Effect on Particulate and Gas Emissions by Combusting Biodiesel Blend Fuels Made from Different Plant Oil Feedstocks in a Liquid Fuel Burner
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials and Chemicals
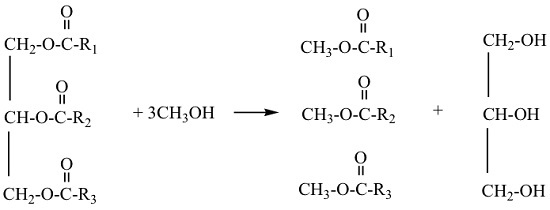
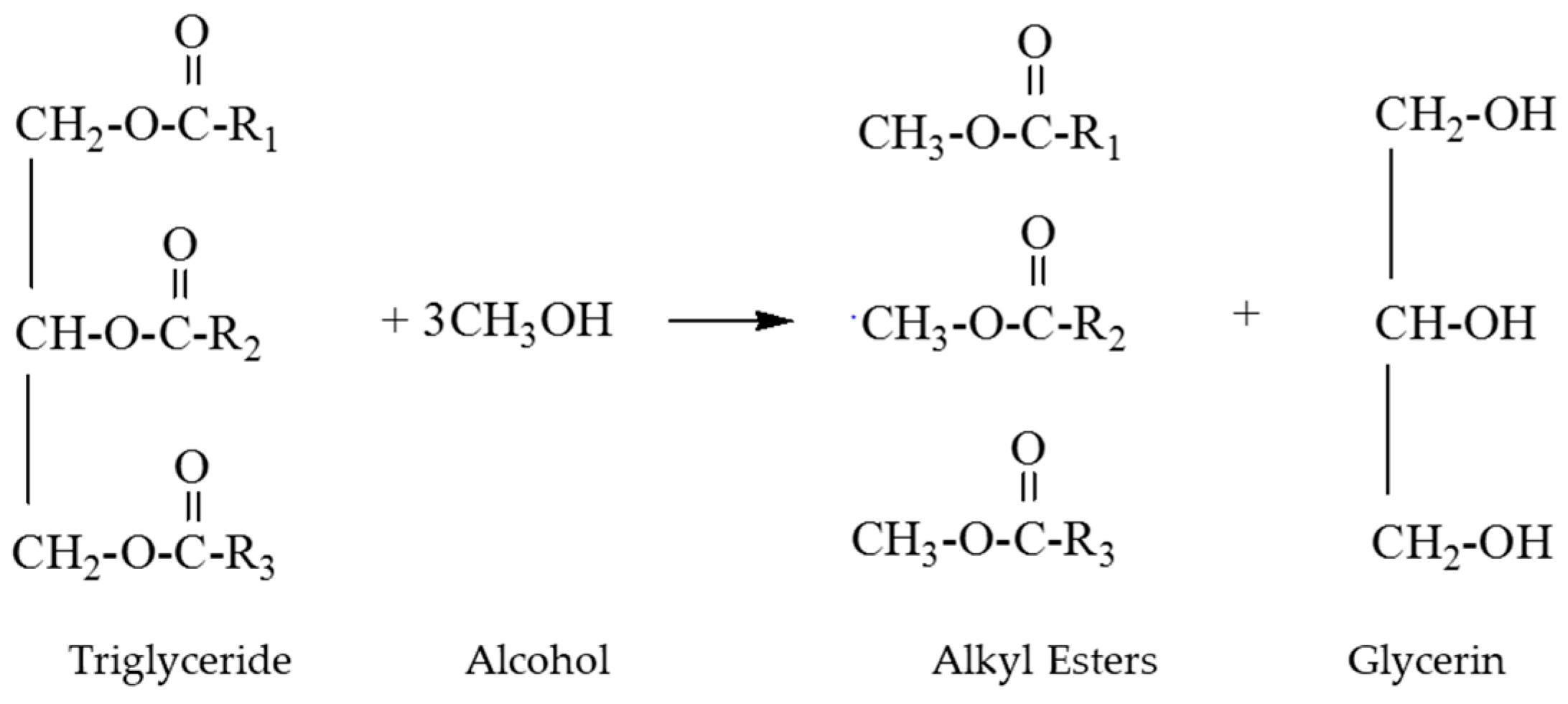
2.2. Biodiesel Production Procedures
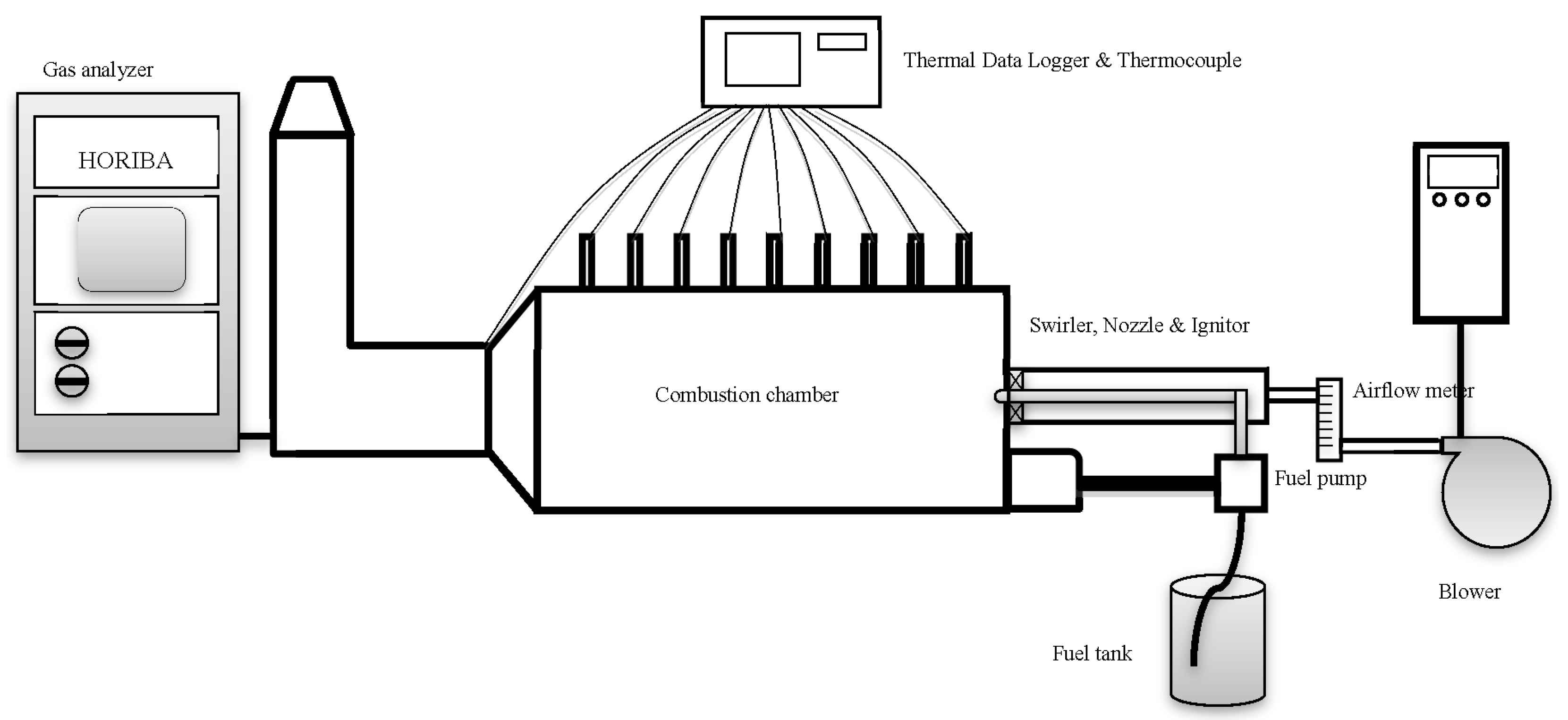
3. Experimental Work
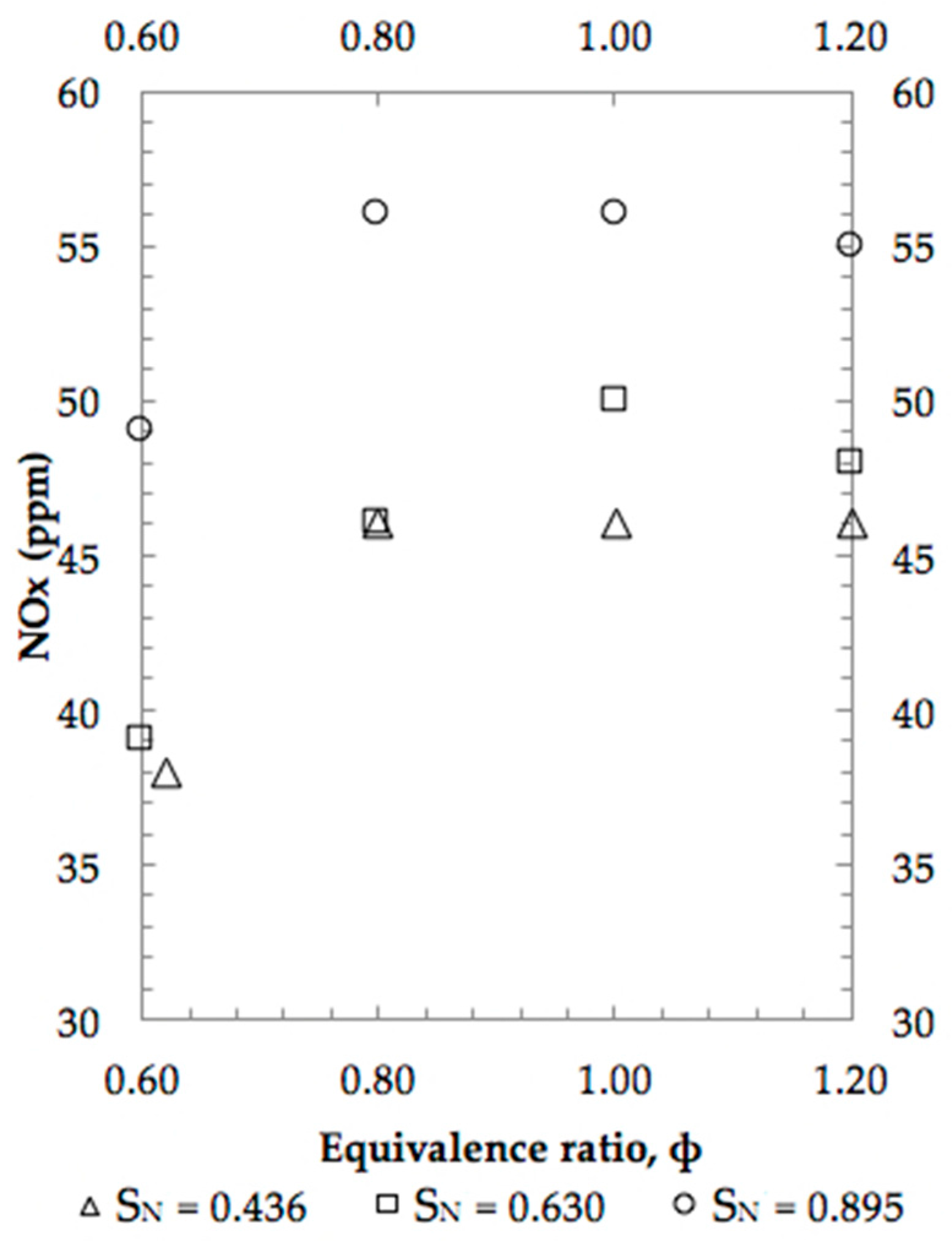
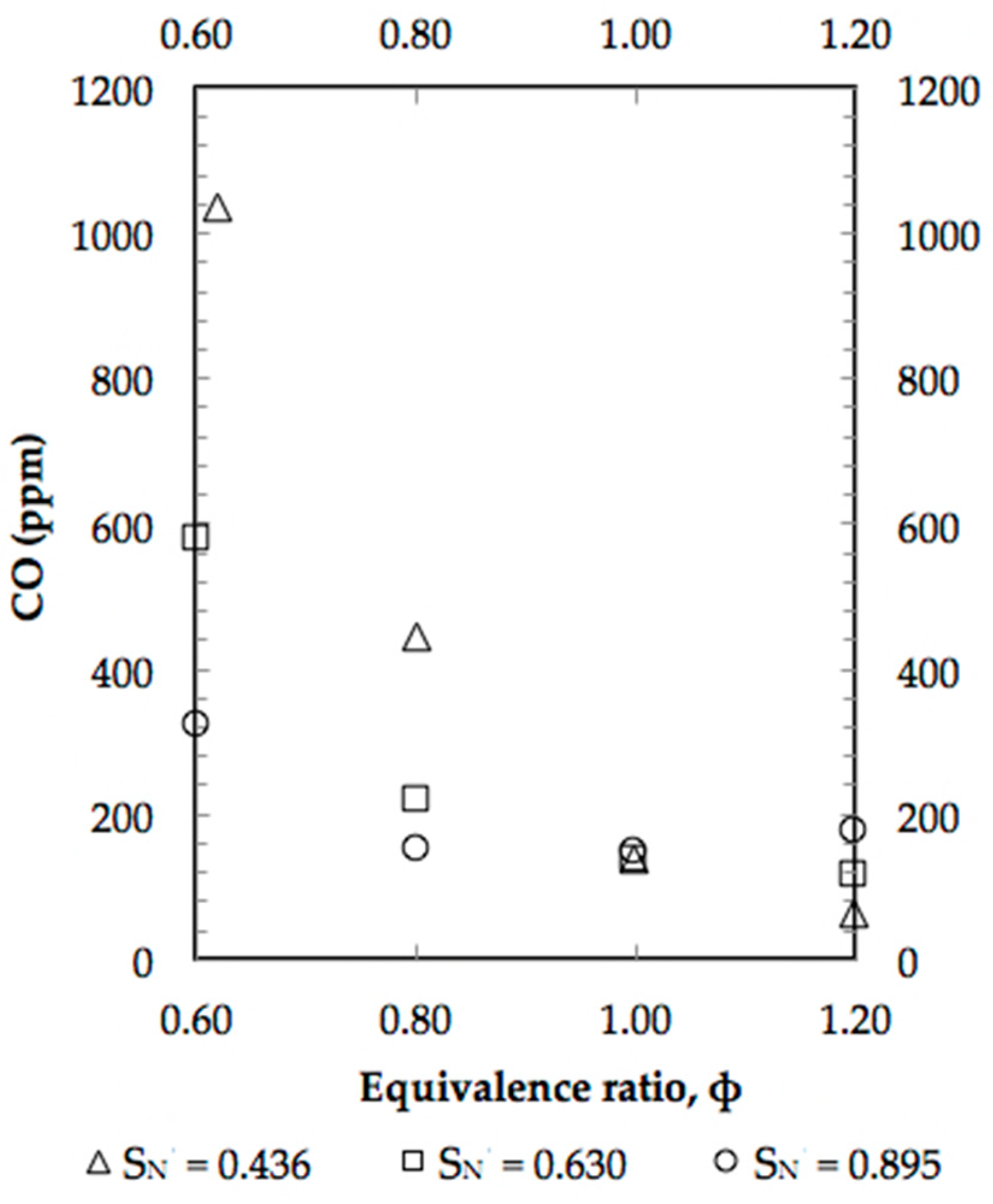
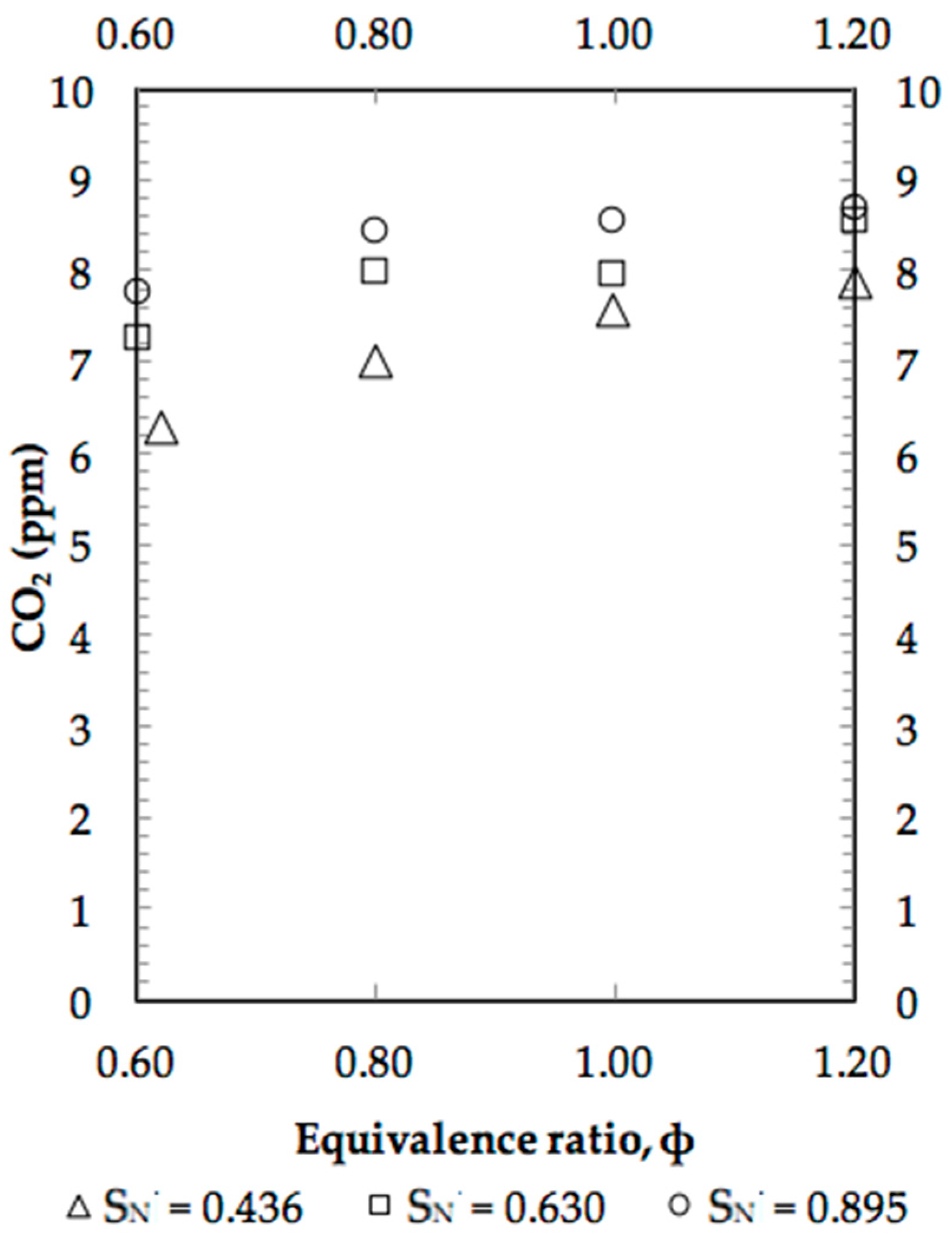
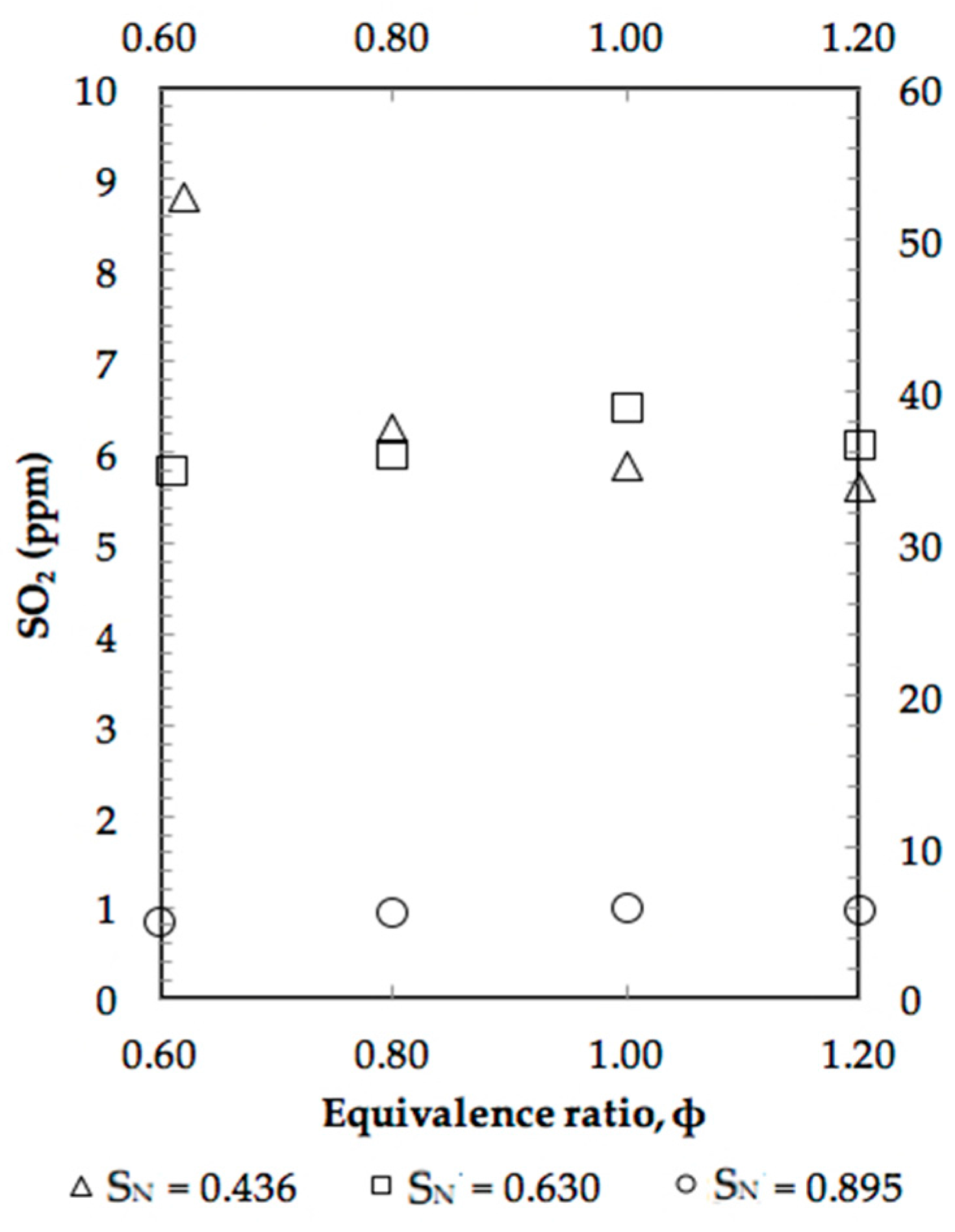
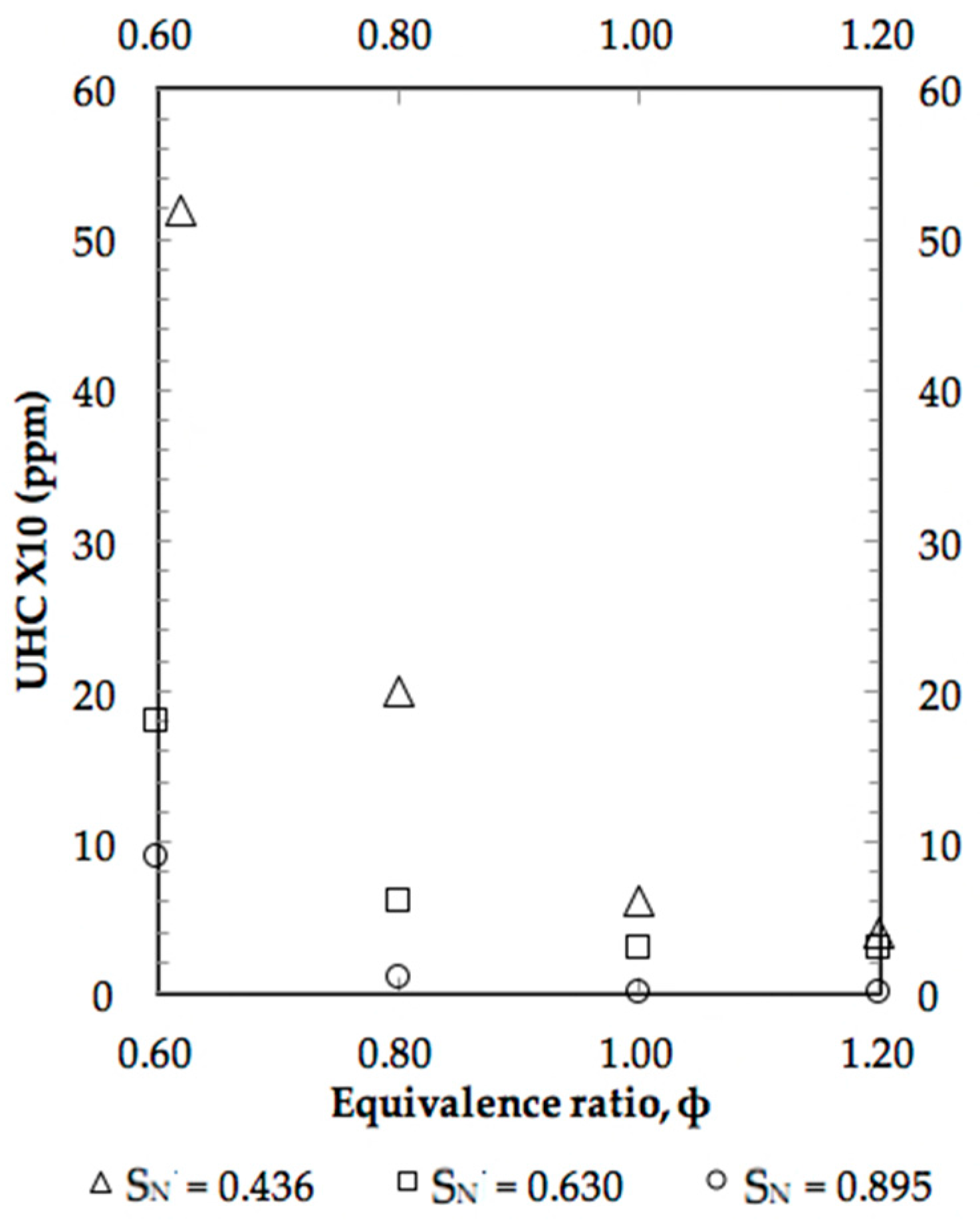
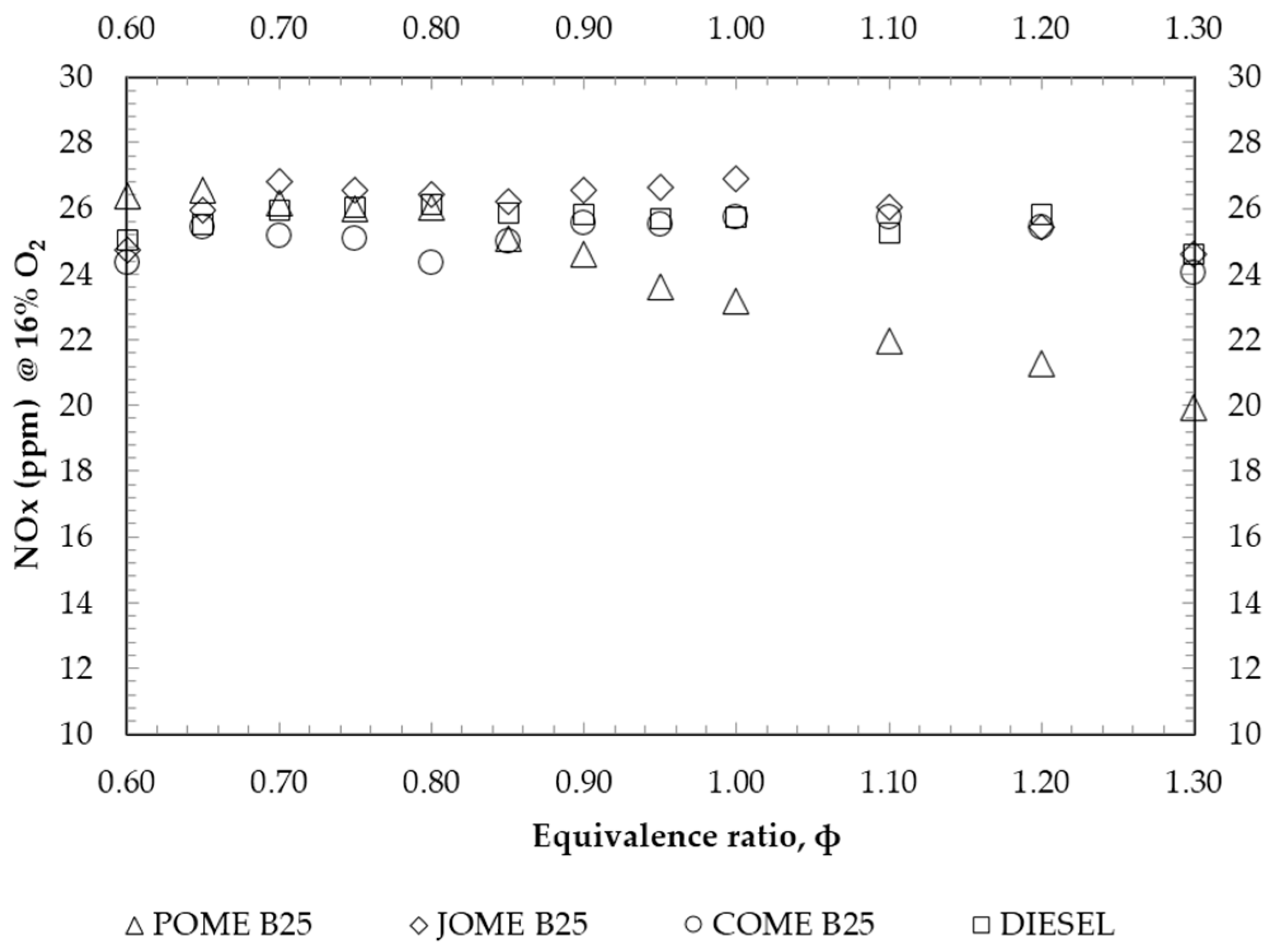
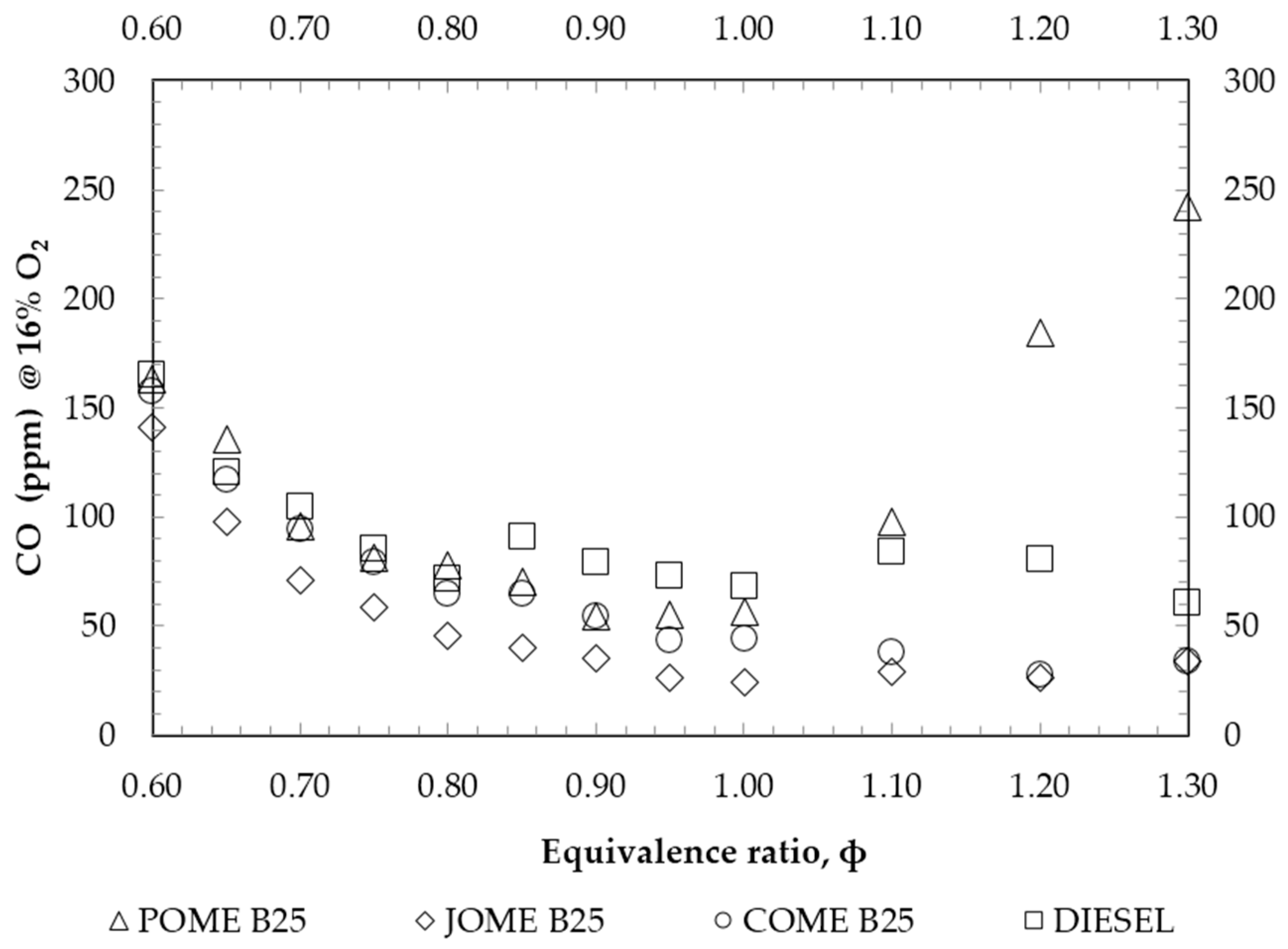
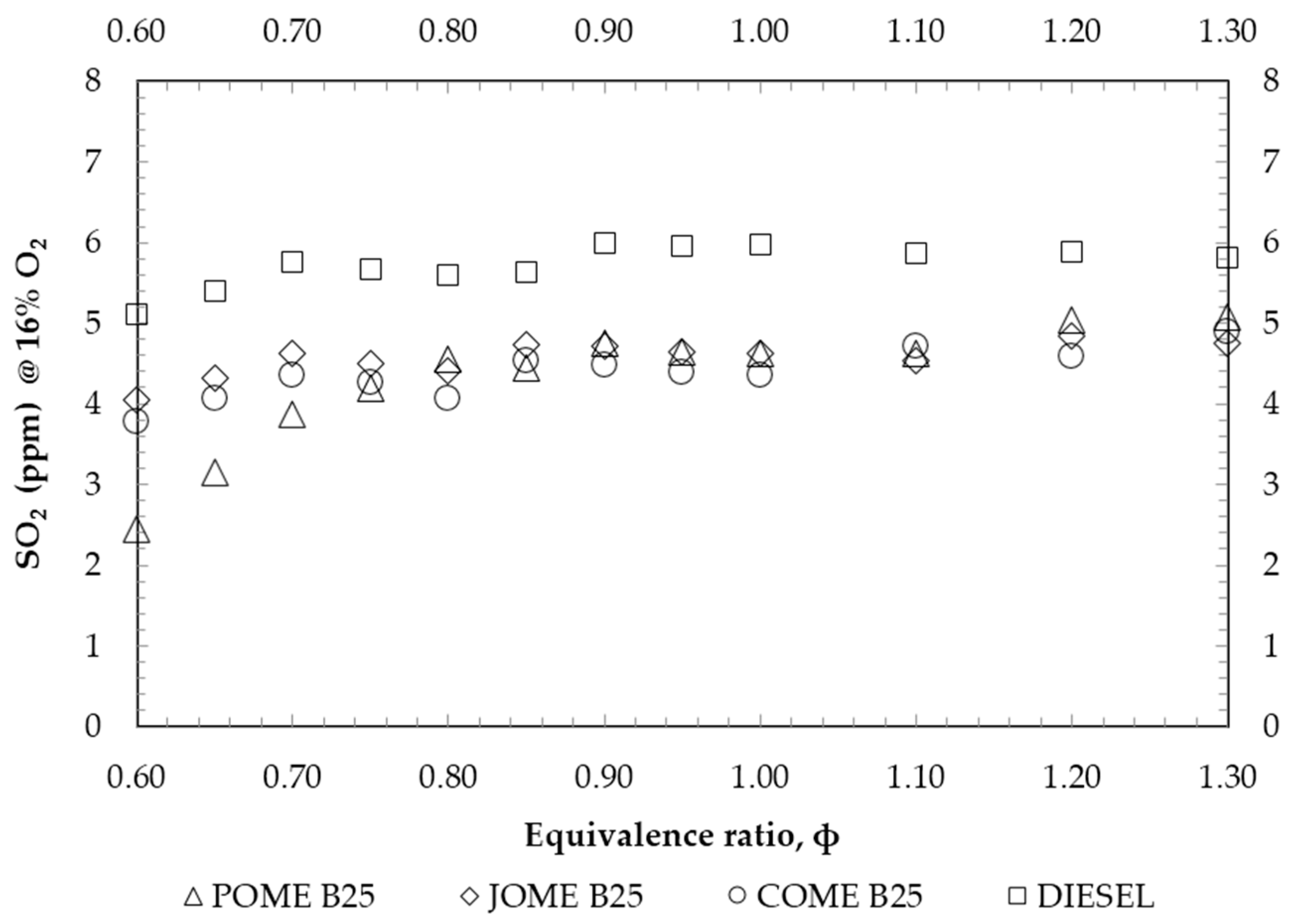
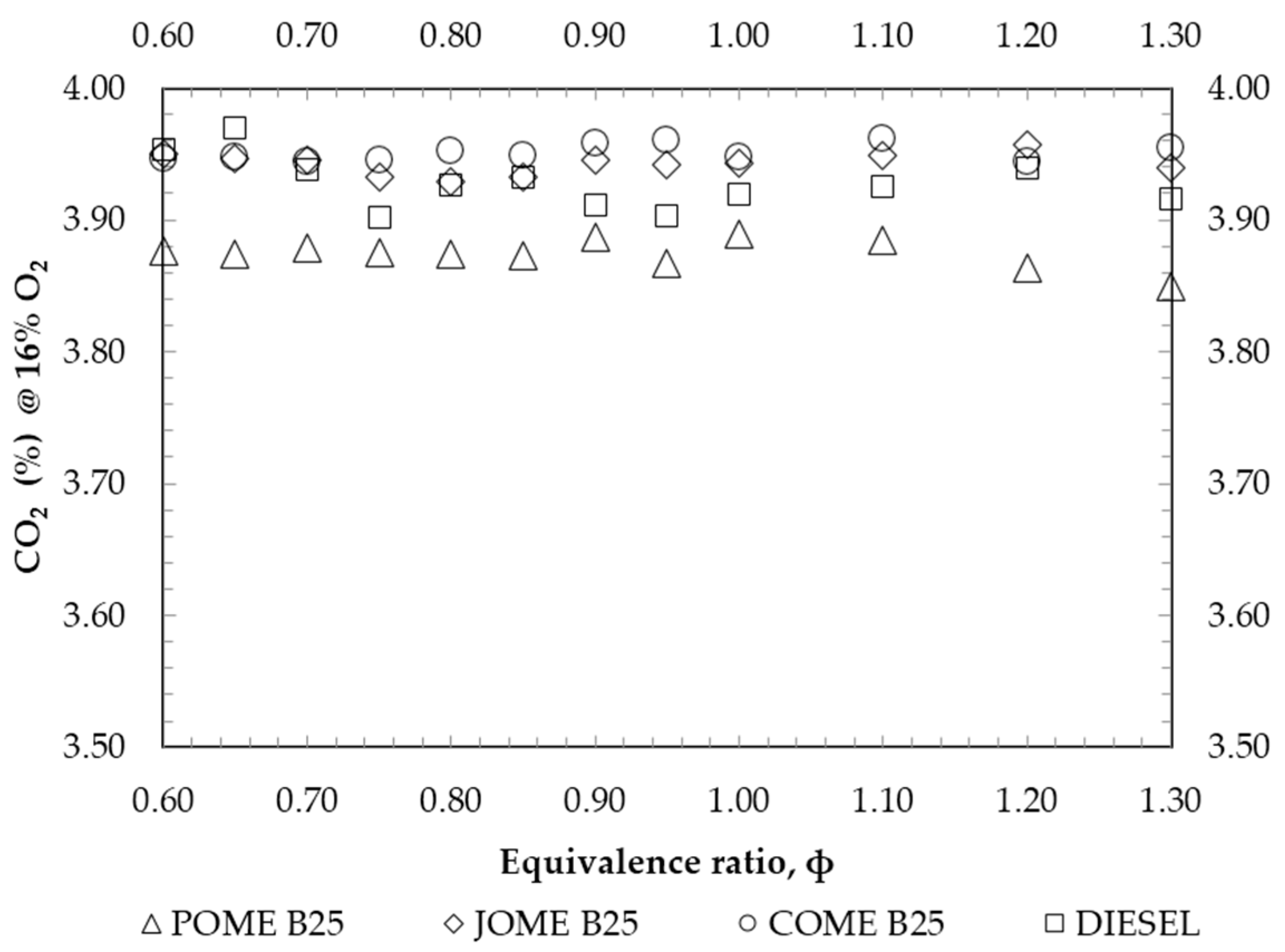
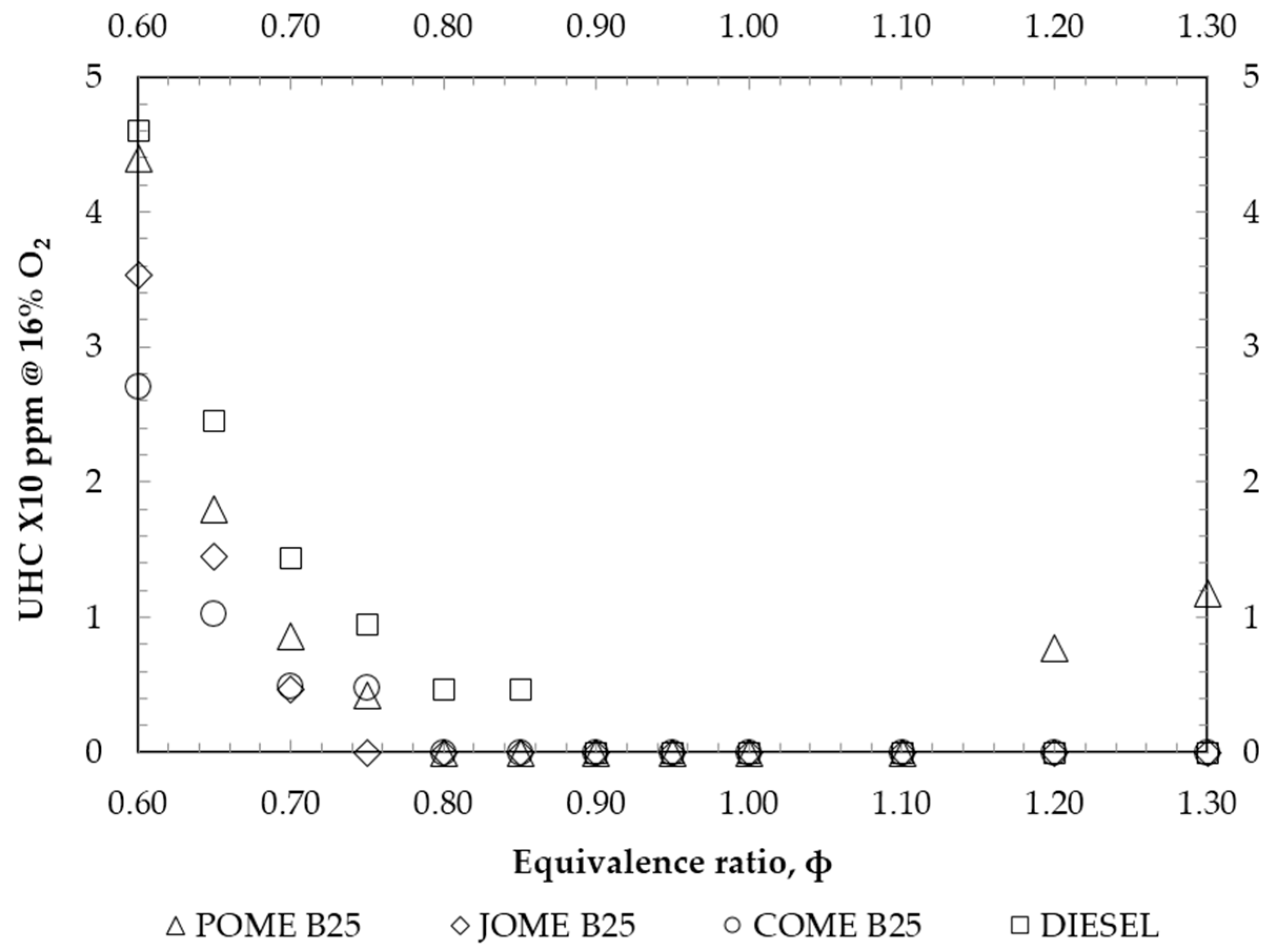
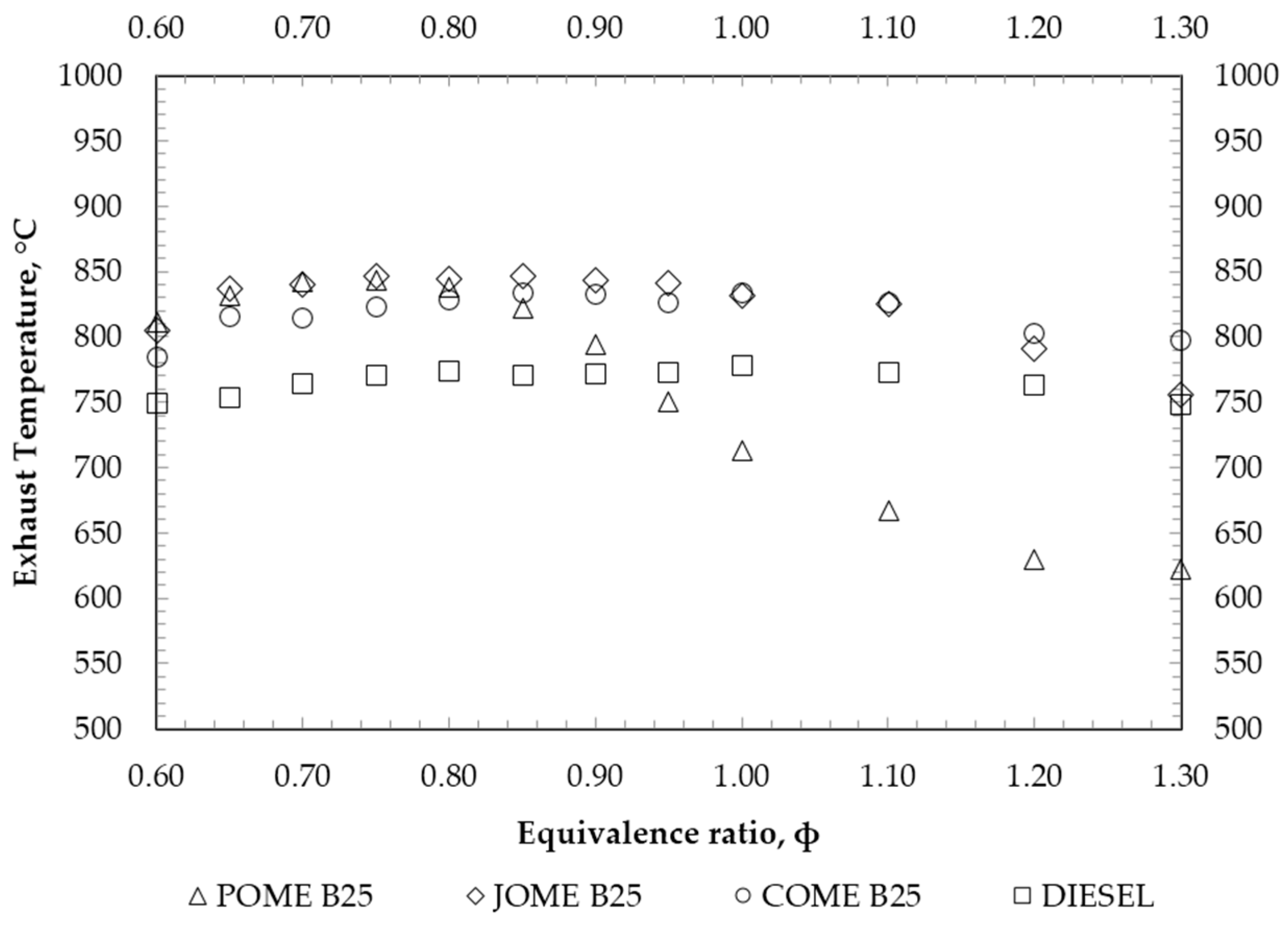
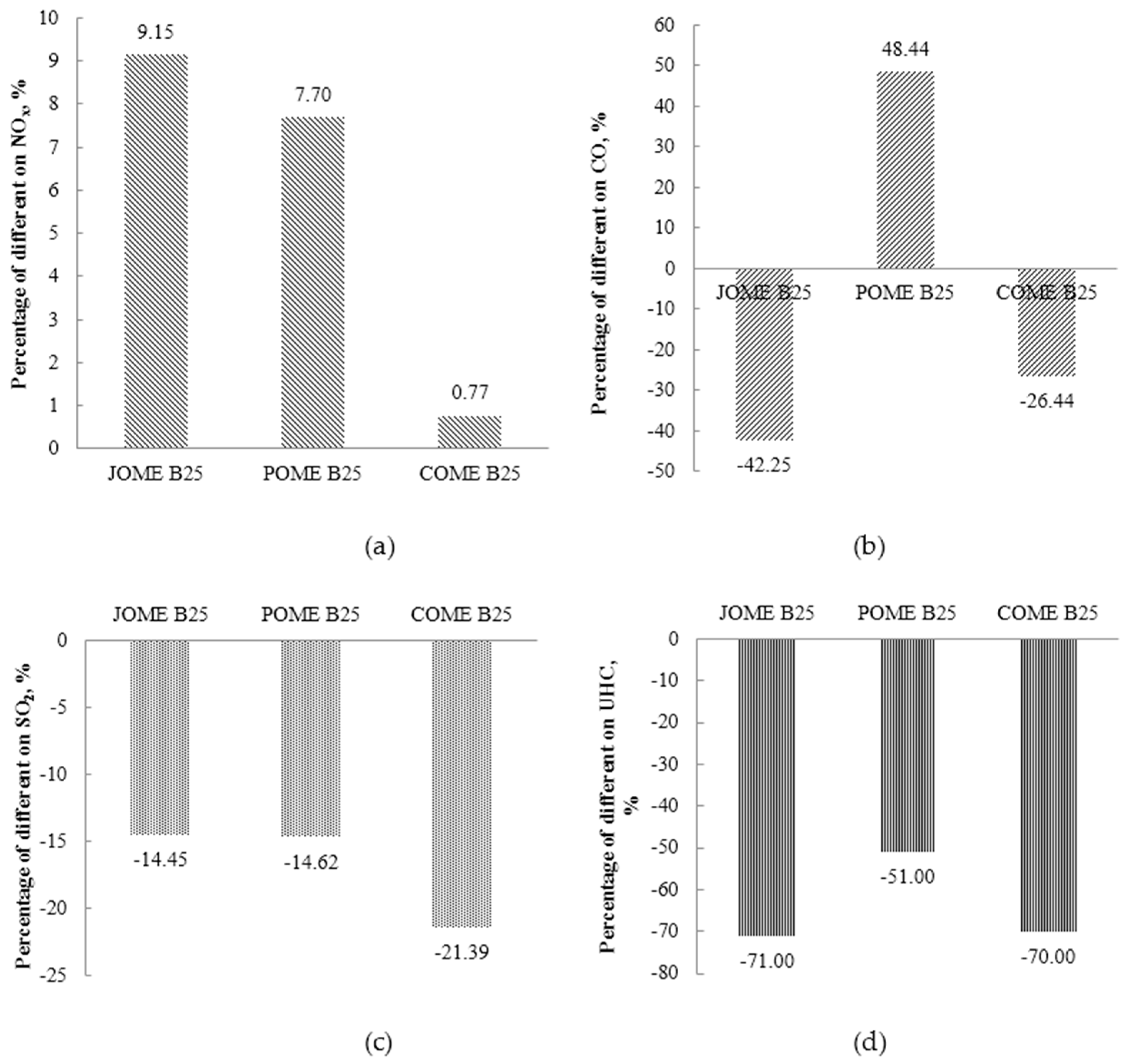
4. Results and Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ng, H.K.; Gan, S. Combustion performance and exhaust emissions from the non-pressurised combustion of palm oil biodiesel blends. Appl. Therm. Eng. 2010, 30, 2476–2484. [Google Scholar] [CrossRef]
- Yang, P.M.; Lin, K.C.; Lin, Y.C.; Jhang, S.R.; Chen, S.C. Emission evaluation of a diesel engine generator operating with a proportion of isobutanol as a fuel additive in biodiesel blends. Appl. Therm. Eng. 2016, 100, 628–635. [Google Scholar] [CrossRef]
- Anand, K.; Sharma, R.P.; Mehta, P.S. A comprehensive approach for estimating thermo-physical properties of biodiesel fuels. Appl. Therm. Eng. 2011, 31, 235–242. [Google Scholar] [CrossRef]
