Results and Discussion
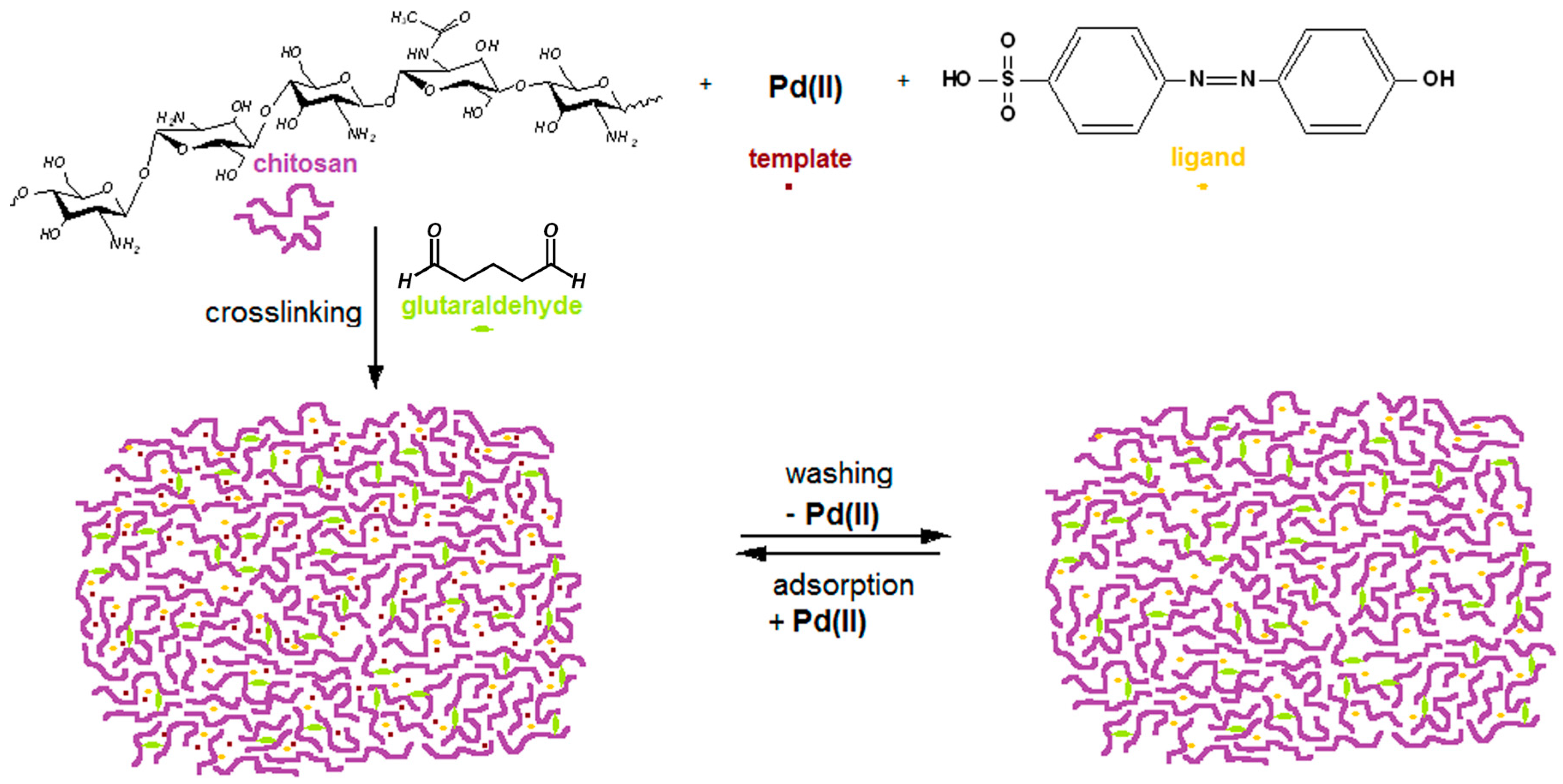
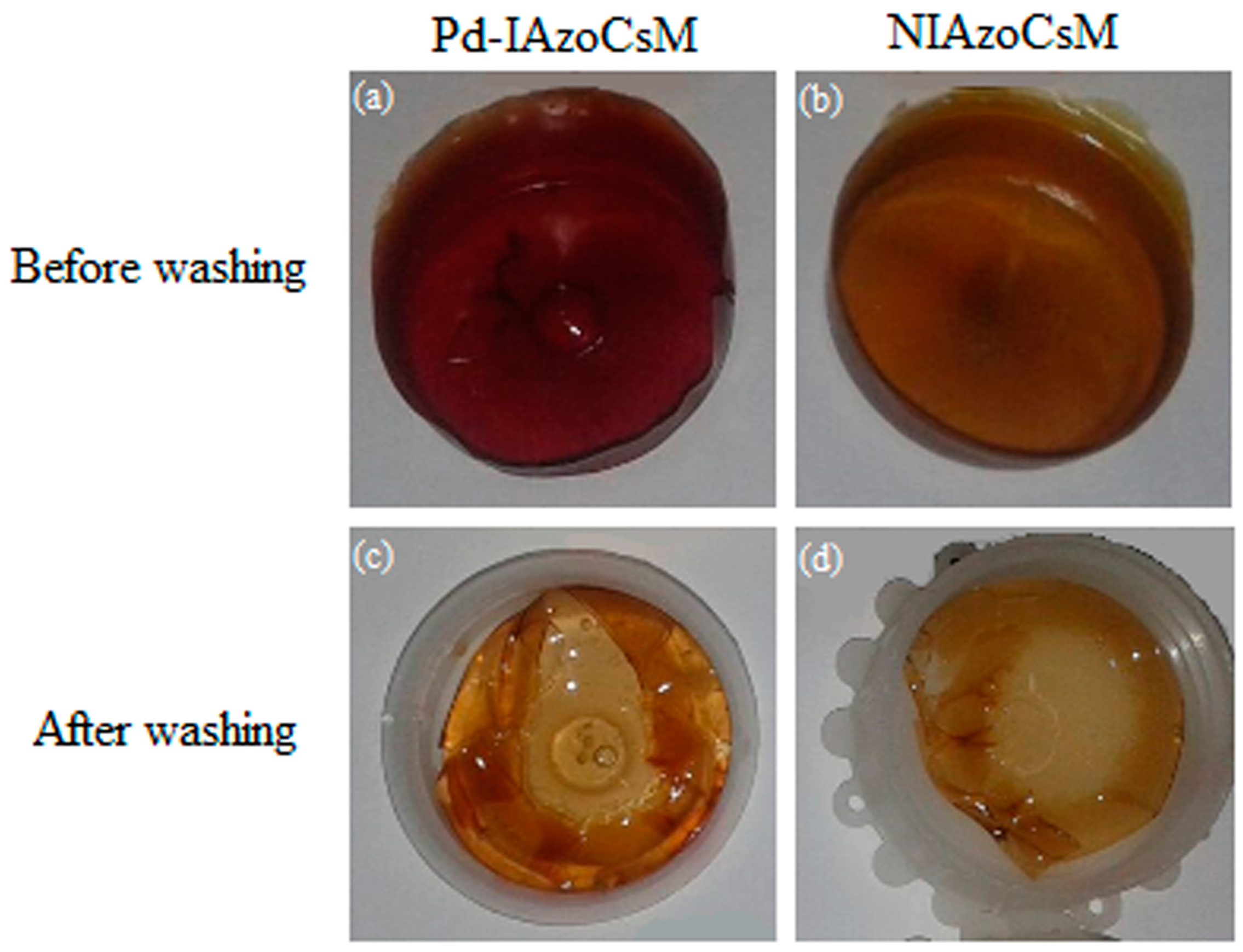
2.1. Modified Chitosan Membranes Synthesis
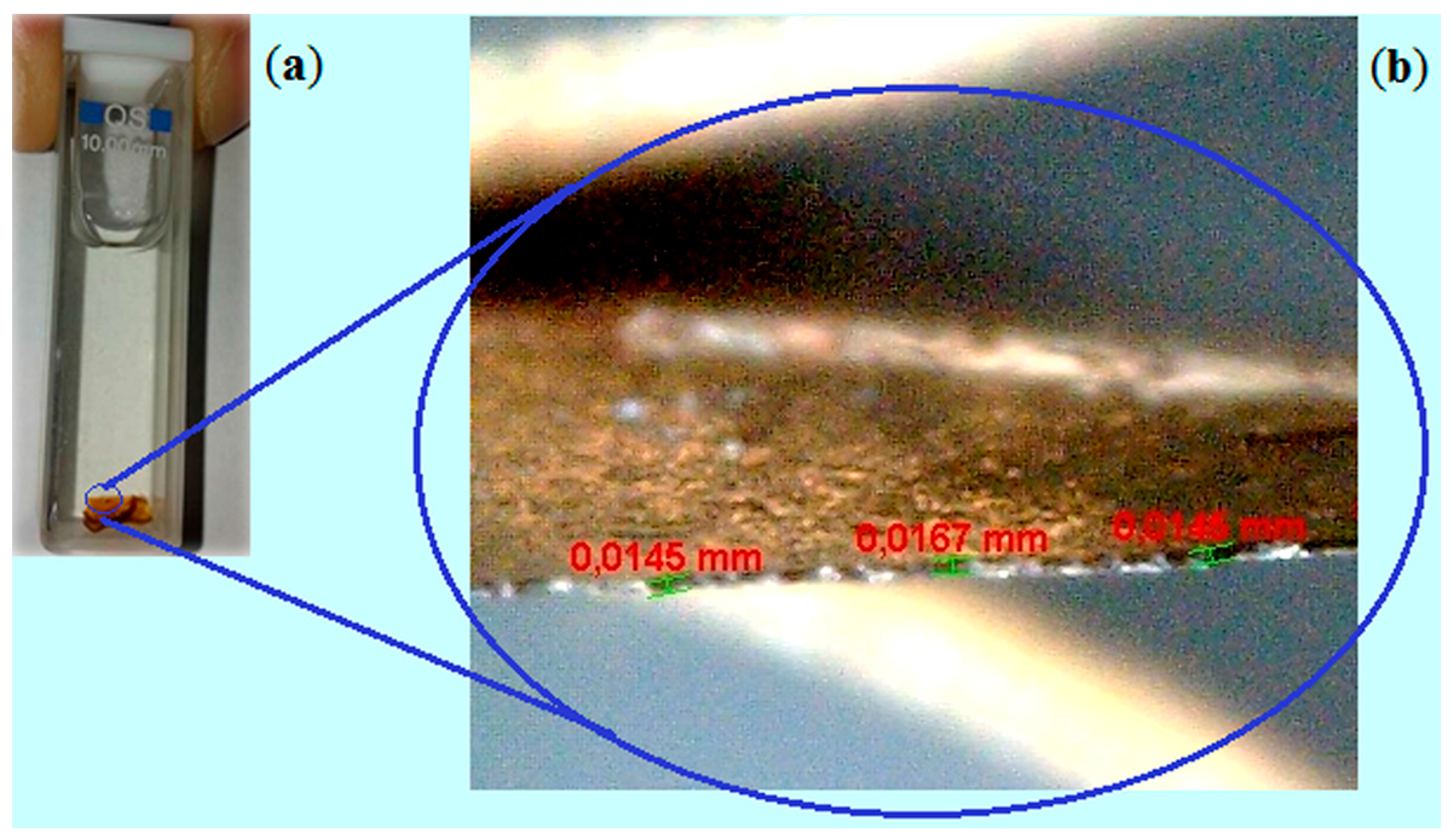
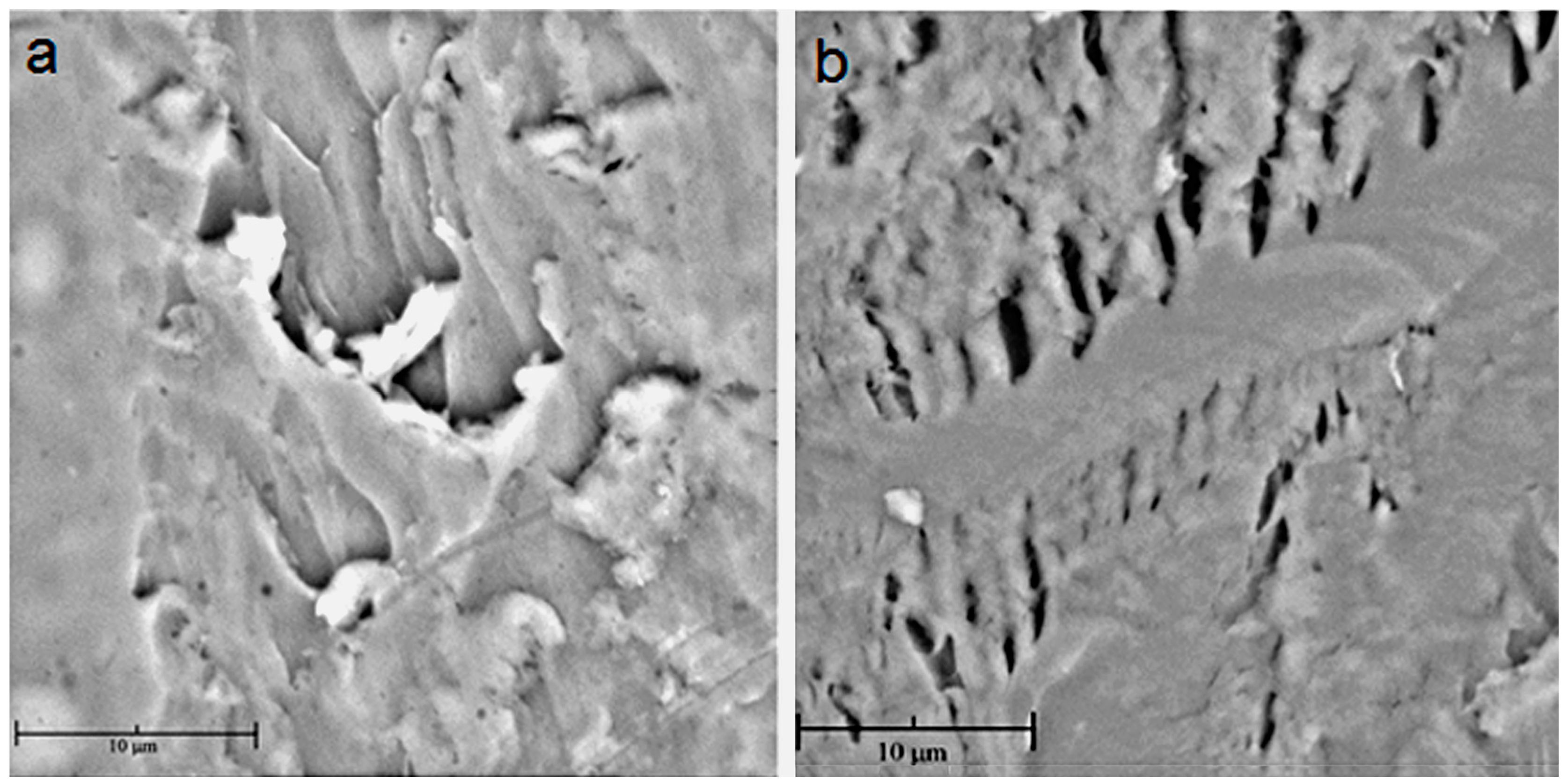
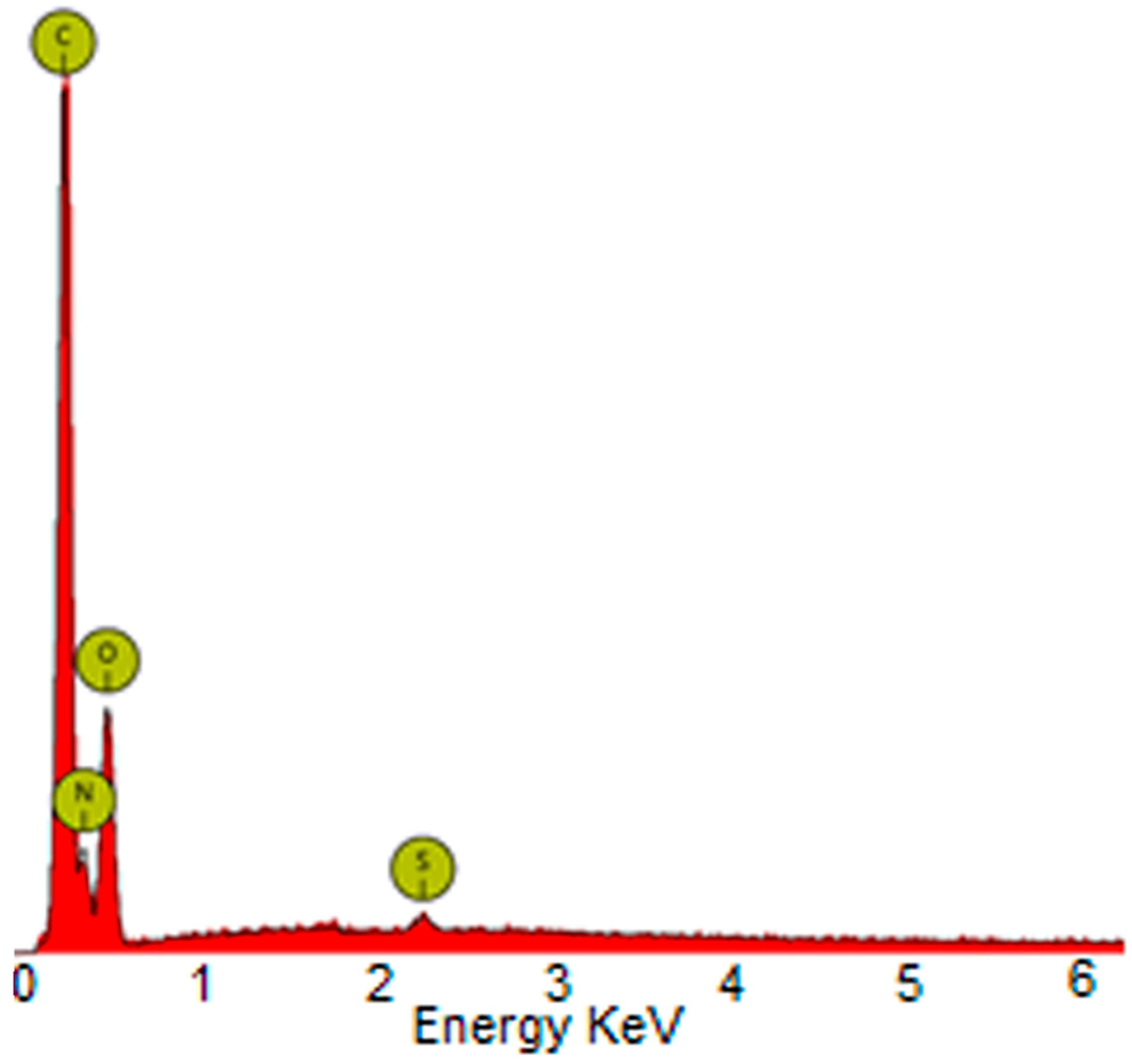
2.2. Membranes Characterization
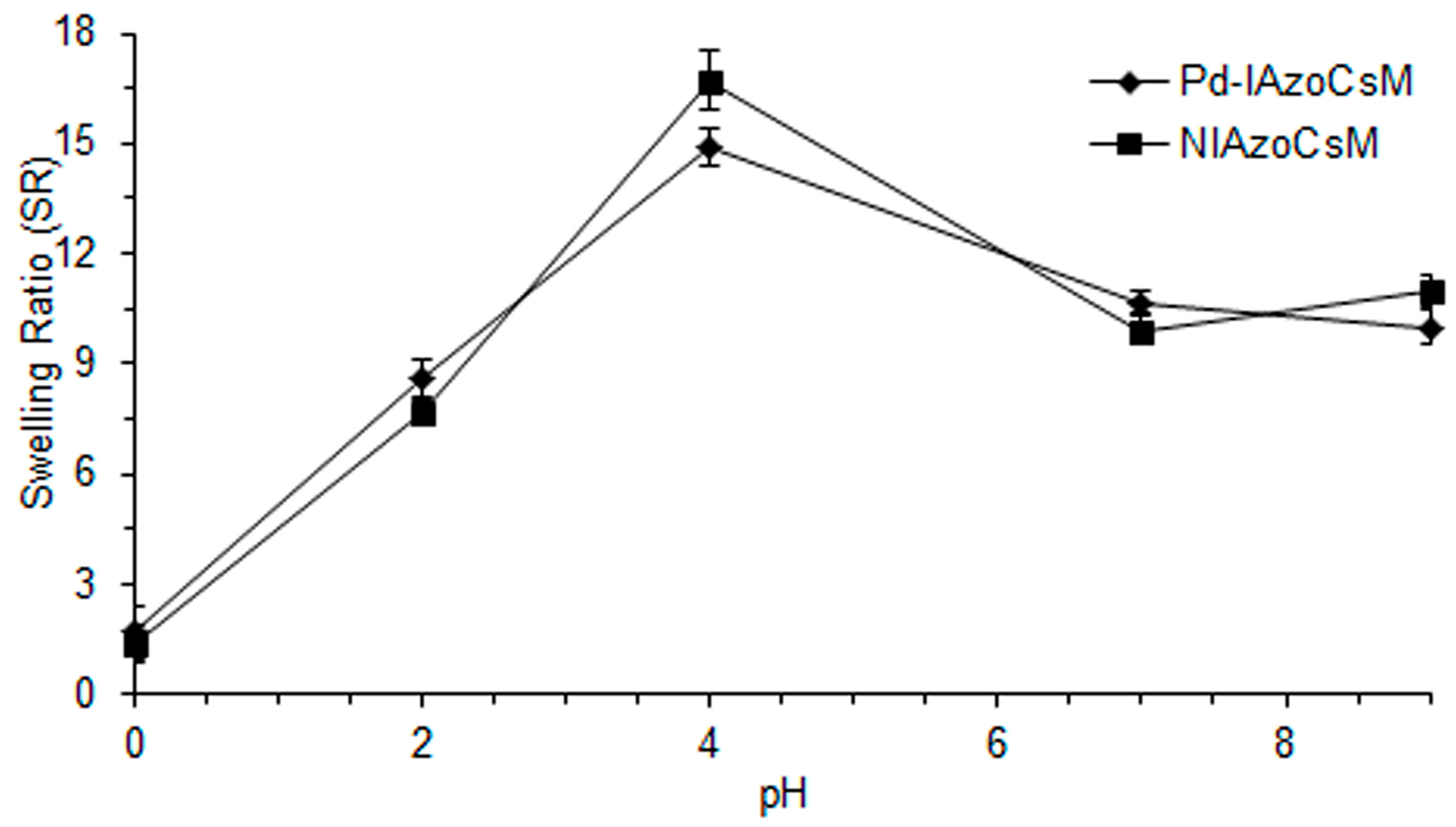
2.3. Swelling Studies
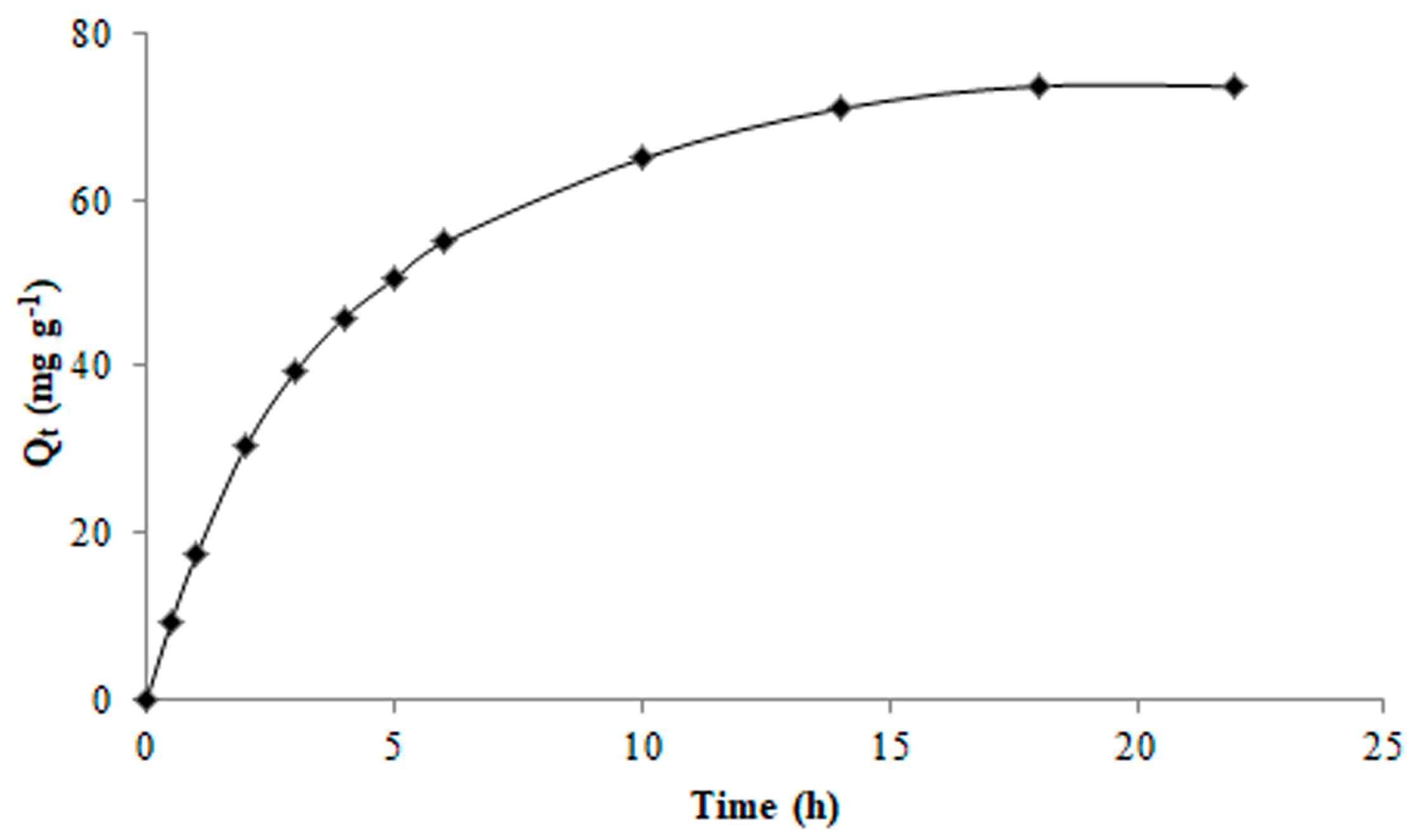
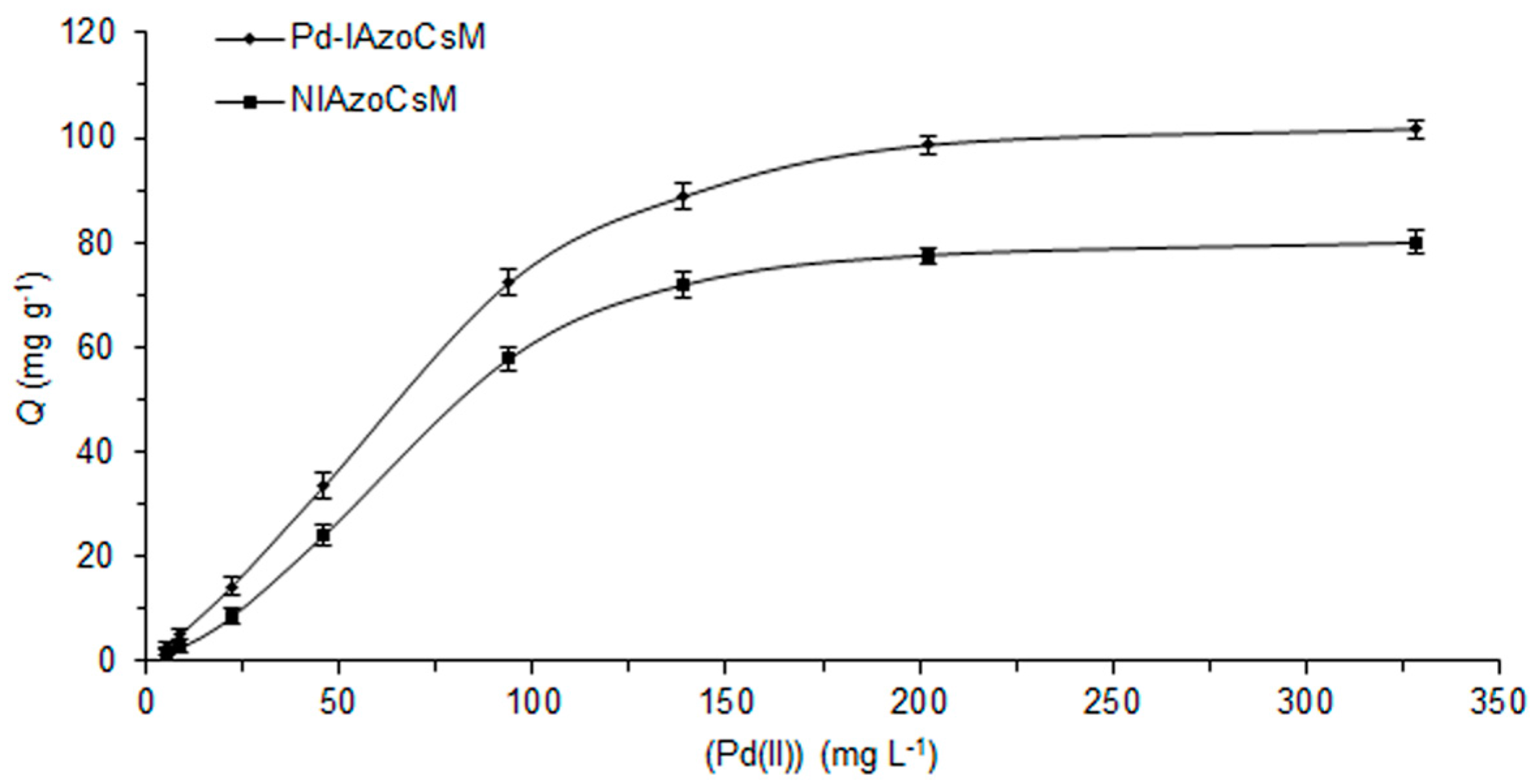
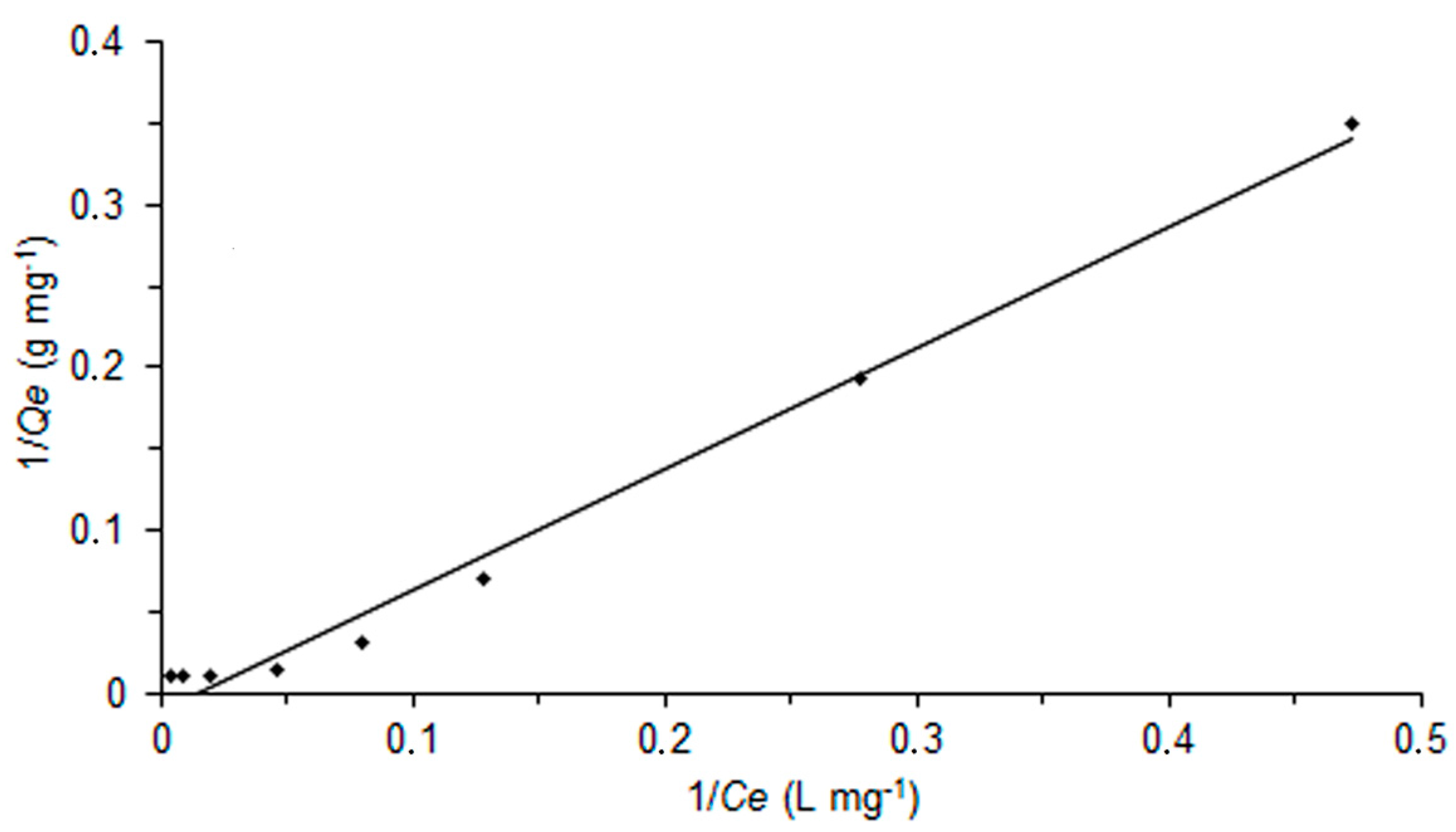
2.4. Adsorption Behaviour
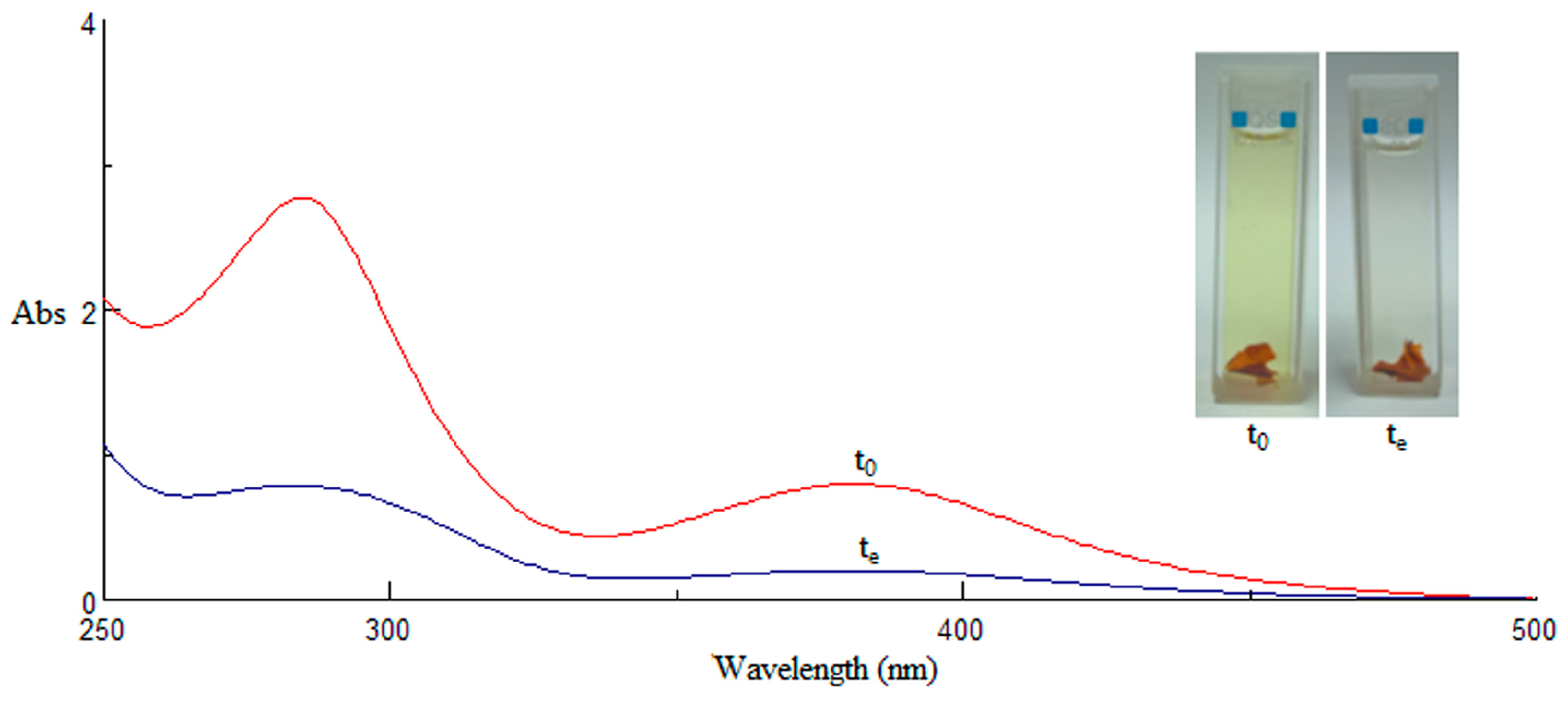
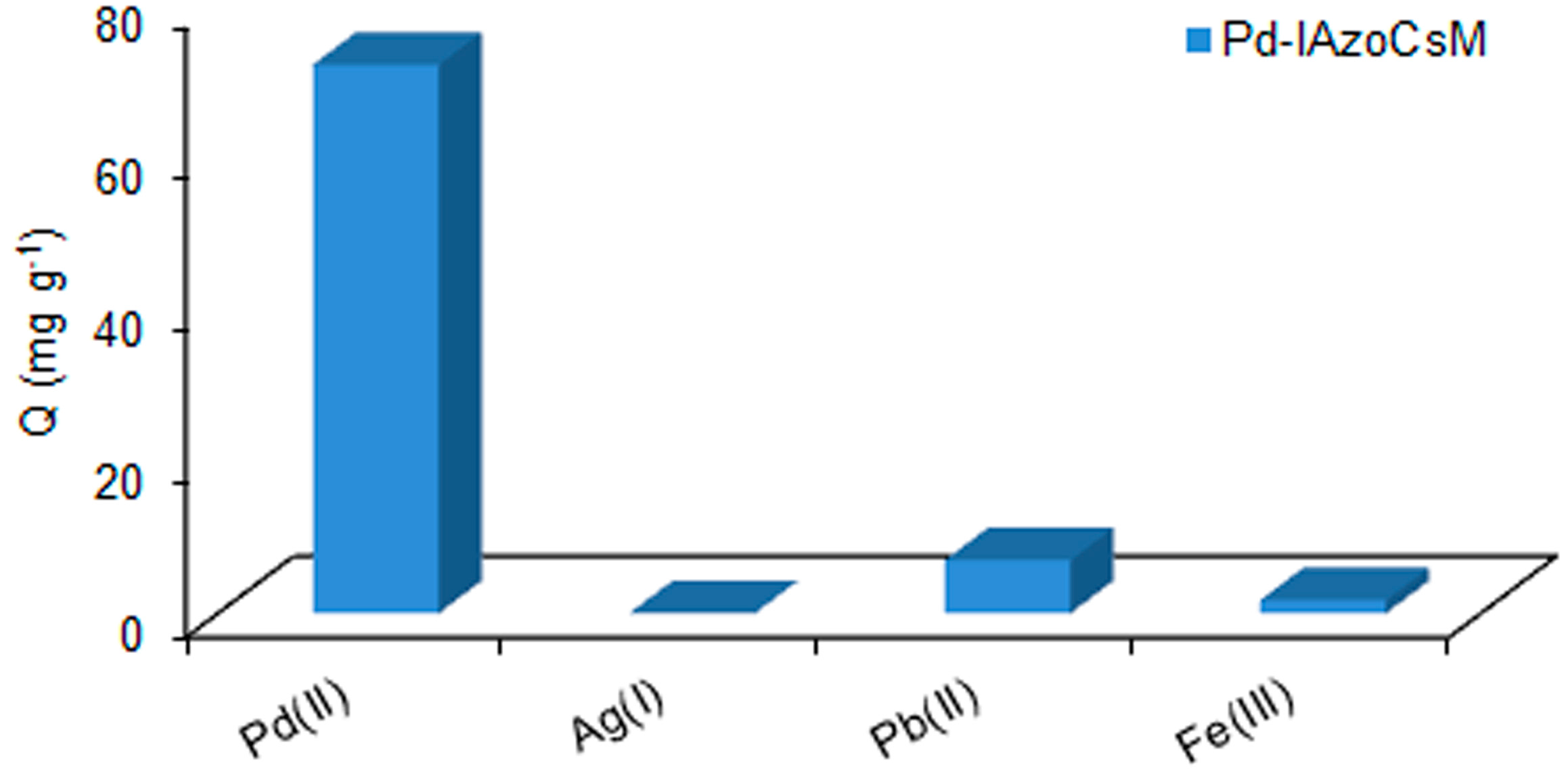
2.5. Selectivity Studies
3. Materials and Methods
3.1. Materials and Apparatus
3.2. Preparation of Pd(II)-Imprinted, Azobenzene-Modified Chitosan Membrane (Pd-IAzoCsM)
3.3. Swelling Studies
3.4. Adsorption Kinetic
3.5. Batch Adsorption Experiments
3.6. Selectivity Studies
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Beletskaya, I.P.; Cheprakov, A.V. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef] [PubMed]
- Han, F.S. Transition-metal-catalyzed Suzuki–Miyaura cross-coupling reactions: A remarkable advance from palladium to nickel catalysts. Chem. Soc. Rev. 2013, 42, 5270–5298. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, D.; Wang, W.Y.; Fan, Y.Q.; Li, J.; Han, H.P. Highly selective determination of palladium(II) after preconcentration using Pd(II)-imprinted functionalized silica gel sorbent prepared by a surface imprinting technique. Microchim. Acta 2007, 157, 7–11. [Google Scholar] [CrossRef]
- Liang, P.; Zhao, E.; Li, F. Dispersive liquid–liquid microextraction preconcentration of palladium in water samples and determination by graphite furnace atomic absorption spectrometry. Talanta 2009, 77, 1854–1857. [Google Scholar] [CrossRef] [PubMed]
- Bruzzoniti, M.C.; Mucchino, C.; Tarasco, E.; Sarzanini, C. On-line preconcentration, ion chromatographic separation and spectrophotometric determination of palladium at trace level. J. Chromatogr. A 2003, 1007, 93–100. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Lee, S.Y.; Won, S.W.; Yun, Y.S. Surface modified bacterial biosorbent with poly(allylaminehydrochloride): Development using response surface methodology and use for recovery of hexachloroplatinate(IV) from aqueous solution. Water Res. 2010, 44, 5919–5928. [Google Scholar] [CrossRef] [PubMed]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Hubicki, Z.; Wołowicz, A. A comparative study of chelating and cationic ion exchange resins for the removal of palladium(II) complexes from acidic chloride media. J. Hazard. Mater. 2009, 164, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Weerawat, P.; Nattaphol, V.; Ura, P. Selective recovery of palladium from used aqua regia by hollow fiber supported with liquid membrane. Korean J. Chem. Eng. 2003, 20, 1092–1096. [Google Scholar] [CrossRef]
- Alzahrani, S.; Mohammad, A.W. Challenges and trends in membrane technology implementation for produced water treatment: A review. J. Water Process Eng. 2014, 4, 107–133. [Google Scholar] [CrossRef]