- Jindal, S.; Nandwana, B.P.; Rathore, N.S.; Vashistha, V. Experimental investigation of the effect of compression ratio and injection pressure in a direct injection diesel engine running on Jatropha methyl ester. Appl. Therm. Eng. 2010, 30, 442–448. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B. Development of biodiesel from karanja, a tree found in rural India. Fuel 2008, 87, 1740–1742. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Abedin, M.J.; Masjuki, H.H.; Kalam, M.A.; Sanjid, A.; Ashraful, A.M. Combustion, performance, and emission characteristics of low heat rejection engine operating on various biodiesels and vegetable oils. Energy Convers. Manag. 2014, 85, 173–189. [Google Scholar] [CrossRef]
- Darnoko, D.; Munir, C. Kinetics of Palm Oil Transesterification in a Batch Reactor. J. Am. Oil Chem. Soc. 2000, 77, 1263–1267. [Google Scholar] [CrossRef]
- Moser, B.R. Influence of extended storage on fuel properties of methyl esters prepared from canola, palm, soybean and sunflowers. Renew. Energy 2011, 36, 1221–1226. [Google Scholar] [CrossRef]
- Habibullah, M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Ashraful, A.M.; Mobarak, H.M. Biodiesel production and performance evaluation of coconut, palm and their combined blend with diesel in a single-cylinder diesel engine. Energy Convers. Manag. 2014, 87, 250–257. [Google Scholar] [CrossRef]
- How, H.G.; Masjuki, H.H.; Kalam, M.A.; Teoh, Y.H. An investigation of the engine performance, emissions and combustion characteristics of coconut biodiesel in a high-pressure common-rail diesel engine. Energy 2014, 69, 749–759. [Google Scholar] [CrossRef]
- Nakpong, P.; Wootthikanokkhan, S. High free fatty acid coconut oil as a potential feedstock for biodiesel production in Thailand. Renew. Energy 2010, 35, 1682–1687. [Google Scholar] [CrossRef]
- Banapurmath, N.R.; Tewari, P.G.; Hosmath, R.S. Performance and emission characteristics of a DI compression ignition engine operated on Honge, Jatropha and sesame oil methyl ester. Renew. Energy 2008, 33, 1982–1988. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Jiang, W.; Li, D.; Liu, S.; Liang, B. Biodiesel Production from Crude Jatropha curcas L. Oil with Trace Acid Catalyst. Chin. J. Chem. Eng. 2012, 20, 740–746. [Google Scholar] [CrossRef]
- Deng, X.; Fang, Z.; Liu, Y. Ultrasonic transesterification of Jatropha curcas L. oil to biodiesel by a two-step process. Energy Convers. Manag. 2010, 51, 2802–2807. [Google Scholar] [CrossRef]
- Juan, J.C.; Kartika, D.A.; Wu, T.Y.; Hun, T.Y.Y. Biodiesel production from Jatropha oil by catalytic and non-catalytic approaches: An overview. Bioresour. Technol. 2011, 102, 452–460. [Google Scholar] [CrossRef] [PubMed]
- El-Kasaby, M.; Nemit-allah, M.A. Experimental investigations of ignition delay period and performance of a diesel engine operated with Jatropha oil biodiesel. Alex. Eng. J. 2013, 52, 141–149. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Das, L.M. Combustion analysis of Jatropha, karanja and Polanga based biodiesel as fuel in a diesel engine. Fuel 2009, 88, 994–999. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Zhou, H.; Yang, Y.; Chen, M.; Liang, B. Production of biodiesel from Jatropha curcas L. oil. Comput. Chem. Eng. 2009, 33, 1091–1096. [Google Scholar] [CrossRef]
- Yunus, S.; Rashid, A.A.; Abdullah, N.R.; Mamat, R.; Latip, S.A. Emissions of Transesterification Jatropha-Palm Blended Biodiesel. Procedia Eng. 2013, 68, 265–270. [Google Scholar] [CrossRef]
- Rehman, A.; Phalke, D.R.; Pandey, R. Alternative fuel for gas turbine: Esterified jatropha oil-diesel blend. Renew. Energy 2011, 36, 2635–2640. [Google Scholar] [CrossRef]