- Dodson, J.R.; Parker, H.L.; García, A.M.; Hicken, A.; Asemave, K.; Farmer, T.J.; He, H.; Clark, J.H.; Hunt, A.J. Bio-derived materials as green route for precious & critical metal recovery and re-use. Green Chem. 2015, 17, 1951–1965. [Google Scholar]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Liu, B.; Lv, X.; Meng, X.; Yu, G.; Wang, D. Removal of Pb(II) from aqueous solution using dithiocarbamate modified chitosan beds with Pb(II) as imprinted ions. Chem. Eng. J. 2013, 220, 412–419. [Google Scholar] [CrossRef]
- Shawky, H.A. Synthesis of ion-imprinting chitosan/PVA crosslinked membrane for selective removal of Ag(I). J. Appl. Polym. Sci. 2009, 114, 2608–2615. [Google Scholar] [CrossRef]
- Tasselli, F.; Mirmohseni, A.; Seyed Dorraji, M.S.; Figoli, A. Mechanical, swelling and adsorptive properties of dry–wet spun chitosan hollow fibers crosslinked with glutaraldehyde. React. Funct. Polym. 2013, 73, 218–223. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Han, T.; Zhong, L.; Ma, C.; Zhou, Y.; Han, X. Improvement of Ag(I) adsorption onto chitosan/triethanolamine composite sorbent by an ion-imprinted technology. Appl. Surf. Sci. 2012, 263, 696–703. [Google Scholar] [CrossRef]
- Park, S.I.; Kwak, I.S.; Won, S.W.; Yun, Y.S. Glutaraldehyde-crosslinked chitosan beads for sorptive separation of Au(III) and Pd(II): Opening a way to design reduction-coupled selectivity-tunable sorbents for separation of precious metals. J. Hazard. Mater. 