- Ling, Z.; Zeng, X.; Ren, T.; Xu, H. Establishing a low-NOx and high-burnout performance in a large-scale, deep-air staging laboratory furnace fired by a heavy-oil swirl burner. Appl. Therm. Eng. 2015, 79, 117–123. [Google Scholar] [CrossRef]
- Sáez, A.; Flores-Maradiaga, A.; Toledo, M. Liquid butane as an alternative fuel for diesel oil burners. Appl. Therm. Eng. 2012, 45–46, 1–8. [Google Scholar] [CrossRef]
- Feyz, M.E.; Esfahani, J.A.; Pishbin, I.; Modarres Razavi, S.M.R. Effect of recess length on the flame parameters and combustion performance of a low swirl burner. Appl. Therm. Eng. 2015, 89, 609–617. [Google Scholar] [CrossRef]
- Ghorbani, A.; Bazooyar, B.; Shariati, A.; Jokar, S.M.; Ajami, H. A comparative study of combustion performance and emission of biodiesel blends and diesel in an experimental boiler. Appl. Energy 2011, 88, 4725–4732. [Google Scholar] [CrossRef]
- Buyukkaya, E. Effects of biodiesel on a DI diesel engine performance, emission and combustion characteristics. Fuel 2010, 89, 3099–3105. [Google Scholar] [CrossRef]
- Lin, B.F.; Huang, J.H.; Huang, D.Y. Experimental study of the effects of vegetable oil methyl ester on DI diesel engine performance characteristics and pollutant emissions. Fuel 2009, 88, 1779–1785. [Google Scholar] [CrossRef]
- Hashimoto, N.; Ozawa, Y.; Mori, N.; Yuri, I.; Hisamatsu, T. Fundamental combustion characteristics of palm methyl ester (PME) as alternative fuel for gas turbine. Fuel 2008, 87, 3373–3378. [Google Scholar] [CrossRef]
- Sharon, H.; Karuppasamy, K.; Soban Kumar, D.R.; Sundaresan, A. A test on DI diesel engine fueled with methyl esters of used palm oil. Renew. Energy 2012, 47, 160–166. [Google Scholar] [CrossRef]