2013, 248–249, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bao, C.; Li, F.; Kong, X.; Xu, J. Preparation and application of 4-amino-4′-nitro azobenzene modified chitosan as a selective adsorbent for the determination of Au(III) and Pd(II). Microchim. Acta 2010, 168, 99–105. [Google Scholar] [CrossRef]
- Zhou, X.; Nie, J.; Du, B. 4-(2-Pyridylazo)-resorcinol functionalized thermosensitive ionic microgels for optical detection of heavy metal ions at nanomolar level. ACS Appl. Mater. Interfaces 2015, 7, 21966–21974. [Google Scholar] [CrossRef] [PubMed]
- Di Bello, M.P.; Mergola, L.; Scorrano, S.; Del Sole, R. Towards a new strategy of a chitosan-based molecularly imprinted membrane for removal of 4-nitrophenol in real water samples. Polym. Int. 2017, 66, 1055–1063. [Google Scholar] [CrossRef]
- Vasapollo, G.; Del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [PubMed]
- Del Sole, R.; Scardino, A.; Lazzoi, M.R.; Vasapollo, G. Molecularly imprinted polymer for solid phase extraction of nicotinamide in pork liver samples. J. Appl. Polym. Sci. 2011, 120, 1634–1641. [Google Scholar] [CrossRef]
- Del Sole, R.; De Luca, A.; Catalano, M.; Mele, G.; Vasapollo, G. Noncovalent imprinted microspheres: Preparation, evaluation and selectivity of DBU template. J. Appl. Polym. Sci. 2007, 105, 2190–2197. [Google Scholar] [CrossRef]
- Scorrano, S.; Mergola, L.; Del Sole, R.; Vasapollo, G. Synthesis of molecularly imprinted polymers for amino acid derivates by using different functional monomers. Int. J. Mol. Sci. 2011, 12, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Mergola, L.; Scorrano, S.; Del Sole, R.; Lazzoi, M.R.; Vasapollo, G. Developments in the synthesis of a water compatible molecularly imprinted polymer as artificial receptor for detection of 3-nitro-l-tyrosine in neurological diseases. Biosens. Bioelectron. 2013, 40, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, S.; Mergola, L.; Di Bello, M.P.; Lazzoi, M.R.; Vasapollo, G.; Del Sole, R. Molecularly imprinted composite membranes for selective detection of 2-deoxyadenosine in urine samples. Int. J. Mol. Sci. 2015, 16, 13746–13759. [Google Scholar] [CrossRef] [PubMed]
- Branger, C.; Meouche, W.; Margaillan, A. A Recent advances on ion-imprinted polymers. React. Funct. Polym. 2013, 73, 859–875. [Google Scholar] [CrossRef]
- Mergola, L.; Scorrano, S.; Bloise, E.; Di Bello, M.P.; Catalano, M.; Vasapollo, G.; Del Sole, R. Novel polymeric sorbents based on imprinted Hg(II)-diphenylcarbazone complexes for mercury removal from drinking water. Polym. J. 2016, 48, 73–79. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Turner, A.P.F. New materials based on imprinted polymers and their applications in optical sensors. In Optical Biosensors: Present and Future; Ligler, F.S., Rowe Taitt, C.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 397–425. ISBN 9780080524085. [Google Scholar]