- Özcanli, M.; Serin, H.; Saribiyik, O.Y.; Aydin, K.; Serin, S. Performance and Emission Studies of Castor Bean (ricinus Communis) Oil Biodiesel and Its Blends with Diesel Fuel. Energy Sources A 2012, 34, 1808–1814. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A. High quality biodiesel and its diesel engine application: A review. Renew. Sustain. Energy Rev. 2012, 14, 1999–2008. [Google Scholar] [CrossRef]
- Rakopoulos, C.D.; Antonopoulos, K.A.; Rakopoulos, D.C.; Hountalas, D.T.; Giakoumis, E.G. Comparative performances and emissions study of a direct injection Diesel engine blends of Diesel fuel with vegetables or bio-diesels of various origins. Energy Convers. Manag. 2006, 47, 3271–3287. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Varman, M.; Masjuki, H.H.; Kalam, M.A.; Hossain, S.; Sayeed, I. Tailoring fuel properties using jatropha, palm and coconut biodiesel to improve CI engine performance and emissions characteristics. J. Clean. 2015, 101, 262–270. [Google Scholar] [CrossRef]
- Palash, S.M.; Kalam, M.A.; Masjuki, H.H.; Masum, B.M.; Rizwanul Fattah, I.M.; Mofijur, M. Impacts of biodiesel combustion on NOx emissions and their reduction approaches. Renew. Sustain. Energy Rev. 2013, 23, 473–490. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Das, L.M.; Babu, M.K.G.; Naik, S.N. Biodiesel development from high acid value polanga seed oil and performance evaluation in a CI engine. Fuel 2007, 86, 448–454. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Atabani, A.E.; Rizwanul Fatah, I.M.; Mobarak, H.M. Comparative evaluation of performance and emission characteristics of Moringa oleifera, and Palm oil based biodiesel in a diesel engine. Ind. Crop. Prod. 2014, 53, 78–84. [Google Scholar] [CrossRef]
- Bojan, S.G.; Durairaj, S.K. Producing Biodiesel from High Free Fatty Acid Jatropha curcas Oil by A Two Step Method-An Indian Case Study. J. Sustain. Energy Environ. 2012, 3, 63–66. [Google Scholar]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Baroutian, S.; Aroua, M.K.; Abdul Raman, A.A.; Nik Sulaiman, N.M. Potassium hydroxide catalyst supported on palm shell activated carbon for transesterification of palm oil. Fuel Process. Technol. 2010, 91, 1378–1385. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.E.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- May, C.Y.; Liang, Y.C.; Foon, C.S.; Ngan, M.A.; Hook, C.C.; Basiron, Y. Key fuel properties of palm oil alkyl esters. Fuel 2005, 84, 1717–1720. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Yang, R.; Yan, Y. Biodiesel production from vegetable oil using heterogeneous acid and alkali catalyst. Fuel 2010, 89, 2939–2944. [Google Scholar] [CrossRef]
- Hashimoto, N.; Nishida, H.; Ozawa, Y. Fundamental combustion characteristics of Jatropha oil as alternative fuel for gas turbines. Fuel 2014, 126, 194–201. [Google Scholar] [CrossRef]
- Beer, J.M.; Chigier, N.A. Combustion Aerodynamics; Applied Science Publishers: London, UK, 1974. [Google Scholar]
- Lilley, D.G. Prediction of Inert Turbulent Swirl Flows. AIAA J. 1973, 11, 955–960. [Google Scholar] [CrossRef]
- Lefebvre, A.H. The role of fuel preparation in low emissions combustion. J. Eng. Gas Turbines Power 1995, 117, 617–654. [Google Scholar] [CrossRef]
- Gumus, M.; Sayin, C.; Canakci, M. The impact of fuel injection pressure on the exhaust emissions of a direct injection diesel engine fueled with biodiesel-diesel fuel blends. Fuel 2012, 95, 486–494. [Google Scholar] [CrossRef]