- Jiang, Y.; Kim, D. Synthesis and selective adsorption behavior of Pd(II)-imprinted porous polymer particles. Chem. Eng. J. 2013, 232, 503–509. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.A.; El-Reash, Y.G.A. Ion-imprinted modified chitosan resin for selective removal of Pd(II) ions. J. Colloid. Interface Sci. 2016, 469, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Chooaksorn, W.; Nitisoravut, R. Heavy Metal Removal from Aqueous Solutions by Chitosan Coated Ceramic Membrane. In Proceedings of the 4th International Conference on Informatics, Environment, Energy and Applications, Pattaya, Thailand, 28–29 March 2015. [Google Scholar]
- Xu, L.; Huang, Y.A.; Zhu, O.J.; Ye, C. Chitosan in molecularly-imprinted polymers: Current and future prospects. Int. J. Mol. Sci. 2015, 16, 18328–18347. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Xu, X.; Zheng, X.; Zhang, F.; Liu, E.; Li, C. An ion imprinted macroporous chitosan membrane for efficiently selective adsorption of dysprosium. Sep. Purif. Technol. 2017, 189, 288–295. [Google Scholar] [CrossRef]
- Zhu, Y.; Bai, Z.; Luo, W.; Wang, B.; Zhai, L. A facile ion imprinted synthesis of selective biosorbent for Cu2+ via microfluidic technology. J. Chem. Technol. Biotechnol. 2017, 92, 2009–2022. [Google Scholar] [CrossRef]
- Zheng, X.F.; Lian, Q.; Wu, H.; Liu, H.; Song, S. Molecularly imprinted polymer for L-tyrosine recognition and controlled release. Russ. J. Appl. Chem. 2015, 88, 160–168. [Google Scholar] [CrossRef]