- Kalam, M.A.; Masjuki, H.H.; Jayed, M.H.; Liaquat, A.M. Emission and performance characteristics of an indirect ignition diesel engine fuelled with waste cooking oil. Energy 2011, 36, 397–402. [Google Scholar] [CrossRef]
| Feedstock | Kinematic Viscosity (cSt, at 40 °C) | Density (g/cm3) | Saponification Number | Iodine Value | Acid Value (mg KOH/g) | Cetane Number | Heating Value (MJ/kg) | References |
|---|---|---|---|---|---|---|---|---|
| Palm | 4.5–4.66 | 855–898.4 | 196.4–206 | 58–61 | 0.24–3.6 | 50–65 | 37.2–41.3 | [6,7,33,34] |
| Coconut | 2.726–4.1 | 807.3–877.1 | - | - | 0.106 | 55.0–60 | 36.98–38.10 | [6,11,34] |
| Soybean | 4.039–4.1 | 885–913.8 | - | 0.266 | 128–143 | 37.9–51 | 37.3–39.76 | [6,33] |
| Rapeseed | 4.44–4.7 | 882–887 | - | - | - | 53 | 37 | [6,33] |
| Corn | 3.62–5.8 | 873–913 | - | 103–140 | 0.34 | 37.6–49 | 41.18 | [25,35] |
| Jatropha | 4.4–4.75 | 869.2–880 | 192.6 | 93.8 | 0.27–3.8 | 53.5–57.1 | 38.5–41.17 | [7,36,37] |
| Karanja | 6.13 | 931 | - | - | 0.3–5.06 | 55 | 34–43.42 | [6,36] |
| Polanga | 4 | 888.6 | - | - | - | 57.3 | 39.25 | [34] |
| Moringa Oliefera | 5.05 | 869.6 | 199 | 77.5 | 8.62 | 56.3 | 40.05 | [38] |
| Property | Unit | Test Method | JOME | POME | COME | DIESEL | Standard Limits |
|---|---|---|---|---|---|---|---|
| Density at 15 °C | kg/m3 | ASTM D1298 | 0.8717 | 0.865 | 0.862 | 0.835 | 860–900 |
| Kinematic viscosity at 40 °C | (mm2/s) | ASTM D445 | 4.521 | 4.488 | 2.903 | 3.619 * | 1.9–6 |
| Acid value | (mg KOH/g) | ASTM D664 | 0.515 | 0.545 | 0.265 | - | 0.5 maximum |
| Flash point | °C | ASTM D93 | 170 | 174 | 106 | 77 * | >130 °C minimum |
| Pour point | °C | ASTM D97 | −6 | 15 | −3 | −20 * | −15 to −16 |
| Cloud Point | °C | ASTM D2500 | 6 | 11 | 6 | −35 * | −3 to −12 |
| Cetane number | - | ASTM-D613 | 54.8 | 59.2 | 64.6 | 48 * | 47 minimum |