- Bhattarai, N.; Ramay, H.R.; Gunn, J.; Matsen, F.A.; Zhang, M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J. Control. Release 2005, 103, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Lazaridis, N.K.; Kostoglou, M. Modelling the effect of pre-swelling on adsorption dynamics of dyes by chitosan derivatives. Chem. Eng. Sci. 2012, 81, 220–230. [Google Scholar] [CrossRef]
- Sharififard, H.; Ashtiani, F.Z.; Soleimani, M. Adsorption of palladium and platinum from aqueous solutions by chitosan and activated carbon coated with chitosan. Asia-Pac. J. Chem. Eng. 2013, 8, 384–395. [Google Scholar] [CrossRef]
- Ghorbani-Kalhor, E.; Behbahani, M.; Abolhasani, J. Application of Ion-Imprinted Polymer Nanoparticles for Selective Trace Determination of Palladium Ions in Food and Environmental Samples with the Aid of Experimental Design Methodology. Food Anal. Methods 2015, 8, 1746–1757. [Google Scholar] [CrossRef]
- Yunus, I.S.; Tsai, S.L. Designed biomolecule—Cellulose complexes for palladium recovery and detoxification. RSC Adv. 2015, 5, 20276–20282. [Google Scholar] [CrossRef]
- Wang, L.; Xing, R.; Liu, S.; Yu, H.; Qin, Y.; Li, K.; Feng, J.; Li, R.; Li, P. Recovery of silver (I) using a thiourea-modified chitosan resin. J. Hazard. Mater. 2010, 180, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Igberase, E.; Osifo, P.; Ofomaj, A. The adsorption of Pb, Zn, Cu, Ni, and Cd by modified ligand in a single component aqueous solution: Equilibrium, kinetic, thermodynamic, and desorption studies. Int. J. Anal. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Hua, T.; Chen, X. Selective adsorption of lead on grafted and crosslinked chitosan nanoparticles prepared by using Pb2+ as template. J. Hazard. Mater. 2016, 308, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ngah, W.S.W.; Ghani, S.A.; Kamari, A. Adsorption behaviour of Fe(II) and Fe(III) ions in aqueous solution on chitosan and cross-linked chitosan beads. Bioresour. Technol. 2005, 96, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Ren, F.L.; Tao, C.Y. Adsorption behavior of Fe(II) and Fe(III) ions on thiourea cross-linked chitosan with Fe(III) as template. Molecules 2012, 17, 4388–4399. [Google Scholar] [CrossRef] [PubMed]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bello, M.P.; Lazzoi, M.R.; Mele, G.; Scorrano, S.; Mergola, L.; Del Sole, R. A New Ion-Imprinted Chitosan-Based Membrane with an Azo-Derivative Ligand for the Efficient Removal of Pd(II). Materials 2017, 10, 1133. https://doi.org/10.3390/ma10101133
Di Bello MP, Lazzoi MR, Mele G, Scorrano S, Mergola L, Del Sole R. A New Ion-Imprinted Chitosan-Based Membrane with an Azo-Derivative Ligand for the Efficient Removal of Pd(II). Materials. 2017; 10(10):1133. https://doi.org/10.3390/ma10101133
Chicago/Turabian StyleDi Bello, Maria Pia, Maria Rosaria Lazzoi, Giuseppe Mele, Sonia Scorrano, Lucia Mergola, and Roberta Del Sole. 2017. "A New Ion-Imprinted Chitosan-Based Membrane with an Azo-Derivative Ligand for the Efficient Removal of Pd(II)" Materials 10, no. 10: 1133. https://doi.org/10.3390/ma10101133