| CFPP Cold Filter Plugging Point) | °C | ASTM D6371 | 1 | 13 | 1 | 44.7 * | Max + 5 |
| Calorific value | (kJ/kg) | ASTM-D240 | 39.7 | 39.4 | 37.6 | - | Report |
| Oxidation stability, 110 °C | hours | EN 15751 | 2.1 | 7.5 | 18.8 | - | 3 h minimum |
| Carbon residue (on 100% sample) | % m/m | ASTM D4530 | 0.01 | 0.01 | 0.01 | - | 0.050 maximum |
| Carbon | %wt | ASTM PS 121 | 76.7 | 76.2 | 72.6 | - | 77 |
| Hydrogen | %wt | ASTM PS 121 | 12.6 | 12.7 | 12.7 | - | 12 |
| Nitrogen | %wt | ASTM PS 121 | <0.1 | 0.1 | <0.1 | - | - |
| Oxygen | %wt | ASTM PS 121 | 10.8 | 11.0 | 14.7 | - | 11 |
| Total sulphur | mg/kg | ASTM 5453-12 | 1.8 | 2.2 | 1.9 | - | 15 maximum |
| Parameter | Measuring Range | Measurement Method |
|---|---|---|
| NOx | 200–5000 ppm | NDIR |
| SO2 | 200–5000 ppm | NDIR |
| CO | 200–5000 ppm | NDIR |
| CO2 | 5–25 vol% | NDIR |
| O2 | 10–25 vol% | Magneto-pneumatic detection |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Rahim, N.; Mohd Jaafar, M.N.; Sapee, S.; Elraheem, H.F. Effect on Particulate and Gas Emissions by Combusting Biodiesel Blend Fuels Made from Different Plant Oil Feedstocks in a Liquid Fuel Burner. Energies 2016, 9, 659. https://doi.org/10.3390/en9080659
Abdul Rahim N, Mohd Jaafar MN, Sapee S, Elraheem HF. Effect on Particulate and Gas Emissions by Combusting Biodiesel Blend Fuels Made from Different Plant Oil Feedstocks in a Liquid Fuel Burner. Energies. 2016; 9(8):659. https://doi.org/10.3390/en9080659
Chicago/Turabian StyleAbdul Rahim, Norwazan, Mohammad Nazri Mohd Jaafar, Syazwana Sapee, and Hazir Farouk Elraheem. 2016. "Effect on Particulate and Gas Emissions by Combusting Biodiesel Blend Fuels Made from Different Plant Oil Feedstocks in a Liquid Fuel Burner" Energies 9, no. 8: 659. https://doi.org/10.3390/en9080659